Abstract
Organic poultry sector needs high-quality proteins sources to meet specific requirements. The EU’s organic regulation forbids synthetic amino acids; therefore, soybean, with its balanced essential amino acid content, has become the most used protein source, though much of it is imported from non-EU countries, with sustainability and crop competition issues; therefore, it should be substituted with a high-protein-value alternative such as insect meal. In this study, 900 Aviagen Savanna broilers were fed with three different organic diets: soybean only (S100), 50% black soldier fly larvae meal (BSL) and 50% soybean (BSL50), and 100% BSL only (BSL100). Broiler performance, welfare, and fatty acids (FA) were analyzed. BSL50 and BSL100 negatively affected growth, while only BSL100 worsened all of the market-related performances. Meat showed a significant increase in saturated FA (SFA) (p < 0.000) and a corresponding decrease in polyunsaturated FA (PUFA), in BSL50 and BSL100, but α-linolenic acid was not affected by BSL50 treatment (p < 0.000). The SFA increase could represent a negative aspect for human health (e.g., cardiovascular diseases), but, as reported by other authors, medium chain SFA, (i.e., lauric acid), may show beneficial effects as well (i.e., antibacterial, anti-inflammatory and antioxidant properties).
Keywords:
organic broiler; protein feed; insect meal; lauric acid; meat quality; nutritional indices 1. Introduction
Soybean is the most used protein source in animal feeding, especially in poultry production, thanks to its high amino acid profile [1]. However, increasing demand for soybean production brings up some important issues linked to economic, environmental, and social challenges. Fluctuating prices, deforestation, excessive water use, increased carbon dioxide (CO2) emissions for long transport distances, and competition with human crops make the current level of soybean production unsustainable [2,3]. In organic poultry production, where synthetic amino acids are not allowed, soybean is the most widely used protein source, and among amino acids, its contents of lysine and methionine, two limiting amino acids for birds, have special weights [1]. Among EU countries, organic poultry production shows higher annual growth rates (11%) than other types of animal production and, at the same time, the feed industry’s demand for high-quality proteins is increasing (A Decade of Organic Growth Organic Farming in the EU N°20 Agricultural Market Brief, 2023). Consequently, European soybean production cannot cover animal needs, meaning that organic poultry production depends on imports from non-EU countries, leading to it being afflicted by issues related to its sustainability. Therefore, alternative proteins are necessary to replace soybean in poultry feeds. The most promising protein alternative is insect meal, which has an amino acid profile close to that of soybean, in terms of some essential (i.e., leucine, lysine, valine, and histidine) or non-essential amino acids (glutamic and aspartic acids) [4]. Insects can transform huge biomass, coming from byproducts of food industries, into high-value protein, fatty acids, mineral compounds, and vitamins well suited for feeding poultry, fish and pigs, and they could help to reduce the EU’s protein deficit [5]. Besides their nutritional value, insects contain bioactive molecules that contribute to improving the health of livestock animals, such as lauric acid, α-helical-peptides, cysteine-rich peptides, proline-rich peptides, glycine-rich peptides (antimicrobial peptides), chitin and chitosan [6]. Insects are authorized by the EU for use as feed for poultry and pigs in Regulations EC 2021/1372, amending Annex IV of Regulation EC 2001/999. So far, insects have not been included in organic certification even if, according to Regulation EU 2018/848 art. 20 and 21, insects and insect meal can legally be used in organic farming, albeit only if reared and produced according to the rules of organic production. Among insects used for poultry feeding, Black Soldier fly (BS) is considered to be the most used and available insect species [5,7,8,9]. Compared to other insect species, BS needs the lowest rearing space, water, and energy resources and has the lowest substrate requirements; thus, it can be used on a large scale and in continuous industrial production [10,11]. Nogales-Mérida et al. [12] acknowledged that BS Larvae (BSL) meal has a desirable nutritional composition that includes not only favorable amino acid levels but also good antimicrobial properties. The nutrient composition of BSL varies depending on the breeding substrate [13]; in any case, BSL meal generally contains high levels of protein, ranging from 49 to 59% of dry matter (DM), with lysine, cysteine, methionine, threonine, and other nutrients, such as calcium and phosphorous, that are important for animal nutrition [14,15]. However, despite its good amino acid profile, many studies have shown that BSL has limited soybean substitution power due to its high saturated fatty acid content [11,16,17,18,19,20]. Generally, defatted insect meal is preferred for maintaining nutrient balance and ensuring uniform feed formulation. However, even if defatted, BSL meal is rich in medium chain saturated fatty acids, such as lauric acid (C12:0), produced by insects from unsaturated fatty acids (UFAs) [13,21,22,23,24]. During its lifetime, BSL bioaccumulates oleic acid (C18:1 n-9), linoleic acid (C18:2 n-6) (LA), and α-linolenic acid or ALA (C18:3 n-3), and it mainly metabolizes them to create lauric acid via a system of regulatory enzymes [25,26]. Lauric acid has improved the intestinal microbiota of poultry inhibiting the grow of pathogens, (i.e., C. perfringens strains), by the separation of the inner and the outer membranes [27,28]. However, from a food quality point of view, lauric acid can substantially affect meat quality in terms of fatty acid profile at the expense of polyunsaturated fatty acids (PUFA), that are significantly reduced, with risk the related risks for quality and health [18,29,30,31].
Considering the positive and negative effects of the inclusion of BSL meal in broiler’s diet, the actual interest, in organic system production, would be to maximize the substitution of soybean to overcome the unsustainability of imported soybean or, at least, to reduce the import volume in EU. Our study responds to a precise issue raised by organic broiler producers. Imported soybean reached high prices, being very dependent on market fluctuations and dumping, and it is difficult to keep away non-compliant soybean (e.g., GMO). As general principle, organic production, according to Regulation EU 848/2018, should decrease external inputs, especially if they are unsustainable. According to the Analytical Brief N° 2 “EU imports of organic agri-food products” of July 2023, [32] the import of organic soybean increases proportionally to the demand for organic poultry needs. Other vegetal protein feed sources, already used, have less protein content and more antinutritional factors [33]. Therefore, the most interesting alternatives come from the animal kingdom or from the sea [33]. Insects are promising because they can be raised everywhere, and they can transform huge quantity of vegetal byproduct coming from the food industry or from agricultural processing. They have been long tested from a growth performance point of view, even though studies on meat quality are limited and/or performed under different conditions or methods. We focused on the study of the fatty acid profile and the impact of high-level inclusion of Hermetia illucens meal on meat quality. A second goal was to explore what was the maximum rate of soybean substitution with no substantial changes in meat quality.
Our work explored and analyzed the potential beneficial/unfavorable effects associated with the high replacement of organic soybean with defatted BSL meal (50%, and 100%) on performance, animal welfare, meat quality, nutritional indices, and potential side effects.
2. Materials and Methods
2.1. Animals, Management, and Feed
The experimental trial, submitted to CREA Ethic Committee named OPBA (Organismo Preposto al Benessere Animale, Roma, Italy), was approved with approval number 17433 of 29 February 2024.
The broiler rearing cycle was carried out at a commercial poultry farm managed by the Council for Agricultural Research and Analysis of Agricultural Economics (CREA) in Monterotondo (RM), Italy.
The poultry house had a 153 m2 (18 m × 8.5 m) area equipped with automatic feeding and watering systems. Environmental conditions and animal behavior were detected using a robot equipped with cameras and sensors (Scout®, Farm Robotics and Automation, Vilanova i la Geltrù, Spain).
An aisle situated on the long side of the poultry house was used to monitor the animals without directly interfering with them.
