Evaluation of Increasing Levels of Acacia mearnsii Tannins on Growth Performance and Intestinal Morphometrics of Broiler Chickens Undergoing a Salmonella Heidelberg Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Housing, Experimental Feeds, and S. Heidelberg Challenge
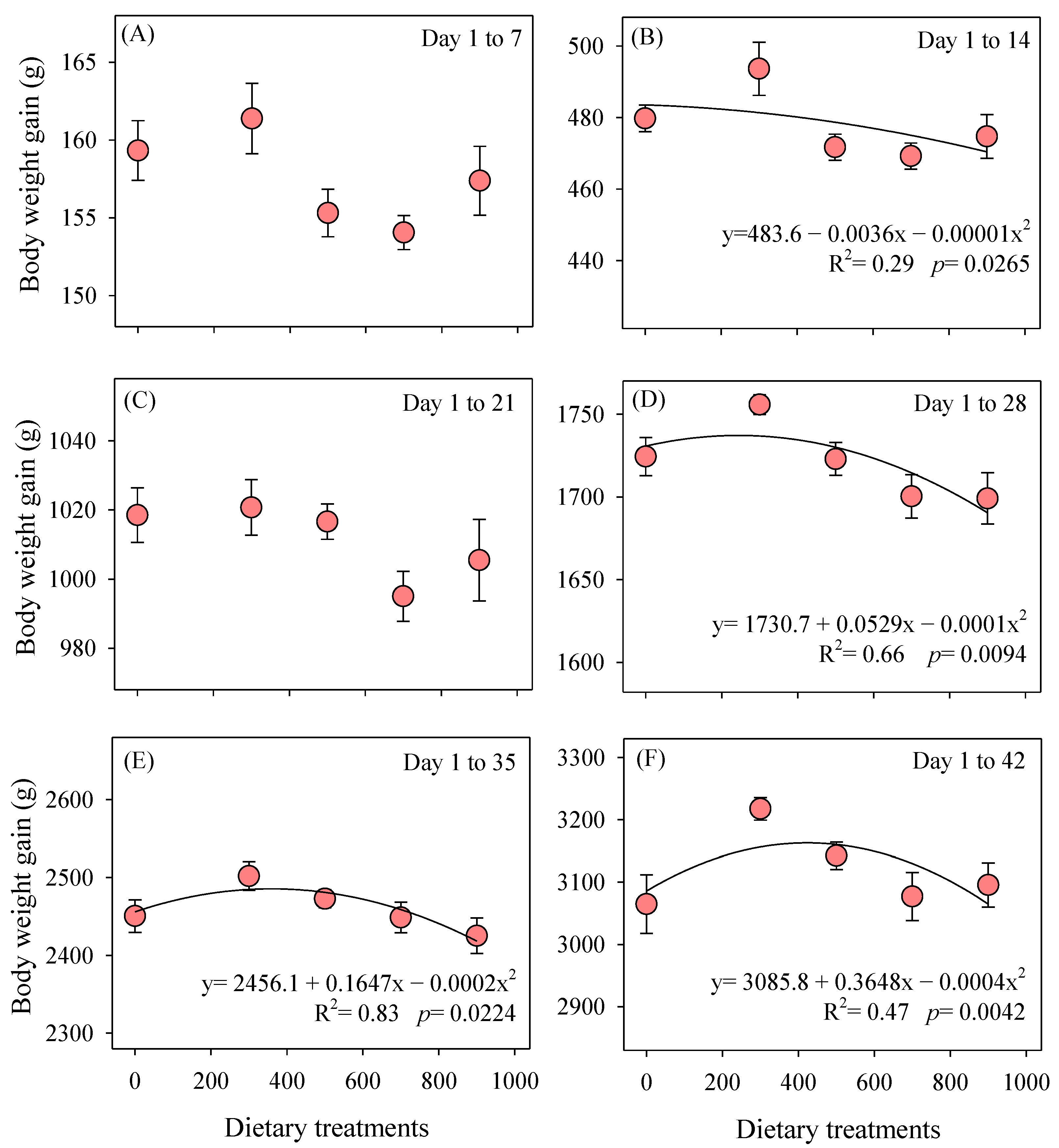
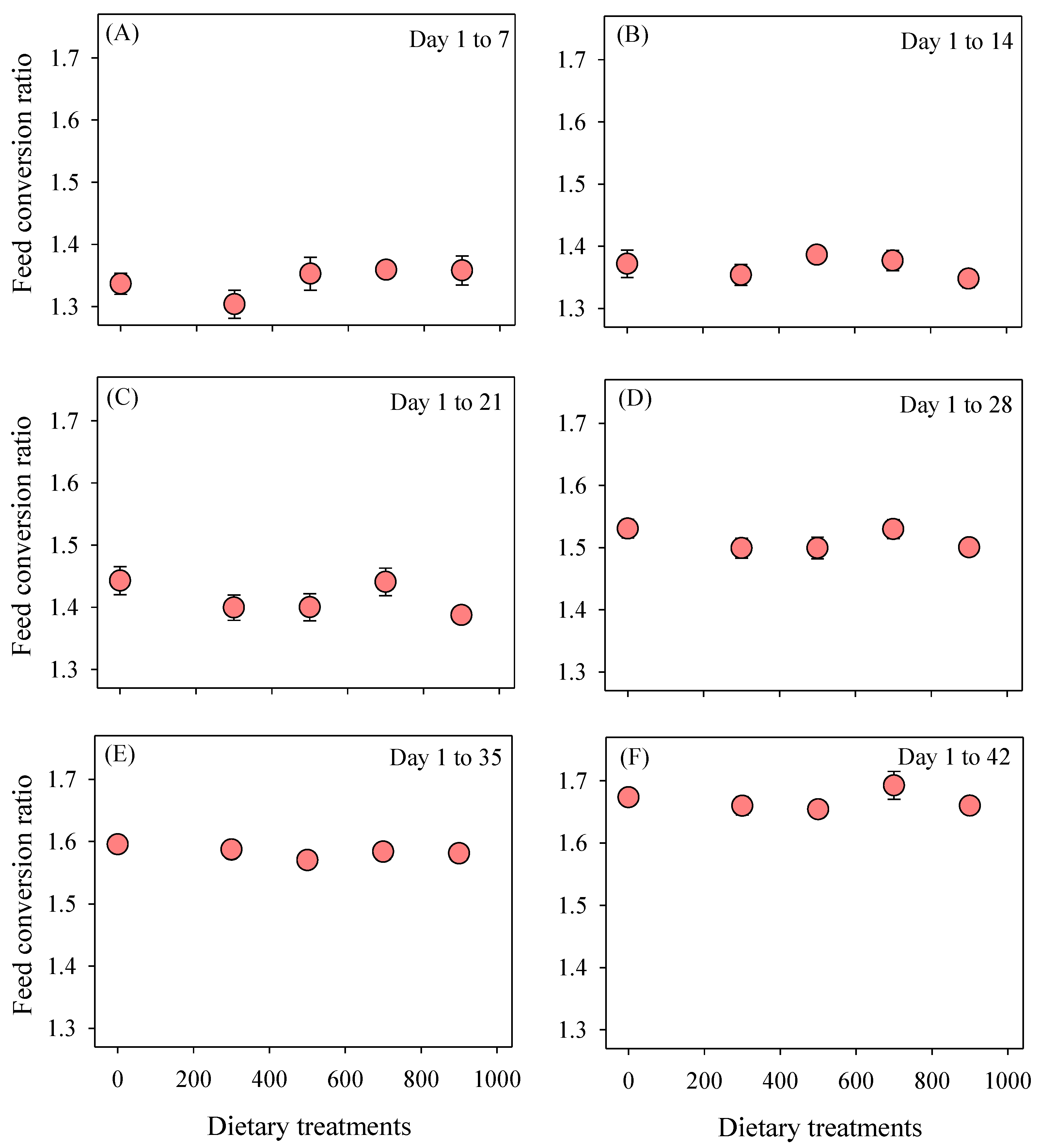
2.2. Growth Performance
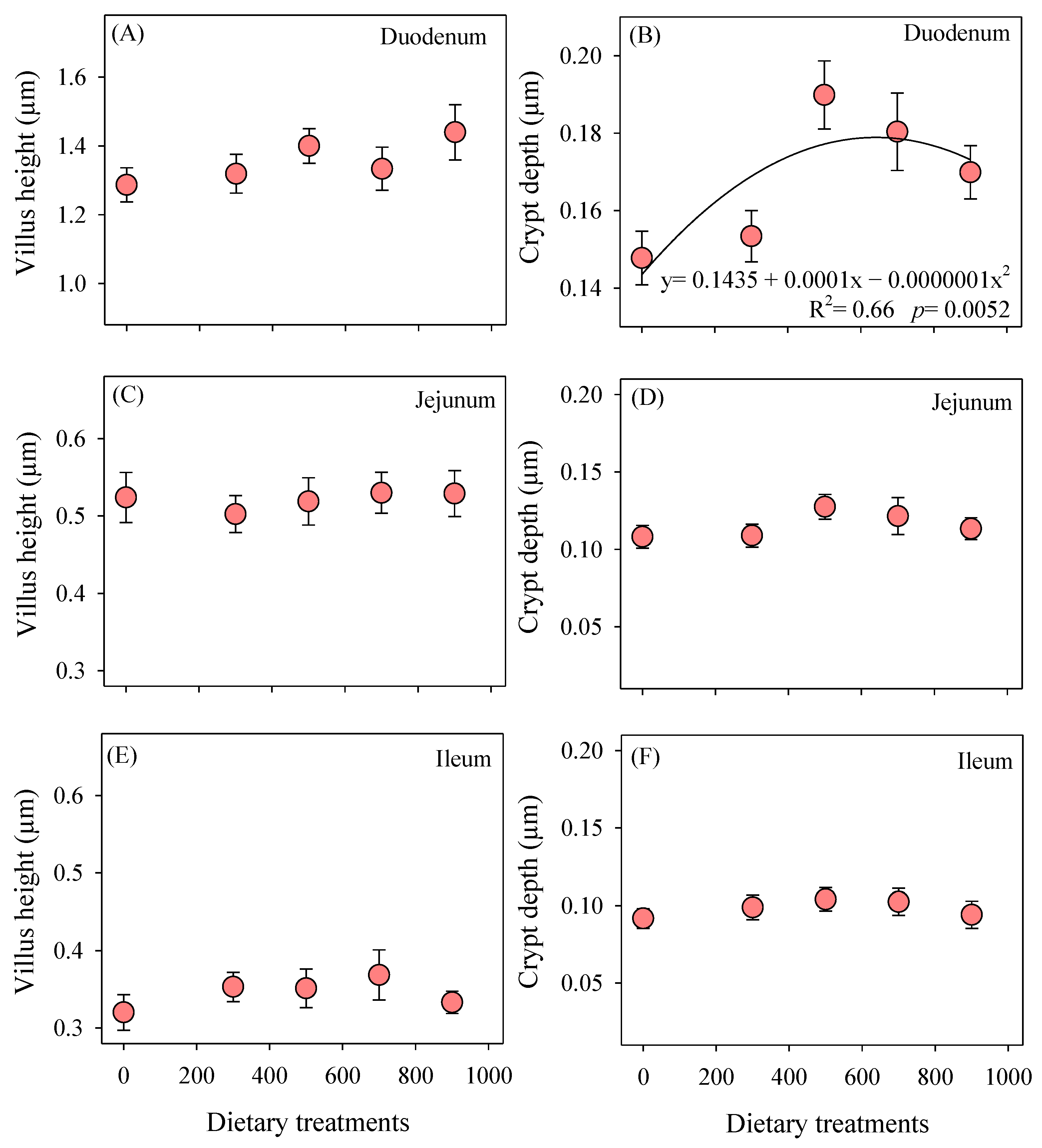
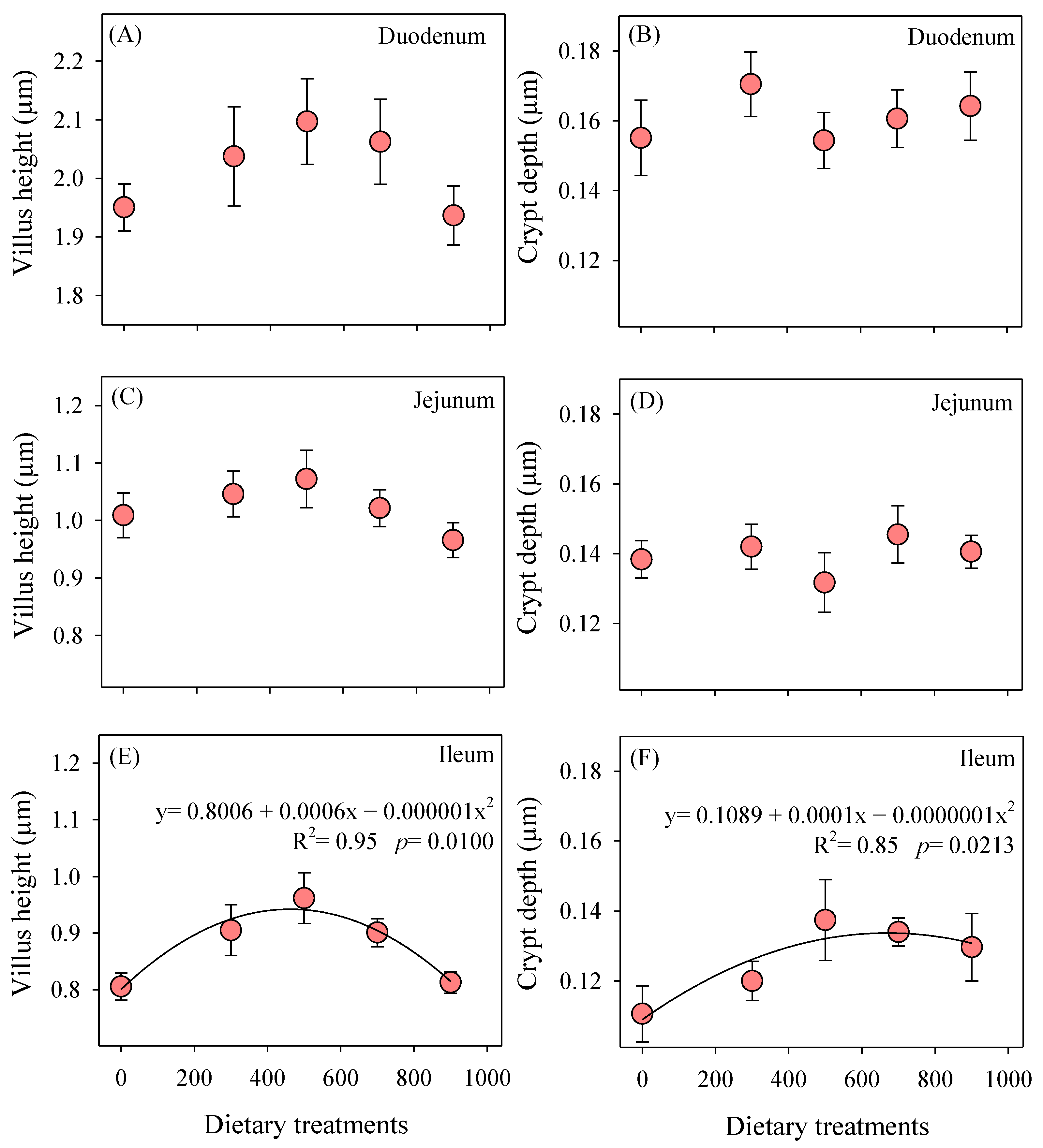
2.3. Intestinal Measurements
2.4. Litter Moisture and Cecal Content Collection
2.5. Statistical Analysis
3. Results
3.1. Cumulative Growth Performance
3.2. Intestinal Measurements
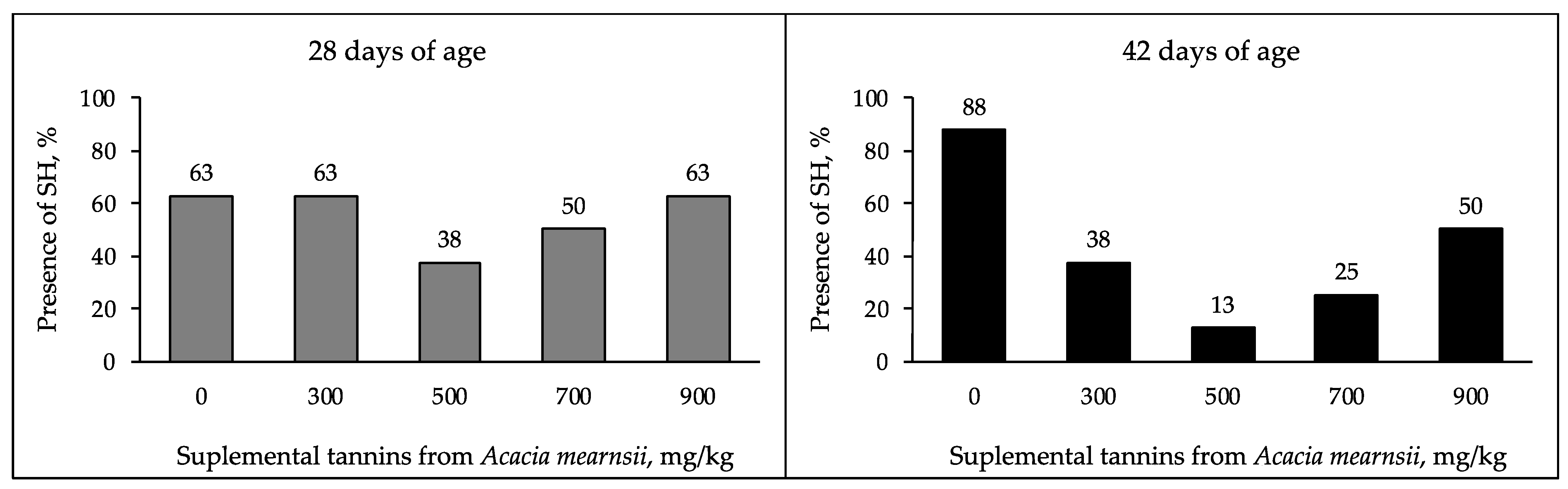
3.3. Litter Moisture and Cecal Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ochoa, I.M.F.; Rodríguez, A.V. Mecanismos moleculares de patogenicidad de Salmonella sp. Rev. Latinoam. Microbiol. 2005, 47, 25–42. [Google Scholar]
- Barrow, P.A.; Neto, O.C.F. Pullorum disease and fowl typhoid-new thoughts on old diseases: A review. Avian Pathol. 2011, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Colla, F.L.; Rodrigues, L.B.; Dickel, E.L.; Borsoi, A.; Nascimento, V.P.d.; Santos, L.R.d. Avaliação in vitro de clorexidina, amônia quaternária e ácido peracético frente a amostras de Salmonella Heidelberg isoladas de abatedouro avícola em 2005 e 2009. Pesqui. Vet. Bras. 2012, 32, 289–292. [Google Scholar] [CrossRef]
