The Cost of Cutbacks: How Reduction in Development Assistance for Health May Affect Progress Made in HIV/AIDS Control in Africa
Abstract
1. Introduction
2. Materials and Methods
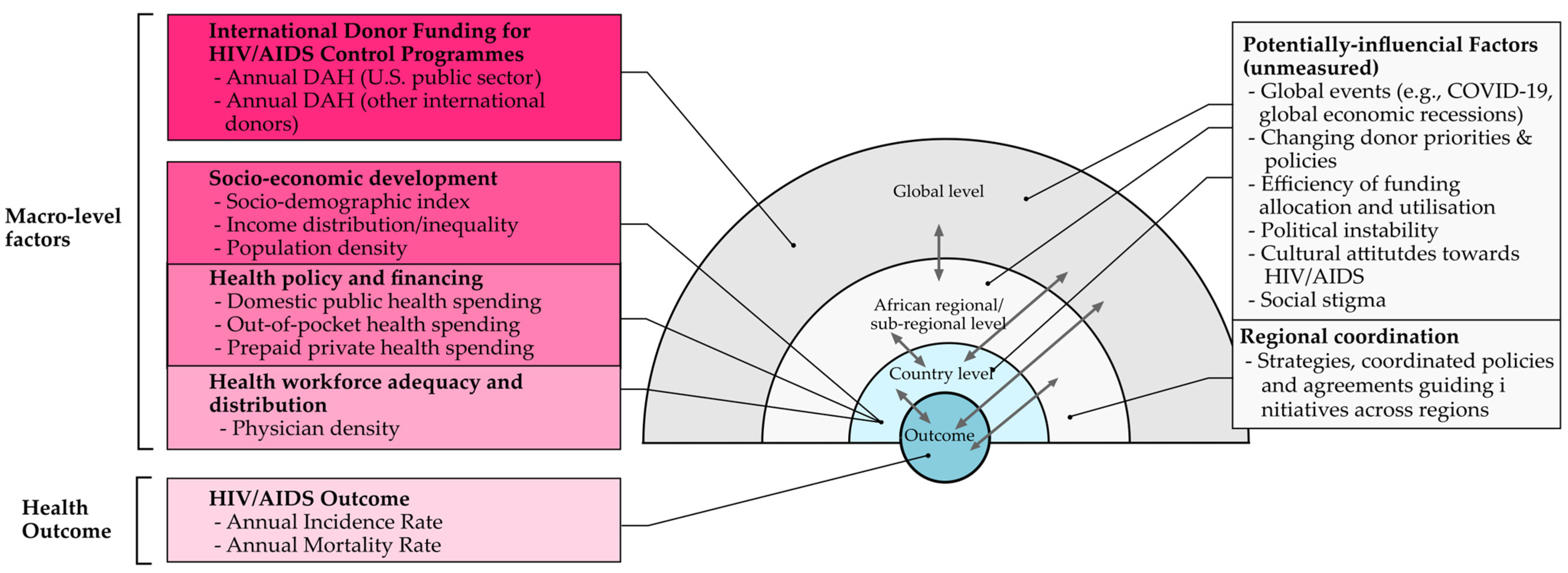
2.1. Conceptual Framework
2.2. Target Region and Countries
2.3. Variables and Data Source
2.4. Data Analysis
2.5. Robustness Assessment
3. Results
3.1. HIV/AIDS-Specific Development Assistance for Health Allocation to Africa
3.2. Association Between DAH and HIV/AIDS Incidence and Mortality
3.3. Additional Results
4. Discussion
4.1. DAH Allocation and Its Effects on HIV/AIDS Incidence and Mortality
4.2. Policy Implications and Recommendations
4.3. Robustness of the Results
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHPC | Allied Health Professions Council |
| AIC | Akaike Information Criterion |
| BIC | Bayesian Information Criterion |
| BLUP | Best Linear Unbiased Prediction |
| CAR | Central African Republic |
| CI | Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| DAH | Development Assistance for Health |
| DRC | Democratic Republic of the Congo |
| GBD | Global Burden of Disease |
| GHES | Domestic General Government Health Expenditure per capita |
| GMM | Generalised Method of Moments |
| HIV/AIDS | Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome |
| ICC | Interclass Correlation |
| IHME | Institute for Health Metrics and Evaluation |
| IQR | Interquartile Range |
| IV | Instrumental Variable |
| MLE | Maximum Likelihood Estimation |
| MTCT | Mother-to-Child Transmission |
| OOP | Out-of-Pocket Health Expenditure |
| PEPFAR | President’s Emergency Plan for AIDS Relief |
| PPP | Purchasing Power Parity |
| PPPS | Pre-paid Private Health Spending |
| RMLE | Restricted Maximum Likelihood Estimation |
| SD | Standard Deviation |
| SDI | Socio-Demographic Index |
| SE | Standard Error |
| SSA | Sub-Saharan Africa |
| SSARSD | Sub-Saharan Africa Research for Sustainable Development |
| TB | Tuberculosis |
| UHC | Universal Health Coverage |
| UN | United Nations |
| UNAIDS | Joint United Nations Programme on HIV/AIDS |
| U.S. | United States |
| USAID | United States Agency for International Development |
| WID | World Identity Database |
References
- Chang, A.Y.; Cowling, K.; Micah, A.E.; Chapin, A.; Chen, C.S.; Ikilezi, G.; Sadat, N.; Tsakalos, G.; Wu, J.; Younker, T.; et al. Past, Present, and Future of Global Health Financing: A Review of Development Assistance, Government, out-of-Pocket, and Other Private Spending on Health for 195 Countries, 1995–2050. Lancet 2019, 393, 2233–2260. [Google Scholar] [CrossRef] [PubMed]
- Micah, A.E.; Su, Y.; Bachmeier, S.D.; Chapin, A.; Cogswell, I.E.; Crosby, S.W.; Cunningham, B.; Harle, A.C.; Maddison, E.R.; Moitra, M.; et al. Health Sector Spending and Spending on HIV/AIDS, Tuberculosis, and Malaria, and Development Assistance for Health: Progress towards Sustainable Development Goal 3. Lancet 2020, 396, 693–724. [Google Scholar] [CrossRef] [PubMed]