In total, 900 Aviagen® Ranger SAVANNA, purchased from the breeder farmm Aglietto Natura, Brianzé, Italy, female chicks were housed, in compliance with organic Regulation EU 848/2018, at one day of age on a 5 cm thick shaved wood litter, and they were vaccinated against coccidia, Marek’s disease, pseudo-plague, infectious bronchitis, and Gumboro’s disease. The poultry house was divided into 6 boxes (3.5 m × 6 m) of 150 animals each (two replicates per treatment), under strictly controlled conditions (temperature, humidity, air flow, lighting, CO2, NH3, feeding and drinking), through the robot. The six groups were located along the short side of the poultry house. No differences were found within randomized blocks and so data for the same treatments have been pooled to perform statistics. From each group, 15 animals were randomly selected to analyze post-mortem performances and fatty acid profile. The poultry house was heated with non-dazzle medium-wave heating infrared lamps (Syner Progetti, Mantova, Italy) 48 h before the animals’ arrival, ultimately reaching a temperature of 30 °C. The room temperature was decreased to 20 °C until day 27. The birds had access to a 4 m2 per bird outdoor area, divided into 6 sectors and covered by grass and trees, from the 28th day of age onward.
The animals were all fed ad libitum for their entire lifetimes. Until the 30th day of their lives (1° period), the chickens were fed with the same organic feed, purchased via the market, which had a protein content of 21.6 g/100 g. The protein source was only soybean, representing 33.80% of feed composition (Table 1 and Table 2).

Table 1.
1° and 2° period’s feed ingredients.

Table 2.
1° and 2° period’s feed composition (as is base).
Starting from the 31st day of life (2° period), the groups were fed 3 different diets. In Group 1, also known as the control group, animals were fed with an organic diet, with only soybean making up the high protein matter (S100); group 2 was fed a diet of 50% soybean replaced with BSL defatted meals (BSL50), while group 3 was fed 100% BSL defatted meal as a protein source (BSL100) (Table 1). The feed was formulated in collaboration with an Italian organic feed mill. Table 2 shows the chemical composition of each diet. BSL, in dehydrated and defatted form, was purchased from Hexafly (Ireland), and its protein content was 53.10% (Table 3).

Table 3.
BSL meal analytical components (as is base).
During rearing, only the normal routine activities of a commercial farm were performed, and no interventional operation was performed on the animals.
2.2. Sample Collection and Analysis
At 75 days old, the broilers were slaughtered in an EC-authorized, commercial slaughterhouse in the presence of a veterinarian. The animals were slaughtered before the 81st day of life to ensure compliance with Regulation 848/2018.
Some welfare parameters, such as the cleanliness of plumage, pododermatitis, hock burning, breast reddening, and breast lesions, which were extrapolated from the “Welfare Quality® assessment protocol for poultry”, were measured at the slaughterhouse. Litter quality and lameness were assessed in the poultry house once per week during the last three weeks. The scores for all ABMs indicators were 0 for the best condition and 3 for the worst condition, the score for the litter condition was 0 for the best and 4 for the worst.
After slaughtering, at the slaughterhouse, 30 carcasses were randomly selected from each diet group, out of a total of 90 animals, before being weighed and dissected in CREA laboratories in Monterotondo (RM). The thigh and breast muscles were separated from the carcasses, before being homogenized and stored at −80 °C until the fatty acid analysis was performed.
Total fat was extracted according to the method of Folch et al. [34]. In total, 100 mg of fat extract were methylated via the addition of methanolic potassium hydroxide (2N), based on the IUPAC procedure (1992), to obtain fatty acids methyl ester (FAME). The C19:0 fatty acid was used as the internal standard. Methyl esters were injected into a GC-FID gas chromatograph (GC 6890 N, Agilent, Inc., Santa Clara, CA, USA) using a CP-Sil88 100 m 0.25 (0.2) column (Agilent Technologies) under the operating conditions described by Failla et al. [35]. Fatty acid methyl ester standards, such as Supelco 37 Component FAME Mix, C22:4 n-6, C22:5 n-3 DPA, and C19:0 (Sigma Aldrich (Oakville, ON, Canada), were used to identify fatty acids. Fatty acids were expressed as the percentage of total FAME. Furthermore, we calculated the principal fatty acid classes as the sum of the saturated fatty acids (ƩSFA), the sum of the monounsaturated fatty acids (ƩMUFA), the sum of the polyunsaturated fatty acids (ƩPUFA), the sum of the n-6 fatty acids (Ʃn-6 PUFA), the sum of the n-3 fatty acids (Ʃn-3 PUFA), the n-6-to-n-3 ratio (n-6/n-3), and the PUFA-to-SFA ratio (PUFA/SFA).
Some nutritional indices were calculated besides the PUFA-to-SFA ratio (PUFA/SFA), which is normally used as the atherogenicity indicator of food.
The index of atherogenicity (IA) takes into consideration the following three hypercholesterolaemic SFA [36]:
IA = [C12:0 + (4 × C14:0) + C16:0]/ƩUFA.
The index of thrombogenicity (IT), as developed by Ulbritcht and Southgate, in 1991 [36]:
IT = (C14:0 + C16:0 + C18:0)/[(0.5 × ƩMUFA) + (0.5 × Ʃn-6 PUFA) + (3 × Ʃn-3 PUFA) + (n-3/n-6)].
The hypocholesterolemic/hypercholesterolemic ratio (hH), a nutritional index proposed for the first time by Santos-Silva et al. [37], with a formula optimized by Mierlita [38], adding C12:0 in hypercholesterolemic fatty acid:
hH = (cis-C18:1 + ƩPUFA)/(C12:0 + C14:0 + C16:0).
The health-promoting index (HPI) is the inverse of the atherogenic index proposed by Chen et al. [39]:
HPI = ƩUFA/[C12:0 + (4 × C14:0) + C16:0]
The following four nutritional indices assess the unsaturated fatty acid content. UI indicates the degree of unsaturation in lipids, and it is calculated as the sum of the percentage of each of the unsaturated fatty acids multiplied by the number of double bonds within that FA [40]:
UI = 1 × (% monoenoics) + 2 × (% dienoics) + 3 × (% trienoics) + 4 × (% tetraenoics) + 5 × (% pentaenoics) + 6 × (% hexaenoics)
FLQ, which was used originally for fish lipid quality [41], calculates the sum of the EPA and DHA as a percentage of the total fatty acids:
FLQ = 100 × (C22: 6 n × 3 + C20: 5 n × 3)/ƩFA
The LA/ALA ratio is one of the traditional indices used to evaluate food quality with two of the most representative n-6 and n-3 fatty acids.
2.3. Statistical Analysis
Data from 30 animals for each treatment (n = 30), randomly selected, were analyzed, via ANOVA (SAS Inst. Inc., Cary, NC, USA) using a mono-factorial model, and significant differences were evaluated using Tukey’s test, with p < 0.05 being the significance limit.
3. Results
3.1. Animal Performance
Performances are described in Table 4. The live weight was significantly lower in animals fed with BSL50 and BSL100 (p = 0.001), which were 3672.67 and 3656.87 g, respectively, compared to the control weight of 3887.53 g.

Table 4.
Broilers’ live weight at slaughter (75 days), daily gain, carcass, thigh and breast weight and feed conversion rate.
The carcass weight showed no significant change between the control and BSL50, as well as a significant decrease between BSL50 and BSL100 (p = 0.014).
The thigh weight follows the same trend: the weight decreased in animals fed with BSL50 and BSL100 (p = 0.001).
The breast weight of BSL100 is significantly different to those of the control (S100) and BSL50 (p = 0.022), and there were no significant differences between the control (S100) and BSL50.