- Pulido-Landínez, M.; Sánchez-Ingunza, R.; Guard, J.; Nascimento, V.P.d. Assignment of serotype to Salmonella enterica isolates obtained from poultry and their environment in southern Brazil. Lett. Appl. Microbiol. 2013, 57, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Voss-Rech, D.; Kramer, B.; Silva, V.S.; Rebelatto, R.; Abreu, P.G.; Coldebella, A.; Vaz, C.S.L. Longitudinal study reveals persistent environmental Salmonella Heidelberg in Brazilian broiler farms. Vet. Microbiol. 2019, 233, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Dutil, L.; Irwin, R.; Finley, R.; Ng, L.K.; Avery, B.; Boerlin, P.; Bourgault, A.M.; Cole, L.; Daignault, D.; Desruisseau, A.; et al. Ceftiofur resistance in Salmonella enteric serovar Heidelberg from chicken meat and humans, Canada. Emerg. Infect. Dis. 2010, 16, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Reynolds, J.; Karp, B.E.; Tate, H.; Fedorka-Cray, P.J.; Plumblee, J.R.; Hoekstra, R.M.; Whichard , J.M.; Mahon, B.E. Ceftriaxone-Resistant nontyphoidal Salmonella from humans, retail meats, and food animals in the United States, 1996–2013. Foodborne Pathog. Dis. 2017, 14, 74–83. [Google Scholar] [CrossRef]
- EFSA. Salmonella control in poultry flocks and its public health impact. EFSA J. 2019, 17, 5596. [Google Scholar] [CrossRef]
- Neves, G.B.; Pick, E.; Giuriatti, J.; Araujo, D.N.; Stefani, L.M. A comparative study on Salmonella Enteritidis, S. Heidelberg and S. typhimurium of poultry origin from Southern Brazil. Ann. Med. Med. Res. 2020, 3, 1027. [Google Scholar]