- The Global Fund Annual Financial Report. The Global Fund: Geneva, Switzerland, 2023; pp. 1–65.
- Dieleman, J.L.; Schneider, M.T.; Haakenstad, A.; Singh, L.; Sadat, N.; Birger, M.; Reynolds, A.; Templin, T.; Hamavid, H.; Chapin, A.; et al. Development Assistance for Health: Past Trends, Associations, and the Future of International Financial Flows for Health. Lancet 2016, 387, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- U.S. President’s Emergency Plan for AIDS Relief Monitoring, Evaluation, and Reporting Indicator Reference Guide; PEPFAR: Washington, DC, USA, 2022; Volume 2.6.1, pp. 154–196.
- Bendavid, E. Past and Future Performance: PEPFAR in the Landscape of Foreign Aid for Health. Curr. HIV/AIDS Rep. 2016, 13, 256–262. [Google Scholar] [CrossRef]
- Bekker, L.G.; Alleyne, G.; Baral, S.; Cepeda, J.; Daskalakis, D.; Dowdy, D.; Dybul, M.; Eholie, S.; Esom, K.; Garnett, G.; et al. Advancing Global Health and Strengthening the HIV Response in the Era of the Sustainable Development Goals: The International AIDS Society—Lancet Commission. Lancet 2018, 392, 312–358. [Google Scholar] [CrossRef]
- Viriyathorn, S.; Sachdev, S.; Wanwong, Y.; Patcharanarumol, W.; Tangcharoensathien, V. The Impact of HIV Spending on Health Outcomes in Thailand. World J. AIDS 2021, 11, 85–100. [Google Scholar] [CrossRef]
- Kim, S.; Tadesse, E.; Jin, Y.; Cha, S. Association between Development Assistance for Health and Disease Burden: A Longitudinal Analysis on Official Development Assistance for HIV/AIDS, Tuberculosis, and Malaria in 2005–2017. Int. J. Environ. Res. Public Health 2022, 19, 14091. [Google Scholar] [CrossRef]
- Hsiao, A.J.; Emdin, C.A. The Association between Development Assistance for Health and Malaria, HIV and Tuberculosis Mortality: A Cross-National Analysis. J. Epidemiol. Glob. Health 2015, 5, 41–48. [Google Scholar] [CrossRef]
- Xie, S.; Du, S.; Huang, Y.; Luo, Y.; Chen, Y.; Zheng, Z.; Yuan, B.; Xu, M.; Zhou, S. Evolution and Effectiveness of Bilateral and Multilateral Development Assistance for Health: A Mixed-Methods Review of Trends and Strategic Shifts (1990-2022). BMJ Glob. Health 2025, 10, e017818. [Google Scholar] [CrossRef]
- Neel, A.H.; Rodríguez, D.C.; Sikazwe, I.; Pillay, Y.; Barron, P.; Pereira, S.K.; Makakole-Nene, S.; Bennett, S.C. HIV Programme Sustainability in Southern and Eastern Africa and the Changing Role of External Assistance for Health. Health Policy Plan. 2024, 39, I107–I117. [Google Scholar] [CrossRef]
- Dzinamarira, T.; Rwibasira, G.; Mwila, L.; Moyo, E.; Mangoya, D.; Moyo, P.; Oladele, E.; Akinjeji, A.; Chimene, M.; Muvunyi, C.M. Advancing Sustainable HIV Services Through Integration in Primary Healthcare in Sub-Saharan Africa: A Perspective on Practical Recommendations. Healthcare 2025, 13, 192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Building Health System Resilience to Public Health Challenges Guidance for Implementation in Countries; World Health Organisation: Geneva, Switzerland, 2024; pp. 1–58. [Google Scholar]