3.2. Animal Welfare
Animal-based measures (ABMs) assessed in the slaughterhouse and in the poultry house (lameness), showed a good degree of welfare. The percentage of animals scoring 0 (best condition) for cleanliness of plumage, pododermatitis, hock burning, breast reddening, breast lesions and lameness was 100% of the examined animals. The litter scored 0 (flacky and dry) for all the groups at day 60, 1 (dry but not easy to move with foot) at day 67 and at day 74.
3.3. Fatty Acid Composition of BSL Meal
The fatty acid composition is shown in Table 5. Among SFA (69.31% of total FAs), lauric acid accounted for 44.70%, followed, in decreasing order, by palmitic and myristic acids, with compositions of 13.72% and 8.77%, respectively. Oleic acid was not detected (amount less than 0.01). Lower amounts were shown for MUFAs and PUFAs, i.e., 13.66 and 15.51%, respectively. Among PUFAs, the only one linoleic acid (C18:2 n-6) was detectable, accounting for 14,27% of the total, while the n-3 class (1.24%) was, in practice, exclusively represented by ALA (α-linolenic acid, C18:3 n-3).

Table 5.
BSL meal fatty acid profile (percentage of total FAME 1).
3.3.1. Fatty Acids in the Thigh
An insect diet showed significant increased levels of SFA (Figure 1 and Table 6), namely 28.17%, 31.83%, and 34.64% for S100, BSL50, and BSL100, respectively (p < 0.001). Within SFA, palmitic (C16:0), lauric (C12:0), and myristic (C14:0) acids increased with the inclusion of BSL in feed, with the highest value being found in BSF100 (p < 0.0001). In particular, lauric acid increased from 0.30 (S100) to 4.32% in BSL100. Stearic fatty acid (C18:0) BSL50 was significantly different from S100 and BSL100, while the last two were not significantly different.
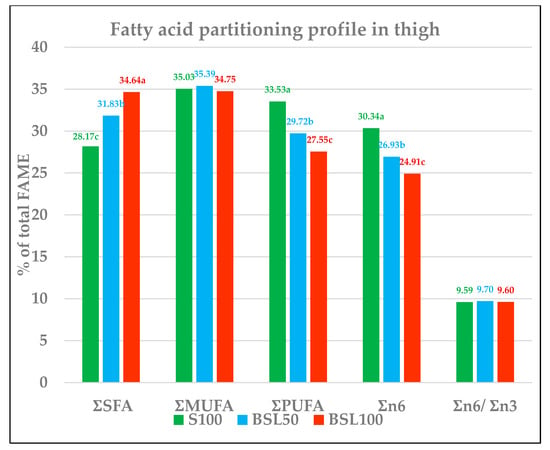
Figure 1.
Fatty acid profile in the thigh (percentage of total FAME). FAME = fatty acid methyl esters. S100 = 100% soybean feed; BSL50 = 50% soybean and 50% black soldier fly larvae meal; BSL100 = 100% black soldier fly larvae meal. ƩSFA = Ʃ (C12:0, C14:0, C16:0, C18:0). ƩMUFA = Ʃ(C14:1, C16:1 n-7, C18:1 n-9, C18:1 n-7). ƩPUFA = Ʃ(C18:2 n-6, C18:3 n-6, C18:3 n-3, C20:2 n-6, C20:3 n-6, C20:4 n-6, C20:5 n-3, C22:5 n-3, C22:6 n-3). Ʃn-6 = Ʃ(C18:2 n-6, C18:3 n-6, C20:2 n-6, C20:3 n-6, C20:4 n-6). Ʃn-3 = Ʃ(C18:3 n-3, C20:5 n-3, C22:5 n-3, C22:6 n-3). a–c Different letters in the same row indicate significant differences.Other: non-detected, complement to 100.

Table 6.
Fatty acid profile in the thigh (percentage of total FAME 1). n = 30.
Unlike SFA, MUFAs have not been significantly affected by BSL inclusion. The most representative fatty acid among MUFAs is oleic acid (C18:1 n9), while the control and BSL50 were not different from each other, though a significant lower amount was found in BSL100 (p < 0.001).
PUFA, total n-6, and total n-3 significantly decreased in proportion to the level of the inclusion insect meals in feed (p < 0.001). PUFAs decreased from 33.53% in S100 to 27.55% in BSL100 (p < 0.001), total n-6 decreased from 30.34% in S100 to 24.91% in BSL100, (p < 0.001), total n-3 decreased from 3.18% in S100 to 2.64% in BSL100 (p < 0.001). As among PUFA, linoleic acid (C18:2 n-6) is the prevailing acid present, its trend is the same as that of PUFA, decreasing from 27.58% in S100 to 22.26% in BSL100 (p < 0.001).
ALA followed the same decreasing trend (p < 0.001). EPA did not appear to be influenced by BSL feed, while the DPA and DHA contents were lower (p < 0.001) for the diet with the inclusion of BSL (both for 50% and 100% substitution).
3.3.2. Fatty Acids in the Breast
Also, in the breast, total SFA significantly increased with the administration of a BSL-based diet (p < 0.001), even though no differences were noted between BSL50 and BSL100 (Figure 2 and Table 7). As in the thigh, the breast showed significant proportional increases in lauric and myristic acids, ranging from S100 (0.35 and 0.62%) to maximum replacement (BSL100) (3.09 and 1.53%) (p < 0.001), while palmitic acid only revealed a significant rise in BSL50. Having a different trend to that of the thigh, stearic fatty acid in BSL100 was significantly different from S100 and BSL50, while the last two were not significantly different from each other.
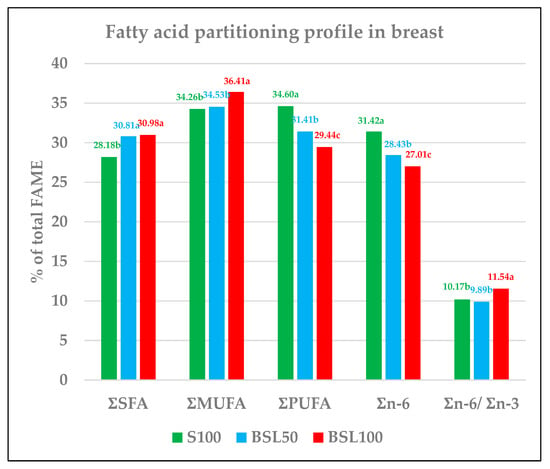
Figure 2.
Fatty acid profile in the breast (percentage on total FAME). FAME = fatty acid methyl esters. S100 = 100% soybean feed; BSL50 = 50% soybean and 50% black soldier fly larvae meal; BSL100 = 100% black soldier fly larvae meal. ƩSFA = Ʃ (C12:0, C14:0, C16:0, C18:0). ƩMUFA = Ʃ(C14:1, C16:1 n-7, C18:1 n-9, C18:1 n-7). ƩPUFA = Ʃ(C18:2 n-6, C18:3 n-6, C18:3 n-3, C20:2 n-6, C20:3 n-6, C20:4 n-6, C20:5 n-3, C22:5 n-3, C22:6 n-3). Ʃn-6 = Ʃ(C18:2 n-6, C18:3 n-6, C20:2 n-6, C20:3 n-6, C20:4 n-6). Ʃn-3 = Ʃ(C18:3 n-3, C20:5 n-3, C22:5 n-3, C22:6 n-3). a–c Different letters in the same row indicate significant differences. Other: non-detected, complement to 100.

Table 7.
Fatty acid profile in the breast (percentage on total FAME 1). n = 30.