- Bragg, R.; Jansen, A.; Coetzee, M.; van der Westhuizen, W.; Boucher, C. Bacterial resistance to quaternary ammonium compounds (QAC) disinfectants. In Infectious Diseases and Nanomedicine II; Adhikari, R., Thapa, S., Eds.; Springer: Kathmandu, Nepal; New Delhi, India, 2014; pp. 1–13. [Google Scholar] [CrossRef]
- Mc Carlie, S.; Boucher, C.E.; Bragg, R.R. Molecular basis of bacterial disinfectant resistance. Drug Resist. Update 2020, 48, 100672. [Google Scholar] [CrossRef]
- Toshitsugu, T.; Takashi, T.; Isao, K. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Reyes, A.W.B.; Hong, T.G.; Hop, H.T.; Arayan, L.T.; Huy, T.X.N.; Min, W.; Lee, H.J.; Lee, K.S.; Kim, S. The in vitro and in vivo protective effects of tannin derivatives against Salmonella enterica serovar Typhimurium infection. Microb. Pathog. 2017, 109, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Godoy, G.L.; Rodrigues, B.N.; Agilar, J.C.; Biselo, V.; Brutti, D.D.; Maysonnave, G.S.; Stefanello, C. Effects of Acacia mearnsii tannins on growth performance, footpad dermatitis, nutrient digestibility, intestinal permeability, and meat quality of broiler chickens. Anim. Feed Sci. Technol. 2024, 308, 115875. [Google Scholar] [CrossRef]
- Chan, J.M.; Day, P.; Feely, J.; Thompson, R.; Little, K.M.; Norris, C.H. Acacia mearnsii industry overview: Current status, key research and development issues. Southern For. A J. For. Sci. 2015, 77, 19–30. [Google Scholar] [CrossRef]