- Elsey, H.; Agyepong, I.; Huque, R.; Quayyem, Z.; Baral, S.; Ebenso, B.; Kharel, C.; Shawon, R.A.; Onwujekwe, O.; Uzochukwu, B.; et al. Rethinking Health Systems in the Context of Urbanisation: Challenges from Four Rapidly Urbanising Low-Income and Middle-Income Countries. BMJ Glob. Health 2019, 4, e001501. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, G.; Garabrant, A.; Adyas, A.; Muga, R.; Otoo, N. Moving towards Universal Health Coverage: Health Insurance Reforms in Nine Developing Countries in Africa and Asia. Lancet 2012, 380, 933–943. [Google Scholar] [CrossRef]
- The White House Presidential Actions—Reevaluating and Realigning United States Foreign AID. Available online: https://www.whitehouse.gov/presidential-actions/2025/01/reevaluating-and-realigning-united-states-foreign-aid/ (accessed on 22 March 2025).
- Kobayashi, Y.; Heinrich, T.; Bryant, K.A. Public Support for Development Aid during the COVID-19 Pandemic. World Dev. 2021, 138, 105248. [Google Scholar] [CrossRef]
- Deng, P.; Chen, M.; Si, L. Temporal Trends in Inequalities of the Burden of HIV/AIDS across 186 Countries and Territories. BMC Public Health 2023, 23, 981. [Google Scholar] [CrossRef]
- Ataguba, J.E.O.; Birungi, C.; Cunial, S.; Kavanagh, M. Income Inequality and Pandemics: Insights from HIV/AIDS and COVID-19—A Multicountry Observational Study. BMJ Glob. Health 2023, 8, e013703. [Google Scholar] [CrossRef]
- Oladosu, A.O.; Chanimbe, T.; Anaduaka, U.S. Effect of Public Health Expenditure on Health Outcomes in Nigeria and Ghana. Health Policy OPEN 2022, 3, 100072. [Google Scholar] [CrossRef]
- Madigan, E.A.; Curet, O.L.; Zrinyi, M. Workforce Analysis Using Data Mining and Linear Regression to Understand HIV/AIDS Prevalence Patterns. Hum. Resour. Health 2008, 6, 2. [Google Scholar] [CrossRef]
- Odugbesan, J.A.; Rjoub, H. Relationship among HIV/AIDS Prevalence, Human Capital, Good Governance, and Sustainable Development: Empirical Evidence from Sub-Saharan Africa. Sustainability 2019, 11, 1348. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Toward an Experimental Ecology of Human Development. Am. Psychol. 1977, 32, 513–531. [Google Scholar] [CrossRef]
- Dahlgren, G.; Whitehead, M. The Dahlgren-Whitehead Model of Health Determinants: 30 Years on and Still Chasing Rainbows. Public Health 2021, 199, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Sola, O.; Irwin, A. A Conceptual Framework for Action on the Social Determinants of Health. In Social Determinants of Health Discussion Paper 2 (Policy and Practice); World Health Organisation: Geneva, Switzerland, 2010; pp. 1–76. [Google Scholar]
- Nketia, R. The Sub-Saharan Africa Research for Sustainable Development (SSARSD) Project. Available online: https://ssarsdproject.org/ (accessed on 27 January 2025).