In BSL100, MUFAs were significantly different to S100 and BSL50 (p < 0.001). The increasing percentage of MUFAs was due to the content of oleic acid, and the fatty acid was most abundant among MUFAs, the trends of which remained unchanged up to BSL50 and significantly increased in BSL100 (p < 0.001).
PUFA, total n-6, and total n-3 showed the same trend and were negatively correlated with the inclusion of insect meals in feed, decreasing from 34.60% in S100 to 29.44% in BSL100.
Also, breast linoleic acid is the prevailing acid content among PUFAs, with a significant decrease recorded as BSL increased in feed from S100 (27.37%) to BSL100 (24.85%). ALA was not influenced by the feed up to BSL50, decreasing only in BSL100 (p < 0.001).
EPA showed a dissimilar behavior in the breast because BSL100 was not significantly different to S100, but it was different to BSL50. DPA and DHA were adversely affected by inclusions at any level (p < 0.001).
Unlike in the thigh, where no differences were detected, n-6/n-3 only significantly increased from BSL50 to BSL100 (p < 0.001).
3.4. Nutritonal Indeces
3.4.1. Thigh
The nutritional indices in the thigh are shown in Table 8. ƩPUFA/ƩSFA, hH, HPI, and UI indices reveal the same trend: a significant decrease from S100 to BSL100 (p < 0.001). Instead, FLQ indices only significantly decrease up to BSL50. In contrast, IA and IT revealed an opposite trend, as they significantly increase from S100 to BSL100 (p < 0.001).

Table 8.
Nutritional indices in the thigh (%).
3.4.2. Breast
As for the thigh, a significant decrease was found in the breast for the PUFA/SFA, hH, HPI, and UI indices, starting from S100 to BSL100 (p < 0.001) (Table 9). Unlike the thigh, the same decrease was also observed for FLQ indices, ranging from S100 to BSL100 (p < 0.001). IA significantly increased up to BSL100; conversely, in the thigh, IT significantly increased up to BSL50.

Table 9.
Nutritional indices in the breast (%).
4. Discussion
4.1. Growth Performances
Our study shows the negative impact of the large dietary substitution (50% and 100% of soybean) of BSL on broiler live weight (Table 5). This result confirmed the differences found by Facey et al. [19], using the same BSL replacements for soybean meal (50 and 100%). However, they did not find significant differences in live weight with lower substitution rates (12.5 and 25% BSL meal). Also, Heuel et al. [18] demonstrated that the total replacement of soybean with defatted BSL meal in animals reared for 63 days caused a significant decrease in live weight. Schiavone et al. [20] and Dabbou et al. [7], with a high inclusion level of BSL (66% replacing soybean), also found significant decreases in live weight. In addition, live weight significantly decreased in a study in which broilers were fed with BSL meal substituted for soybean at proportions of between 29% and 58% [17]. In contrast, Altmann et al. [42] and Pieterse et al. [43], using 47.4% BSL meal and BSF pre-pupae 55% meal, respectively, as substitutes for soybean meal, did not find differences compared to the control. Similarly, Cullere et al. [44] reported no changes in live weight when feeding broiler quails with diet containing a less than 24.8% replacement of soybean meal with defatted BSL meal.
Kim et al. [17], who observed a decline in carcass characteristics with 50% BSL substitution, hypothesized that the decline might have been caused by anti-nutritional factors such as chitin content [14], which increase in BSLM diets, or other anti-nutritional factors that still need to be identified. To support this hypothesis, Dabbou et al. [7] observed negative gut development in terms of short villi, deep crypts, and a reduced villus height-to-crypt-depth ratio upon replacing soybeans with about 67% BSL with respect to other the lower replacement levels. In contrast, Islam et al. [45] suggested that chitin fragments had potential as prebiotic for the improvement of the overall health and performance of chicken, while Tabata et al. [46] found that the chitin hydrolyzing enzyme (acidic chitinase, Chia) is constitutively and predominantly expressed in chicken glandular stomach tissues, and, consequently, insects could be used in diet for broilers. It should also be noted that there are differences in the chemical and nutritional composition of BLS according to the rearing substrate [47,48] and biological phase (i.e., larva, pupa, and adult), as reported by Liu et al. [49], as well as larval development [48], and this might explain the variability in the results available in the literature.
Carcass weight was negatively influenced by BSL100 (p = 0.014). The same significant decrease in carcass weight was confirmed by Heuel et al. [18], who experimented with the total replacement of soybean with BSL meal (100%) compared to the control, and by Schiavone et al. [20], who performed 66% BSL replacement. Kim et al. [17] also found significant differences from the control with lower replacement (29% of BSL meal). Unlike those results, no significant differences in carcass weight were observed by Altmann et al. [38] with 47% BSL meal replacement; Pieterse et al. [43] with up to 55% BSL meal replacement; or Cullere et al. [44] with up to 25% BSL meal replacement.
Thigh weight decreased with BSL meal inclusion. These results are different to those of other studies using BSL in different percentages, which showed no significant differences in thigh weight compared to control [20,42,43].
Breast weight was only influenced by BSL100. This result was confirmed by Heuel et al. [18], who experimented with the complete replacement of soybean meal with BSL, noting a significant decrease in breast weight. As in our study, studies using the partial substitutions of BSL below 66% did not lead to significant reductions in breast weight [20,42,43,44]. The addition of BSL to control feed, on the other hand, led to significant losses in breast weight in the study of Seyedalmoosavi et al. [50]. As the major significative differences were only noted when the soybean was totally substituted with BSF meal, 50% substitution did not change commercial performances.
4.2. Fatty Acids in BSL Meal
Insects are known to be reservoirs of proteins suitable for poultry nutrition. However, the content of fatty acid, especially SFA, is not indifferent. It is well known the importance of fatty acid profile for a healthy and high-quality food. Omega 3 fatty acids are positively related to the nutritional value. Generally speaking, the composition of insect meal fatty acid depends on the feeding substrates, stage of growth, and insect physiology; however, in any case, BSL is always rich in SFA, particularly lauric acid [13,51]. In our study, SFA in BSL meal were 69.31% of the total FA, with lauric acid being more than half of the SFA (64.49%), which is similar to the value reported by other studies [13,18,52,53,54,55]. Also, the other two macro categories of fatty acids (MUFA and PUFA) were comparable with data from the literature, in modulus [13,18]. Interestingly, in our data they are quite balanced (13.66% vs. 15.51%), without a clear prevalence of one over the other and this could represent a positive trait for meat quality. Indeed, as dietary fatty acids are supposed to directly influence the meat of monogastrics, a varied apport of unsaturated fatty acids (i.e., MUFA + PUFA) might increase the positive effect of fat desaturation degree of meat with a partial control of the oxidation susceptibility attributable to PUFAs alone.
4.3. Fatty Acid and Meat Quality
Monounsaturated supply from the diet, at the expense of the PUFAs, has been related to lower greasiness and oxidation in pork [56] and chicken [57], with limited or no undesirable side effects on carcass and meat quality characteristics. Dietary MUFAs are also able to lower plasma cholesterol in humans. MUFA concentrations in broilers are dependent on either endogenous synthesis or gut absorption from the diets [58]. In our study MUFA content of the breast seems to vary according to the level of BSFL in the diet (Table 7) while PUFAs decrease significantly. BSFL 100 resulted in the highest values of all detected monounsaturated fatty acids, whereas BSFL 50 ranked intermediate, except for C18:1n7 (vaccenic acid), which was very similar in both BSFL 50 and 100, and quite abundant in BSFL meal (Table 5). Poultry actively synthesizes fatty acids in the liver [59] and vaccenic acid might be retro-converted into C16:1 n-7 (palmitoleic acid) by hepatic peroxisomal β-oxidation and accumulated in other tissues [60]. No evident effect was observed in the thigh, with the exception of palmitoleic acid.