- Botha, J.J.; Ferreira, D.; Roux, D.G.; Hull, W.E. Condensed tannins: Condensation mode and sequence during formation of synthetic and natural triflavonoids. J. Chem. Soc. Chem. Commun. 1979, 11, 510–512. [Google Scholar] [CrossRef]
- Choi, J.; Kim, W.K. Dietary application of tannins as a potential mitigation strategy for current challenges in poultry production: A Review. Animals 2020, 10, 2389. [Google Scholar] [CrossRef]
- Cobb-Vantress. Breeder Management Guide Cobb 500; Cobb-Vantress: Siloam Springs, AR, USA, 2018. [Google Scholar]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histotechnology—Armed Forces Institute of Pathology (AFIP); American Registry of Pathology: Washington, DC, USA, 1994. [Google Scholar]
- Association of Official Analytical Chemistry. Official Methods of Analysis, 18th ed.; Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Mooijman, K.A.; Pielaat, A.; Kuijpers, A.F.A. Validation of EN ISO 6579-1—Microbiology of the food chain—Horizontal method for the detection, enumeration and serotyping of Salmonella—Part 1 detection of Salmonella spp. Int. J. Food Microbiol. 2019, 288, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Appl. Math. 2014, 5, 2952. [Google Scholar] [CrossRef]
- Andrade, D.F.; Ogliari, P.J. Estatística para as Ciências Agrárias e Biológicas, com Noções de Experimentação; Editora da UFSC: Florianópolis, Brazil, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 21 May 2024).