- Appiah, E.K.; Arko-Achemfuor, A.; Adeyeye, O.P. Appreciation of Diversity and Inclusion in Sub-Sahara Africa: The Socioeconomic Implications. Cogent Soc. Sci. 2018, 4, 1521058. [Google Scholar] [CrossRef]
- The World Bank. The World Bank In Africa: Overview. Available online: https://www.worldbank.org/en/region/afr/overview (accessed on 25 July 2024).
- United Nations Development Programme. The Rise of the South: Human Progress in a Diverse World. Human Development Report 2013; United Nations Development Programme: New York, NY, USA, 2013; pp. 1–28. [Google Scholar]
- World Health Organisation Regional Office for Africa. Atlas of African Health Statistics 2022: Health Situation Analysis of the WHO African Region—Country Profiles; WHO Regional Office for Africa: Brazzaville, Congo, 2022; pp. 1–99. [Google Scholar]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef]
- Chancel, L.; Piketty, T.; Saez, E.; Zucman, G.; Duflo, E.; Banerjee, A. Global Inequality Report. 2022. Available online: https://wir2022.wid.world/ (accessed on 22 January 2025).
- Dieleman, J.; Campbell, M.; Chapin, A.; Eldrenkamp, E.; Fan, V.Y.; Haakenstad, A.; Kates, J.; Liu, Y.; Matyasz, T.; Micah, A.; et al. Evolution and Patterns of Global Health Financing 1995–2014: Development Assistance for Health, and Government, Prepaid Private, and out-of-Pocket Health Spending in 184 Countries. Lancet 2017, 389, 1981–2004. [Google Scholar] [CrossRef]
- Mathers, C.D. History of Global Burden of Disease Assessment at the World Health Organisation. Arch. Public Health 2020, 78, 77. [Google Scholar] [CrossRef]
- Meteyard, L.; Davies, R.A.I. Best Practice Guidance for Linear Mixed-Effects Models in Psychological Science. J. Mem. Lang. 2020, 112, 104092. [Google Scholar] [CrossRef]
- Harrison, X.A.; Donaldson, L.; Correa-Cano, M.E.; Evans, J.; Fisher, D.N.; Goodwin, C.E.D.; Robinson, B.S.; Hodgson, D.J.; Inger, R. A Brief Introduction to Mixed Effects Modelling and Multi-Model Inference in Ecology. PeerJ 2018, 6, e4794. [Google Scholar] [CrossRef]
- Huang, P.H. Asymptotics of AIC, BIC, and RMSEA for Model Selection in Structural Equation Modelling. Psychometrika 2017, 82, 407–426. [Google Scholar] [CrossRef]
- West, R.M. Best Practice in Statistics: The Use of Log Transformation. Ann. Clin. Biochem. 2022, 59, 162–165. [Google Scholar] [CrossRef]
- Morgenstern, H. Ecologic Studies in Epidemiology: Concepts, Principles, and Methods. Annu. Rev. Public Health 1995, 16, 61–81. [Google Scholar] [CrossRef]
- Thomopoulos, N.T. Generating Random Variates. In Essentials of Monte Carlo Simulation; Thomopoulos, N.T., Ed.; Springer: New York, NY, USA, 2013; pp. 15–26. [Google Scholar]
- D’Agostino McGowan, L. Sensitivity Analyses for Unmeasured Confounders. Curr. Epidemiol. Rep. 2022, 9, 361–375. [Google Scholar] [CrossRef]
- Belotti, F.; Peracchi, F. Fast Leave-One-out Methods for Inference, Model Selection, and Diagnostic Checking. Stata J. 2020, 20, 785–804. [Google Scholar] [CrossRef]