The increasing level of SFA recorded in our study was confirmed by Popova et al. [31] using a feed with defatted BSL (22.5% defatted BSL replacing soybean). The same results were confirmed by Altmann et al. [42], who compared the leg meat intramuscular fat of two different broiler genotypes fed with BSL in diet (47% BSL replacing soybean). Different results were found by Kim et al. [17], and they did not demonstrate significant SFA differences in the thigh of animals fed through the replacement of 29% and 58% BSL meal. Among SFA, palmitic (C16:0), lauric (C12:0), and myristic (C14:0) acid contents increased with the inclusion of BSL in feed, confirming the results found by Popova et al. [31] and by Altmann et al. [42]. In our study lauric acid content was up to 14-fold higher in BSL100, (p < 0.001). Popova et al. [31] obtained a 79-fold increase in the thigh with 22.5% soybean substitution, similar to those of Heuel et al. [18], noting an 80-fold increase, with 100% substitution. Even if in our study lauric acid increased accordingly to substitution rate, the increase was smaller than data reported in the literature, probably due to a different insect’s growing substrate [18,52]. Therefore, lauric acid could be beneficial for human health as medium-chain triglycerides are used for skin care, weight control, cholesterol level maintenance, immunomodulatory effect, cardiovascular prevention, and more recently in Alzheimer’s disease treatment as highlighted in the review of Roopashree et al. [61].
In the breast, SFA increased only significantly up to BSL50, and this result was confirmed by other studies, which tested BSL substitutions (from 16% to 25%) [18,28,30]. In contrast, Schiavone et al. [20] found no significant differences in breast SFA when substituting 16%, 41%, and 67% soybean with BSL. ALA decreased significantly only for the highest substitution level (BSL100), and lauric acid increased accordingly but not as much as in other studies on similar or lower inclusion [18,20,29,31]. Therefore, the substitution of 50% of soybean did not bring detrimental effects on meat quality and might have some potential benefits for human health, considering that the breast represents the most abundant part of the edible meat in commercial chickens (about 20% of carcass weight).
The negative influences on meat quality of SFA, lauric, and myristic acids on human health should be compared with the benefic effects of lauric acid. Generally speaking, SFA are considered to be a risk factor of cardiovascular disease. However, lauric acid as medium chain FA could have some benefits for animal and human health. In broiler, lauric acid is known for its beneficial properties, as it is an antibacterial agent with the capacity to prevent necrotic enteritis lesions from inhibiting C. perfringens in broiler gut [62,63].
With regard to human health, it was observed that a long-term diet with high dose lauric triglycerides reduced cholesterol biosynthesis in obese rats [64]. It also can reduce blood pressure and heart rate in normotensive and hypertensive rats and reduce oxidative stress in the heart and kidneys [65]. Ekanayaka et al. [66] considered the influence of lauric acid on cholesterol levels in human blood, suggesting its decrease in a diet with coconut fat with a high percentage of lauric acid. In addition, lauric acid does not have a metabolic inflammatory effect and can contribute to the preservation of skeletal muscle metabolic health [67].
In addition, a higher concentration of SFA brings a lower risk of meat oxidation [16,68], as well as a better shelf life, confirmed by Kim et al. [30] who, using BSL oil as a functional ingredient, found increased antioxidant ability in broiler meat.
Regarding BSL meal’s impact on PUFA, in the thigh and breast, the increases in SFA in our study occurred at the expense of PUFA. These results were confirmed by Popova et al. [31], even at lower percentages of BSL inclusion (22.5% BSL), who did not distinguish between defatted and full-fat BSL, as well as by Altman et al. [40]. Indeed, for PUFA, linoleic acid (C18:2 n-6) is the prevailing acid, and its trend influences PUFA behavior; however, ALA did not change when 50% of soybean was replaced.
In addition, Popova et al. [69] hypothesized that if stearic acid decreased, as it did in our study in both substitutions, the stearoyl-CoA desaturase and elongase activities required to produce polyunsaturated fatty acids should be lower. Liland et al. [52] concluded that PUFA if the PUFA content depended on substrates. Also, Heuel et al. [18] found different proportions of SFA and PUFA on different substrates; therefore, to better balance the SFA/PUFA ratio in feed, it is important to find the right substrates and establish insect feeding protocols to produce more standardized products for the feeding industry. Lauric acid in broiler metabolism is often used for other metabolic pathways and energy utilization [18]; however, all the citied studies found large quantities of lauric acid in the meat. These assumptions would allow producers to better modulate the various ingredients required to rear substrates to balance SFA and PUFA.
Regarding DPA, the significant decrease recorded in our study was confirmed by other studies, with BSL replacement ranging from 16% to 67% [20,31]. For DHA, there were disagreements between studies: some found significant differences [20,29], whereas others did not find significant changes [18,31].
The n-6/n-3 ratio increased significantly in the thigh and breast from BSL50 to BSL100 (p < 0.001). The same result was confirmed by Heuel et al. [18] and Schiavone et al. [20], who found no differences in substitution under 67%. On the contrary, Popova et al. [31] and Cullere et al. [29] found significant evidence even below the 25% BSL replacement level.
The decrease in PUFA, combined with the consequent lower levels of omega 6 and omega 3, could be considered to be a negative nutritional aspect due to the PUFA’ well-known cholesterol-lowering effects. In the human diet, PUFA reduces the risk of cardiovascular and chronic diseases and may regulate the antioxidant signaling pathway and modulate inflammatory processes [70,71]. Furthermore, deficiencies in essential fatty acids, such as linoleic, linolenic, and arachidonic acids, cause several negative effects, such as stunted growth, skin symptoms, malabsorption, and catabolic diseases [11]. One important characteristic of organically raised broilers is the increased amounts of ALA and the three long-chain fatty acids, namely EPA, DHA, and DPA, in meat compared to those solely reared inside the poultry house. ALA is associated with the grass eaten by outdoor animals, and it is the precursor of the three long-chain fatty acids, namely EPA, DHA, and DPA [72,73]. This gained value is lost with the inclusion of BSL, which depreciates the qualitative profile of meat, thus decreasing PUFA.
Fatty acid evaluation, performed through nutritional indices used in recent years, allows us to better assess the meat quality profile [74]. All nutritional indices considered in the present work were negatively affected by the use of BSL in diet. There are very limited studies of the nutritional indices of broilers fed with insect meal. Popova et al. [31] found a significant IA increase, even with lower substitutions (22.5% BSL), but they found no difference for IT. Schiavone at al. [20] agreed with Popova et al. [31] about the significant increase in IA in broiler breast fed with 16%, 41%, and 67% BSL as a substitute for soybean, as well as no differences for IT. The PUFA/SFA ratio, AI, TI, hH, and HPI were used to evaluate the impact of diet on cardiovascular health (CVH). SFA contributes to elevated serum cholesterol levels, while PUFA can depress low-density lipoprotein cholesterol [3]. Moreover, the role of fatty acids in human health is not limited based on the amount of PUFA; in fact, the n-6/n-3 fatty acids ratio should be as low as possible [75]. In our study, the impact of the total substitution negatively affects the breast n-6/n-3 ratio, and the PUFA/SFA ratio is considered to be an important index for broiler meat quality [76]. The AI and TI indices increased with the administration of a BSL diet. Higher AI values involve increasing levels of total cholesterol and LDL-C in human blood plasma, and higher TI values increased the risk of coronary heart disease [77,78]. There are no references regarding broiler meat for the HPI, UI and FLQ indices, which are generally evaluated for milk, fish, and vegetable products; these indices are affected by the presence of PUFA in low quantities.