- Gast, R.K. Diseases of Poultry. In Salmonella Infections, 13th ed.; Wiley-Blackwell: Ames Iowa, IA, USA, 2013; pp. 677–736. [Google Scholar]
- Penha Filho, R.A.C.; Díaz, S.J.A.; Fernando, F.S.; Chang, Y.F.; Andreatti Filho, R.L.; Berchieri, A., Jr. Immunomodulatory activity and control of Salmonella Enteritidis colonization in the intestinal tract of chickens by Lactobacillus based probiotic. Vet. Immunol. Immunopathol. 2015, 167, 64–69. [Google Scholar] [CrossRef]
- Brenner, F.W.; Villar, R.G.; Angulo, F.J.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [CrossRef]
- Sausen, L.; Mendes, A.S.; Sikorski, R.R.; Uliana, R.F.; Zago, C.H.F. Use of organic acid blends to control spread of Salmonella Heidelberg in broilers. Med. Vet. 2022, 16, 49–58. [Google Scholar] [CrossRef]
- Iacob, S.; Iacob, D.G.; Luminos, L.M. Intestinal microbiota as a host defense mechanism to infectious threats. Front. Microbiol. 2019, 9, 3328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Prosdócimo, F.; Batallé, M.; Sosa, N.; De Franceschi, M.; Barrios, H. Determinación in vitro del efecto antibacteriano de un extracto obtenido de quebracho colorado, Schinopsis lorentzii. InVet 2010, 12, 139–143. [Google Scholar]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, R.; Liang, J.B.; Jahromi, M.F.; Shokryazdan, P.; Ebrahimi, M.; Chen, M.L.; Goh, Y.M. Effects of tannic acid on performance and fatty acid composition of breast muscle in broiler chickens under heat stress. Ital. J. Anim. Sci. 2015, 14, 3956. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.Q.; Jin, L.; Xu, Z.J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef]

| Item | Pre-Starter (Day 1 to 7) | Starter (Day 8 to 21) | Grower (Day 22 to 35) | Finisher (Day 36 to 42) |
|---|---|---|---|---|
| Ingredients, % | ||||
| Corn | 52.00 | 57.36 | 66.92 | 66.08 |
| Soybean meal | 40.70 | 35.29 | 26.00 | 26.95 |
| Soybean oil | 2.80 | 3.00 | 3.50 | 3.80 |
| Dicalcium phosphate | 1.98 | 1.75 | 1.16 | 1.15 |
| Limestone | 0.99 | 0.91 | 0.77 | 0.72 |
| Salt | 0.47 | 0.46 | 0.34 | 0.34 |
| Sodium bicarbonate | 0.01 | 0.01 | 0.02 | 0.02 |
| DL-Methionine, 99% | 0.38 | 0.40 | 0.58 | 0.29 |
| Lysine sulphate, 78% | 0.27 | 0.36 | 0.35 | 0.31 |
| L-Threonine, 98.5% | 0.10 | 0.16 | 0.12 | 0.10 |
| Choline chloride, 60% | 0.05 | 0.05 | 0.04 | 0.04 |
| Vitamin and mineral premix 1 | 0.25 | 0.25 | 0.20 | 0.20 |
| Nutrient and energy composition, % or as shown | ||||
| ME, kcal/kg | 2975 | 3050 | 3100 | 3150 |
| Crude protein | 23.10 | 20.80 | 19.06 | 18.17 |
| Ca | 0.90 | 0.84 | 0.78 | 0.76 |
| Av. P | 0.43 | 0.40 | 0.38 | 0.37 |
| Na | 0.21 | 0.20 | 0.19 | 0.18 |
| Choline, mg/kg | 1600 | 1600 | 1500 | 1500 |
| Dig. Lys | 1.25 | 1.12 | 1.02 | 0.97 |
| Dig. Met + Cys | 0.93 | 0.83 | 0.75 | 0.72 |
| Dig. Thr | 0.83 | 0.74 | 0.67 | 0.64 |