- Barber, R.F.; Candès, E.J.; Ramdas, A.; Tibshirani, R.J. Predictive Inference with the Jackknife+. Ann. Stat. 2021, 49, 486–507. [Google Scholar] [CrossRef]
- Imbens, G.W. Instrumental Variables: An Econometricians’s Perspective. Stat. Sci. 2014, 29, 323–358. [Google Scholar] [CrossRef]
- Cui, G.; Norkute, M.; Sarafidis, V.; Yamagata, T. Two-Stage Instrumental Variable Estimation of Linear Panel Data Models with Interactive Effects. Econom. J. 2022, 25, 340–361. [Google Scholar] [CrossRef]
- Ullah, S.; Zaefarian, G.; Ullah, F. How to Use Instrumental Variables in Addressing Endogeneity? A Step-by-Step Procedure for Non-Specialists. Ind. Mark. Manag. 2021, 96, A1–A6. [Google Scholar] [CrossRef]
- Kruiniger, H. GMM Estimation and Inference in Dynamic Panel Data Models with Persistent Data. Econom. Theory 2009, 25, 1348–1391. [Google Scholar] [CrossRef]
- Bun, M.J.G.; Sarafidis, V. Dynamic Panel Data Models; UvA-Econometrics Discussion Paper; University of Amsterdam: Amsterdam, The Netherlands, 2013; pp. 1–36. [Google Scholar]
- Yu, P.; Liao, Q.; Phillips, P.C.B. New Control Function Approaches in Threshold Regression with Endogeneity. Econom. Theory 2023, 40, 1065–1119. [Google Scholar] [CrossRef]
- Wooldridge, J.M.W. Control Function Methods in Applied Econometrics. J. Hum. Resour. 2018, 50, 420–445. [Google Scholar] [CrossRef]
- Kiviet, J.F.; Kripfganz, S. Instrument Approval by the Sargan Test and Its Consequences for Coefficient Estimation. Econ. Lett. 2021, 205, 109935. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 18. StataCorp LLC: College Station, TX, USA, 2023. [Google Scholar]
- Kutzin, J. Health Financing for Universal Coverage and Health System Performance: Concepts and Implications for Policy. Bull. World Health Organ. 2013, 91, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.M.; Kazemian, M.; Motaghed, Z.; Abdi, Z. Systematic Review of Factors Determining Health Care Expenditures. Health Policy Technol. 2021, 10, 100498. [Google Scholar] [CrossRef]
- The Joint United Nations Programme on HIV/AIDS Impact of US Funding Cuts on the Global AIDS Response—28 March 2025 Update. Available online: https://www.unaids.org/en/resources/presscentre/featurestories/2025/march/20250328_sitrep (accessed on 11 May 2025).
- Ndjeka, N.; Kubjane, M.; Abdullah, F.; Mohr-Holland, E.; Subrayen, P.; Loveday, M.; Dube, M.; Boffa, J. Impact of US Funding Cuts and Stop Work Orders on TB Services and Research in South Africa. IJTLD Open 2025, 2, 241–243. [Google Scholar] [CrossRef]
- Atun, R.; De Jongh, T.; Secci, F.; Ohiri, K.; Adeyi, O. Integration of Targeted Health Interventions into Health Systems: A Conceptual Framework for Analysis. Health Policy Plan. 2010, 25, 104–111. [Google Scholar] [CrossRef]
- Biesma, R.G.; Brugha, R.; Harmer, A.; Walsh, A.; Spicer, N.; Walt, G. The Effects of Global Health Initiatives on Country Health Systems: A Review of the Evidence from HIV/AIDS Control. Health Policy Plan. 2009, 24, 239–252. [Google Scholar] [CrossRef]