5. Conclusions
In organic farming, the protein component of the diet plays a very important role in ensuring the sustainability of animal breeding. Since synthetic amino acids are prohibited in organic farming, organic soybean plays a crucial role due to its high amino-acid profile. However, concerns regarding the sustainability of soybean mean that it necessary to find nutritionally viable alternatives for organic broiler without compromising animal growth and meat quality.
The replacement of 50% and 100% of soybean with BSL meal influenced growth performances; but from a commercial perspective, 50% replacement compromised neither commercial performance nor meat quality. The corresponding increase in lauric and myristic acids, as they are medium chain FA, can also bring positive value for humans thanks to the proven effects on skin, immune system, metabolism and some diseases with an improvement on broiler health as well. These promising results may encourage feed industry and broiler producers to start including insect meal in the standard commercial practice.
However, as comparable studies reported some discrepancies in results, in particular about ALA and lauric acid contents, further research on the effect of insect’s growth substrate on FA composition, especially PUFA, and on lauric metabolic pathways to formulate a balanced insect-based diet without decreasing meat quality, are encouraged.
Author Contributions
Conceptualization, M.G.A. and M.C.L.M.; methodology, M.G.A., M.C.L.M. and M.C. (Massimo Calì); investigation M.G.A., M.C. (Massimo Calì) and L.P.J.; analysis M.C. (Michela Contò) and G.R.; writing—original draft preparation, M.G.A. and M.C.L.M.; review and editing, D.M.Z. and M.C. (Michela Contò), project administration, M.G.A.; funding acquisition, M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Agriculture, MASAF, grant number 92011, 21 December 2018, the study is part of PERILBIO Project.
Institutional Review Board Statement
The experimental trial, submitted to CREA Ethic Committee named OPBA (Organismo Preposto al Benessere Animale), was approved with approval number 17433 of 29/02/2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Thanks to the technical staff of CREA-ZA, Natalino Bottan, Delfino Rosati, Gaetano D’Ippoliti.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; the collection, analyses, or interpretation of data; the writing of the manuscript; or the decision to publish the results.
References
- Dei, H.K. Soybean as a Feed Ingredient for Livestock and Poultry; IntechOpen: London, UK, 2011. [Google Scholar]
- Jia, F.; Peng, S.; Green, J.; Koh, L.; Chen, X. Soybean Supply Chain Management and Sustainability: A Systematic Literature Review. J. Clean. Prod. 2020, 255, 120254. [Google Scholar] [CrossRef]
- Román, A.A. EPRS|European Parliamentary Research Service; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Parolini, M.; Ganzaroli, A.; Bacenetti, J. Earthworm as an Alternative Protein Source in Poultry and Fish Farming: Current Applications and Future Perspectives. Sci. Total Environ. 2020, 734, 139460. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; Thongpea, S.; Paengkoum, S.; Purba, R.A.P.; et al. Nutritional Composition of Black Soldier Fly Larvae (Hermetia Illucens L.) and Its Potential Uses as Alternative Protein Sources in Animal Diets: A Review. Insects 2022, 13, 831. [Google Scholar] [CrossRef]
- Veldkamp, T.; Dong, L.; Paul, A.; Govers, C. Bioactive Properties of Insect Products for Monogastric Animals—A Review. J. Insects Food Feed 2022, 8, 1027–1040. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Growth Performance, Blood Traits, Gut Morphology and Histological Features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Dabbou, S.; De Marco, M.; Cullere, M.; Biasato, I.; Biasibetti, E.; Capucchio, M.T.; Bergagna, S.; Dezzutto, D.; Meneguz, M.; et al. Black Soldier Fly Larva Fat Inclusion in Finisher Broiler Chicken Diet as an Alternative Fat Source. Animal 2018, 12, 2032–2039. [Google Scholar] [CrossRef]
- Murawska, D.; Daszkiewicz, T.; Sobotka, W.; Gesek, M.; Witkowska, D.; Matusevičius, P.; Bakuła, T. Partial and Total Replacement of Soybean Meal with Full-Fat Black Soldier Fly (Hermetia Illucens L.) Larvae Meal in Broiler Chicken Diets: Impact on Growth Performance, Carcass Quality and Meat Quality. Animals 2021, 11, 2715. [Google Scholar] [CrossRef]
- van Huis, A. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 9789251075951. [Google Scholar]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-Art on Use of Insects as Animal Feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Nogales-Mérida, S.; Gobbi, P.; Józefiak, D.; Mazurkiewicz, J.; Dudek, K.; Rawski, M.; Kierończyk, B.; Józefiak, A. Insect Meals in Fish Nutrition. Rev. Aquac. 2019, 11, 1080–1103. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty Acid Composition of Black Soldier Fly Larvae (Hermetia Illucens)—Possibilities and Limitations for Modification through Diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Marono, S.; Piccolo, G.; Loponte, R.; Di Meo, C.; Attia, Y.A.; Nizza, A.; Bovera, F. In Vitro Crude Protein Digestibility of Tenebrio Molitor and Hermetia Illucens Insect Meals and Its Correlation with Chemical Composition Traits. Ital. J. Anim. Sci. 2015, 14, 3889. [Google Scholar] [CrossRef]
- Leiber, F.; Gelencsér, T.; Stamer, A.; Amsler, Z.; Wohlfahrt, J.; Früh, B.; Maurer, V. Insect and Legume-Based Protein Sources to Replace Soybean Cake in an Organic Broiler Diet: Effects on Growth Performance and Physical Meat Quality. Renew. Agric. Food Syst. 2015, 32, 21–27. [Google Scholar] [CrossRef]
- de Souza Vilela, J.; Alvarenga, T.I.R.C.; Andrew, N.R.; McPhee, M.; Kolakshyapati, M.; Hopkins, D.L.; Ruhnke, I. Technological Quality, Amino Acid and Fatty Acid Profile of Broiler Meat Enhanced by Dietary Inclusion of Black Soldier Fly Larvae. Foods 2021, 10, 297. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, H.R.; Lee, S.; Baek, Y.-C.; Jeong, J.Y.; Bang, H.T.; Ji, S.Y.; Park, S.H. Effects of Dietary Inclusion Level of Microwave-dried and Press-defatted Black Soldier Fly (Hermetia Illucens) Larvae Meal on Carcass Traits and Meat Quality in Broilers. Animals 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Heuel, M.; Sandrock, C.; Leiber, F.; Mathys, A.; Gold, M.; Zurbrüegg, C.; Gangnat, I.D.M.; Kreuzer, M.; Terranova, M. Black Soldier Fly Larvae Meal and Fat as a Replacement for Soybeans in Organic Broiler Diets: Effects on Performance, Body N Retention, Carcase and Meat Quality. Br. Poult. Sci. 2022, 63, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Facey, H.; Kithama, M.; Mohammadigheisar, M.; Huber, L.-A.; Shoveller, A.K.; Kiarie, E.G. Complete Replacement of Soybean Meal with Black Soldier Fly Larvae Meal in Feeding Program for Broiler Chickens from Placement through to 49 Days of Age Reduced Growth Performance and Altered Organs Morphology. Poult. Sci. 2023, 102, 102293. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Dabbou, S.; Petracci, M.; Zampiga, M.; Sirri, F.; Biasato, I.; Gai, F.; Gasco, L. Black Soldier Fly Defatted Meal as a Dietary Protein Source for Broiler Chickens: Effects on Carcass Traits, Breast Meat Quality and Safety. Animal 2019, 13, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; De Marco, M.; Martínez, S.; Dabbou, S.; Renna, M.; Madrid, J.; Hernandez, F.; Rotolo, L.; Costa, P.; Gai, F.; et al. Nutritional Value of a Partially Defatted and a Highly Defatted Black Soldier Fly Larvae (Hermetia Illucens L.) Meal for Broiler Chickens: Apparent Nutrient Digestibility, Apparent Metabolizable Energy and Apparent Ileal Amino Acid Digestibility. J. Anim. Sci. Biotechnol. 2017, 8, 51. [Google Scholar] [CrossRef]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguz, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or Total Replacement of Soybean Oil by Black Soldier Fly Larvae (Hermetia Illucens L.) Fat in Broiler Diets: Effect on Growth Performances, Feed-Choice, Blood Traits, Carcass Characteristics and Meat Quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Hoc, B.; Genva, M.; Fauconnier, M.-L.; Lognay, G.; Francis, F.; Caparros Megido, R. About Lipid Metabolism in Hermetia Illucens (L. 1758): On the Origin of Fatty Acids in Prepupae. Sci. Rep. 2020, 10, 11916. [Google Scholar] [CrossRef]
- Dalle Zotte, A. Do Insects as Feed Ingredient Affect Meat Quality? Theory Pract. Meat Process. 2021, 6, 200–209. [Google Scholar] [CrossRef]
- Zhu, Z.; Rehman, K.U.; Yu, Y.; Liu, X.; Wang, H.; Tomberlin, J.K.; Sze, S.-H.; Cai, M.; Zhang, J.; Yu, Z.; et al. De Novo Transcriptome Sequencing and Analysis Revealed the Molecular Basis of Rapid Fat Accumulation by Black Soldier Fly (Hermetia Illucens L.) for Development of Insectival Biodiesel. Biotechnol. Biofuels 2019, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Giannetto, A.; Oliva, S.; Ceccon Lanes, C.F.; de Araújo Pedron, F.; Savastano, D.; Baviera, C.; Parrino, V.; Lo Paro, G.; Spanò, N.C.; Cappello, T.; et al. Hermetia Illucens (Diptera: Stratiomydae) Larvae and Prepupae: Biomass Production, Fatty Acid Profile and Expression of Key Genes Involved in Lipid Metabolism. J. Biotechnol. 2020, 307, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Skřivanová, E.; Marounek, M.; Benda, V.V.S.C.; Březina, P. Susceptibility of Escherichia coli, Salmonella sp and Clostridium perfringens to organic acids and monolaurin. Veterinární Medicína 2006, 51, 81–88. [Google Scholar] [CrossRef]
- Kim, S.A.; Rhee, M.S. Marked Synergistic Bactericidal Effects and Mode of Action of Medium-Chain Fatty Acids in Combination with Organic Acids against Escherichia Coli O157: H7. Appl. Environ. Microbiol. 2013, 79, 6552–6560. [Google Scholar] [CrossRef] [PubMed]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Acuti, G.; Marangon, A.; Dalle Zotte, A. Black Soldier Fly as Dietary Protein Source for Broiler Quails: Meat Proximate Composition, Fatty Acid and Amino Acid Profile, Oxidative Status and Sensory Traits. Animal 2018, 12, 640–647. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, D.-H.; Jeong, S.-B.; Lee, J.-W.; Kim, T.-H.; Lee, H.-G.; Lee, K.-W. Black Soldier Fly Larvae Oil as an Alternative Fat Source in Broiler Nutrition. Poult. Sci. 2020, 99, 3133–3143. [Google Scholar] [CrossRef]
- Popova, T.L.; Petkov, E.; Ignatova, M. Effect of Black Soldier Fly. (Hermetia illucens) Meals on the Meat Quality in Broilers. Agric. Food Sci. 2020, 29, 177–188. [Google Scholar] [CrossRef]
- EC. EU Imports of Organic Agri-Food Products, Key Developments in 2022, July 2023; European Commission, DG Agriculture and Rural Development: Brussels, Belgium, 2023; Available online: https://agriculture.ec.europa.eu/system/files/2023-07/analytical-brief-2-eu-organic-imports-2022_en.pdf (accessed on 1 November 2023).
- Guarino Amato, M.; Castellini, C. Adaptability Challenges for Organic Broiler Chickens: A Commentary. Animals 2022, 12, 1354. [Google Scholar] [CrossRef]
- Folch, J.; Mark, L.; Gerald, H.; Sloane, S. A simple method for the isolation and purification of total lipids from animal tissues. J. Boil. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Failla, S.; Buttazzoni, L.; Meo Zilio, D.; Contò, M.; Renzi, G.; Castellini, C.; Guarino Amato, M. An index to measure the activity attitude of broilers in extensive system. Poult. Sci. 2021, 100, 101279. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, J.; Bessa, R.; Santos-Silvã, F.; Silvã, S. Effect of Genotype, Feeding System and Slaughter Weight. on the Quality of Light. Lambs II. Fatty Acid. Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Mierliţă, D. Effects of Diets Containing Hemp Seeds or Hemp Cake on Fatty Acid Composition and Oxidative Stability of Sheep Milk. S. Afr. J. Anim. Sci. 2018, 48, 504–515. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.A.; de Vries, A.L.; Fodor, E.; Cossins, A.R. Lipid compositional correlates of temperature-adaptive interspecific differences in membrane physical structure. J. Exp. Biol. 2000, 203, 2105–2115. [Google Scholar] [CrossRef]
- Krajnović-Ozretic, M.; Najdek, M.; Ozretić, B. Fatty Acids in Liver and Muscle of Farmed and Wild Sea Bass (Dicentrarchus labrax L.). Comp. Biochem. Physiol. Part A Physiol. 1994, 109, 611–617. [Google Scholar] [CrossRef]
- Altmann, B.A.; Geisler, S.; Morthorst, F.; Angeli, S.; Bortolini, S.; Gauly, M.; Hummel, J.; Sünder, A.; Mörlein, D.; Traulsen, I.; et al. Animal Performance and Meat Quality of Two Slow-Growing Chicken Genotypes Fed Insects Reared on Municipal Organic Waste. J. Insects Food Feed 2023, 9, 1445–1459. [Google Scholar] [CrossRef]
- Pieterse, E.; Erasmus, S.W.; Uushona, T.; Hoffman, L.C. Black Soldier Fly (Hermetia Illucens) Pre-Pupae Meal as a Dietary Protein Source for Broiler Production Ensures a Tasty Chicken with Standard Meat Quality for Every Pot. J. Sci. Food Agric. 2019, 99, 893–903. [Google Scholar] [CrossRef]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black Soldier Fly as Dietary Protein Source for Broiler Quails: Apparent Digestibility, Excreta Microbial Load, Feed Choice, Performance, Carcass and Meat Traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- Islam, M.M.; Yang, C.-J. Efficacy of Mealworm and Super Mealworm Larvae Probiotics as an Alternative to Antibiotics Challenged Orally with Salmonella and E. coli Infection in Broiler Chicks. Poult. Sci. 2017, 96, 27–34. [Google Scholar] [CrossRef]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Kino, Y.; Matoska, V.; Bauer, P.O.; Oyama, F. Gastric and Intestinal Proteases Resistance of Chicken Acidic Chitinase Nominates Chitin-Containing Organisms for Alternative Whole Edible Diets for Poultry. Sci. Rep. 2017, 7, 6662. [Google Scholar] [CrossRef]