| Dig. Arg | 1.44 | 1.28 | 1.15 | 1.09 |
| Dig. Val | 0.96 | 0.86 | 0.79 | 0.75 |
| Dig. Ile | 0.90 | 0.80 | 0.72 | 0.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maysonnave, G.S.; Brutti, D.D.; Silva, V.M.d.; Stefanello, C. Evaluation of Increasing Levels of Acacia mearnsii Tannins on Growth Performance and Intestinal Morphometrics of Broiler Chickens Undergoing a Salmonella Heidelberg Challenge. Poultry 2024, 3, 284-297. https://doi.org/10.3390/poultry3030021
Maysonnave GS, Brutti DD, Silva VMd, Stefanello C. Evaluation of Increasing Levels of Acacia mearnsii Tannins on Growth Performance and Intestinal Morphometrics of Broiler Chickens Undergoing a Salmonella Heidelberg Challenge. Poultry. 2024; 3(3):284-297. https://doi.org/10.3390/poultry3030021
Chicago/Turabian StyleMaysonnave, Greicy Sofia, Danielle Dias Brutti, Vitória Mendonça da Silva, and Catarina Stefanello. 2024. "Evaluation of Increasing Levels of Acacia mearnsii Tannins on Growth Performance and Intestinal Morphometrics of Broiler Chickens Undergoing a Salmonella Heidelberg Challenge" Poultry 3, no. 3: 284-297. https://doi.org/10.3390/poultry3030021
APA StyleMaysonnave, G. S., Brutti, D. D., Silva, V. M. d., & Stefanello, C. (2024). Evaluation of Increasing Levels of Acacia mearnsii Tannins on Growth Performance and Intestinal Morphometrics of Broiler Chickens Undergoing a Salmonella Heidelberg Challenge. Poultry, 3(3), 284-297. https://doi.org/10.3390/poultry3030021