- Sparkes, S.P.; Bump, J.B.; Özçelik, E.A.; Kutzin, J.; Reich, M.R. Political Economy Analysis for Health Financing Reform. Health Syst. Reform 2019, 5, 183–194. [Google Scholar] [CrossRef]
- Carlisle, D. AIDS Will Affect Us All Eventually, Mandela Tells London Audience. BMJ 2003, 327, 122. [Google Scholar] [CrossRef]
- The Joint United Nations Programme on HIV/AIDS. The Media and HIV/AIDS: Making a Difference; UNAIDS: Geneva, Switzerland, 2004; pp. 1–25. [Google Scholar]
- Stein, J. HIV/AIDS and the Media: A Literature Review; The Centre for AIDS Development, Research and Evaluation (Cadre): Johannesburg, South Africa, 2001; pp. 1–18. [Google Scholar]
- Lacroix, J.M.; Snyder, L.B.; Huedo-medina, T.B.; Johnson, B.T. Effectiveness of Mass Media Interventions for HIV Prevention, 1986–2013: A Meta-Analysis. J. Acquir. Immune Defic. Syndr. 2014, 66, S329–S340. [Google Scholar] [CrossRef]
- Peltzer, K.; Parker, W.; Mabaso, M.; Makonko, E.; Zuma, K.; Ramlagan, S. Impact of National HIV and AIDS Communication Campaigns in South Africa to Reduce HIV Risk Behaviour. Sci. World J. 2017, 2012, 384608. [Google Scholar] [CrossRef]
- Jones, P.S. “A Test of Governance”: Rights-Based Struggles and the Politics of HIV/AIDS Policy in South Africa. Polit. Geogr. 2005, 24, 419–447. [Google Scholar] [CrossRef]
- Lu, C.; Schneider, M.T.; Gubbins, P.; Leach-Kemon, K.; Jamison, D.; Murray, C.J. Public Financing of Health in Developing Countries: A Cross-National Systematic Analysis. Lancet 2010, 375, 1375–1387. [Google Scholar] [CrossRef] [PubMed]


| United States Public Sector | Other International Donors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Region/Country | 1990 | 2022 | Total (1990–2022) | Median | Interquartile Range (IQR) | 1990 | 2022 | Total (1990–2022) | Median | IQR |
| Central Africa | ||||||||||
| Angola | 220 | 17,048 | 292,450 | 9848 | 647–15,089 | 82 | 10,796 | 259,796 | 4961 | 1622–12,693 |
| Cameroon | 296 | 101,689 | 622,547 | 5524 | 223–27,608 | 88 | 47,896 | 579,146 | 18,300 | 579–30,458 |
| Central African Republic | 69 | 10,192 | 86,471 | 1954 | 75–3061 | 19 | 15,485 | 247,069 | 6393 | 345–12,523 |
| Chad | 159 | 8899 | 111,448 | 1428 | 342–6892 | 2577 | 14,342 | 329,348 | 6965 | 2975–15,469 |
| Congo | 50 | 4152 | 42,420 | 1117 | 39–1922 | 8 | 7841 | 145,867 | 3695 | 515–7563 |
| Democratic Republic of Congo | 980 | 103,010 | 1,065,907 | 17,541 | 1038–61,344 | 10,015 | 55,502 | 1,262,142 | 44,185 | 2738–67,400 |
| Equatorial Guinea | 2 | 103 | 3810 | 1 | 0–103 | 3 | 2608 | 34,081 | 12 | 32,143 |
| Gabon | 21 | 698 | 15,853 | 225 | 12–831 | 2 | 3339 | 61,981 | 1544 | 22–2921 |
| Sao Tome and Principe | 8 | 2023 | 13,885 | 117 | 6–621 | 1 | 4898 | 53,728 | 668 | 25–2647 |
| Sub-region | 1805 | 247,814 | 2,254,791 | 703 | 39–5524 | 12,795 | 162,707 | 2,973,158 | 2975 | 302–11,262 |