- Fuso, A.; Barbi, S.; Macavei, L.I.; Luparelli, A.V.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. [Google Scholar] [CrossRef]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and By-Products as Rearing Substrates for Black Soldier Fly (Hermetia Illucens) Larvae: Effects on Larval Body Composition and Performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; Ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic Changes of Nutrient Composition throughout the Entire Life Cycle of Black Soldier Fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef]
- Seyedalmoosavi, M.M.; Dannenberger, D.; Pfuhl, R.; Görs, S.; Mielenz, M.; Maak, S.; Wolf, P.; Daş, G.; Metges, C.C. Lipid Metabolism, Fatty Acid Composition and Meat Quality in Broilers Supplemented with Increasing Levels of Defrosted Black Soldier Fly Larvae. J. Insects Food Feed 2022, 9, 583–598. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and Fatty Acid Composition of Black Soldier Fly Hermetia Illucens (Diptera: Stratiomyidae) Larvae Are Influenced by Dietary Fat Sources and Levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of Nutrient Composition of Black Soldier Fly (Hermetia Illucens) Larvae by Feeding Seaweed-Enriched Media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the Suitability of a Partially Defatted Black Soldier Fly (Hermetia Illucens, L.) Larvae Meal as Ingredient for Rainbow Trout (Oncorhynchus Mykiss Walbaum) Diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- de Souza-Vilela, J.; Andronicos, N.M.; Kolakshyapati, M.; Hilliar, M.; Sibanda, T.Z.; Andrew, N.R.; Swick, R.A.; Wilkinson, S.; Ruhnke, I. Black Soldier Fly Larvae in Broiler Diets Improve Broiler Performance and Modulate the Immune System. Anim. Nutr. 2021, 7, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, T.; Murawska, D.; Kubiak, D.; Han, J. Chemical Composition and Fatty Acid Profile of the Pectoralis Major Muscle in Broiler Chickens Fed Diets with Full-Fat Black Soldier Fly (Hermetia Illucens) Larvae Meal. Animals 2022, 12, 464. [Google Scholar] [CrossRef]
- Mas, G.; Llavall, M.; Coll, D.; Roca, R.; Díaz, I.; Oliver, M.A.; Gispert, M.; Realini, C.E. Effect of an elevated monounsaturated fat diet on pork carcass and meat quality traits and tissue fatty acid composition from York-crossed barrows and gilts. Meat Sci. 2011, 89, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Rebolé, A.; Rodríguez, M.L.; Ortiz, L.T.; Alzueta, C.; Centeno, C.; Viveros, A.; Brenes, A.; Arija, I. Effect of dietary high-oleic acid sunflower seed, palm oil and vitamin E supplementation on broiler performance, fatty acid composition and oxidation susceptibility of meat. Br. Poult. Sci. 2006, 47, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Eleroğlu, H.; Yıldırım, A.; Işıklı, N.D.; Şekeroğlu, A.; Duman, M. Comparison of meat quality and fatty acid profile in slow-growing chicken genotypes fed diets supplemented with Origanum vulgare or Melissa officinalis leaves under the organic system. Ital. J. Anim. Sci. 2013, 12, e64. [Google Scholar] [CrossRef]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Bloc’h, J.L.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef]
- Hernández-Saavedra, D.; Stanford, K.I. The regulation of lipokines by environmental factors. Nutrients 2019, 11, 2422. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, P.G.; Shetty, S.S.; Kumari, N.S. Effect of medium chain fatty acid in human health and disease. J. Funct. Foods 2021, 87, 104724. [Google Scholar] [CrossRef]
- Borrelli, L.; Varriale, L.; Dipineto, L.; Pace, A.; Menna, L.F.; Fioretti, A. Insect Derived Lauric Acid as Promising Alternative Strategy to Antibiotics in the Antimicrobial Resistance Scenario. Front. Microbiol. 2021, 12, 620798. [Google Scholar] [CrossRef]
- Timbermont, L.; Lanckriet, A.; Dewulf, J.; Nollet, N.; Schwarzer, K.; Haesebrouck, F.; Ducatelle, R.; van Immerseel, F. Control of Clostridium Perfringens-Induced Necrotic Enteritis in Broilers by Target-Released Butyric Acid, Fatty Acids and Essential Oils. Avian Pathol. 2010, 39, 117–121. [Google Scholar] [CrossRef]
- Xia, J.; Yu, P.; Zeng, Z.; Ma, M.; Zhang, G.; Wan, D.; Gong, D.; Deng, S.; Wang, J. High Dietary Intervention of Lauric Triglyceride Might Be Harmful to Its Improvement of Cholesterol Metabolism in Obese Rats. J. Agric. Food Chem. 2021, 69, 4453–4463. [Google Scholar] [CrossRef]
- Alves, N.F.B.; de Queiroz, T.M.; de Almeida Travassos, R.; Magnani, M.; de Andrade Braga, V. Acute Treatment with Lauric Acid Reduces Blood Pressure and Oxidative Stress in Spontaneously Hypertensive Rats. Basic. Clin. Pharmacol. Toxicol. 2017, 120, 348–353. [Google Scholar] [CrossRef]
- Ekanayaka, R.A.I.; Ekanayaka, N.K.; Perera, B.; De Silva, P.G.S.M. Impact of a Traditional Dietary Supplement with Coconut Milk and Soya Milk on the Lipid Profile in Normal Free-Living Subjects. J. Nutr. Metab. 2013, 2013, 481068. [Google Scholar] [CrossRef] [PubMed]
- Sergi, D.; Luscombe-Marsh, N.; Naumovski, N.; Abeywardena, M.; O’Callaghan, N. Palmitic Acid, but Not Lauric Acid, Induces Metabolic Inflammation, Mitochondrial Fragmentation, and a Drop in Mitochondrial Membrane Potential in Human Primary Myotubes. Front. Nutr. 2021, 8, 663838. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive Review on Lipid Oxidation in Meat and Meat Products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Marinova, P.; Kozelov, L. Lipid Deposition and Fatty Acid. Composition of Some Adipose Depots in Lambs Fed. Coconut Oil Supplemented Diet. Arch. Zootech. 2012, 15, 5–14. [Google Scholar]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-Inflammatory and Antioxidant Properties of n-3 PUFA: Their Role in Cardiovascular Protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Mugnai, C.; Mattioli, S.; Rosati, A.; Ruggeri, S.; Ranucci, D.; Castellini, C. Transfer of Bioactive Compounds from Pasture to Meat in Organic Free-Range Chickens. Poult. Sci. 2016, 95, 2464–2471. [Google Scholar] [CrossRef]
- Giampietro-Ganeco, A.; Boiago, M.M.; Mello, J.L.M.; De Souza, R.A.; Ferrari, F.B.; De Souza, P.A.; Borba, H. Lipid Assessment, Cholesterol and Fatty Acid Profile of Meat from Broilers Raised in Four Different Rearing Systems. An. Acad. Bras. Cienc. 2020, 92, e20190649. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Tyagi, P.K.; Biswas, A.K.; Tyagi, P.K.; Mandal, A.B.; Kumar, F.; Sharma, D.; Biswas, A.; Verma, A.K. Inclusion of Flaxseed, Broken Rice, and Distillers Dried Grains with Solubles (DDGS) in Broiler Chicken Ration Alters the Fatty Acid Profile, Oxidative Stability, and Other Functional Properties of Meat. Eur. J. Lipid Sci. Technol. 2018, 120, 1700470. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of Individual Saturated Fatty Acids and Risk of Coronary Heart Disease in US Men and Women: Two Prospective Longitudinal Cohort Studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef] [PubMed]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty Acid Profile of Milk from Saanen and Swedish Landrace Goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).