| Eastern Africa | ||||||||||
| Burundi | 205 | 24,949 | 233,886 | 4201 | 205–12,259 | 96 | 16,753 | 426,658 | 15,111 | 882 - 20,138 |
| Comoros | 11 | 851 | 13,671 | 185 | 33–697 | 1 | 3615 | 57,257 | 733 | 157–2807 |
| Djibouti | 26 | 611 | 33,650 | 628 | 26–1774 | 6 | 3291 | 117,903 | 3516 | 179–6146 |
| Eritrea | 131 | 4177 | 97,458 | 2739 | 143–4186 | 15 | 7617 | 260,522 | 7617 | 856–12,507 |
| Ethiopia | 2426 | 146,934 | 3,492,492 | 82,387 | 4055–187,851 | 3764 | 88,865 | 2,396,746 | 70,536 | 14,179–88,865 |
| Kenya | 3409 | 324,109 | 6,430,490 | 161,959 | 14,219–372,810 | 18,973 | 109,057 | 2,033,820 | 67,565 | 20,892–98,800 |
| Madagascar | 195 | 4015 | 78,617 | 2533 | 212–3476 | 30 | 7807 | 200,763 | 5402 | 232–9378 |
| Malawi | 1308 | 209,916 | 2,013,290 | 35,219 | 11,341–90,054 | 2095 | 84,257 | 2,111,738 | 71,714 | 12,147–114,210 |
| Mauritius | 10 | 428 | 9717 | 16 | 5–411 | 1 | 3219 | 48,454 | 236 | 9–2972 |
| Mozambique | 1992 | 382,558 | 4,042,448 | 63,990 | 2166–244,619 | 3336 | 108,294 | 1,957,063 | 63,016 | 5461–108,294 |
| Rwanda | 313,903 | 86,028 | 2,069,012 | 63,490 | 3504–86,667 | 99,987 | 34,189 | 1,397,188 | 34,189 | 5200–59,871 |
| Seychelles | 0 | 0 | 1886 | 0 | 0–1 | 0 | 0 | 9686 | 0 | 0–72 |
| Somalia | 132 | 4103 | 44,589 | 706 | 93–2240 | 16 | 7497 | 142,612 | 3141 | 118–7933 |
| South Sudan | 154 | 54,466 | 240,929 | 294 | 112–14,301 | 60 | 30,362 | 205,605 | 1098 | 257–11,904 |
| Tanzania | 0 | 314,037 | 5,071,245 | 112,160 | 7850–314,037 | 26,790 | 64,987 | 2,349,792 | 64,229 | 17,352–119,253 |
| Uganda | 2307 | 310,491 | 5,398,838 | 176,254 | 8763–299,376 | 2213 | 93,288 | 1,824,540 | 57,251 | 21,022–86,849 |
| Zambia | 1733 | 270,598 | 4,107,366 | 112,261 | 11,847–228,169 | 2803 | 68,115 | 1,678,906 | 54,344 | 9346–76,743 |
| Zimbabwe | 1439 | 194,929 | 1,778,476 | 34,590 | 7816–97,346 | 11,172 | 81,341 | 2,275,025 | 64,960 | 18,686–115,723 |
| Sub-region | 329,381 | 2,333,200 | 35,158,060 | 3520.5 | 205–70,262 | 171,358 | 812,554 | 19,494,278 | 9234 | 1166–57,740 |
| Northern Africa | ||||||||||
| Algeria | 10 | 389 | 4257 | 5 | 0–137 | 0 | 1770 | 30,251 | 136 | 12–1544 |
| Egypt | 16 | 3517 | 22,844 | 280 | 9–1021 | 0 | 5819 | 59,749 | 1093 | 361–2999 |
| Libya | 3 | 0 | 185 | 0 | 0–3 | 0 | 1282 | 28,053 | 77 | 9–1282 |
| Morocco | 46 | 5121 | 31,935 | 555 | 3–1350 | 0 | 7463 | 122,778 | 3490 | 1905–5514 |
| Sudan | 222 | 12,410 | 203,026 | 2567 | 159–12,244 | 41 | 18,314 | 385,003 | 9045 | 508–17,907 |
| Tunisia | 27 | 1459 | 13,007 | 29 | 0–553 | 0 | 3047 | 44,714 | 420 | 6–2139 |
| Sub-region | 324 | 22,896 | 275,254 | 44 | 0–864 | 41 | 37,695 | 670,548 | 1262 | 45–3371 |
| Southern Africa | ||||||||||
| Botswana | 775 | 34,198 | 1,341,181 | 34,198 | 843–56,662 | 1070 | 5879 | 304,508 | 6494 | 1280–15,802 |
| Eswatini | 0 | 53,531 | 673,237 | 8242 | 0–39,476 | 0 | 6507 | 325,252 | 6797 | 400–16,379 |
| Lesotho | 410 | 65,370 | 577,317 | 3342 | 293–30,313 | 225 | 15,984 | 408,954 | 12,602 | 1291–22,882 |
| Namibia | 707 | 71,082 | 1,347,383 | 32,218 | 707–71,082 | 1385 | 8678 | 486,842 | 12,000 | 2737–21,966 |
| South Africa | 3677 | 522,597 | 8,135,678 | 164,487 | 15,185–468,293 | 3164 | 112,359 | 2,538,446 | 94,203 | 8647–112,496 |
| Sub-region | 5569 | 746,778 | 12,074,796 | 24,625 | 544–60,303 | 5844 | 149,407 | 4,064,002 | 11,356 | 2123–23,821 |
| Western Africa | ||||||||||
| Benin | 200 | 11,866 | 144,016 | 3649 | 262–7583 | 44 | 12,585 | 317,886 | 12,137 | 746–15,581 |
| Burkina Faso | 271 | 11,716 | 172,928 | 2995 | 504–8240 | 85 | 13,410 | 643,460 | 16,076 | 7933–29,425 |
| Cabo Verde | 0 | 571 | 8601 | 115 | 0–482 | 0 | 680 | 29,471 | 680 | 0–1535 |
| Cote d’Ivoire | 677 | 71,696 | 1,512,831 | 33,287 | 526–85,710 | 210 | 25,708 | 531,344 | 13,510 | 3966–25,473 |
| Gambia | 0 | 3114 | 53,611 | 2021 | 0–2790 | 0 | 3825 | 145,775 | 4973 | 286–7306 |
| Ghana | 413 | 30,709 | 604,773 | 19,496 | 6904–28,540 | 108 | 32,464 | 941,254 | 31,352 | 1830–45,967 |
| Guinea | 114 | 15,583 | 132,545 | 3848 | 1495–5148 | 37 | 20,816 | 230,989 | 6548 | 1951–10,859 |
| Guinea-Bissau | 55 | 5120 | 55,448 | 555 | 55–3432 | 279 | 8668 | 164,006 | 2834 | 752–8668 |
| Liberia | 107 | 10,480 | 127,521 | 1959 | 79–7275 | 51 | 9827 | 248,536 | 7661 | 266–12,708 |
| Mali | 158 | 13,360 | 204,609 | 6901 | 2386–8924 | 137 | 18,257 | 343,616 | 11,954 | 1439–16,381 |
| Mauritania | 11 | 1850 | 16,771 | 345 | 9–761 | 3 | 5297 | 70,162 | 1224 | 131–3620 |
| Niger | 148 | 9525 | 57,425 | 1687 | 148–2461 | 45 | 16,178 | 226,402 | 8384 | 657–11,350 |
| Nigeria | 1999 | 216,935 | 5,061,493 | 90,165 | 1999–274,701 | 1441 | 94,471 | 2,286,510 | 52,867 | 9432–112,113 |
| Senegal | 202 | 12,166 | 2,51,025 | 8337 | 2129–12,166 | 67 | 16,173 | 358,767 | 10,832 | 3704–16,506 |
| Sierra Leone | 155 | 18,833 | 1,37,782 | 1956 | 112–7641 | 32 | 17,635 | 302,103 | 8566 | 192–15,796 |
| Togo | 144 | 15,171 | 94,808 | 1784 | 102–4258 | 33 | 15,524 | 234,941 | 6387 | 490–11,908 |
| Sub-region | 4654 | 448,695 | 8,636,187 | 2311 | 170–8042 | 2572 | 3,11,518 | 7,075,222 | 6392 | 752–15,499.5 |
| Overall (Africa) | 341,733 | 3,799,383 | 58,399,088 | 1679 | 93–12,672 | 192,610 | 1,473,881 | 34,277,208 | 5194 | 676–18,036 |
| Effects | HIV/AIDS Incidence | HIV/AIDS Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | 95% CI | p | Coefficient | SE | 95% CI | p | |
| Fixed Effects | ||||||||
| U.S._DAH | −0.047 | 0.017 | −0.081 to−0.013 | 0.006 * | 0.027 | 0.024 | −0.019 to 0.073 | 0.254 |
| Other_DAH | −0.046 | 0.006 | −0.057 to −0.035 | <0.001 * | −0.007 | 0.007 | −0.022 to 0.007 | 0.301 |
| U.S._DAH#Other_DAH | −0.013 | 0.001 | −0.015 to −0.010 | <0.001 * | −0.025 | 0.002 | −0.028 to −0.022 | <0.001 * |
| GHES | 0.108 | 0.021 | 0.066 to 0.150 | <0.001 * | 0.133 | 0.028 | 0.079 to 0.187 | <0.001 * |
| U.S._DAH#GHES | −0.093 | 0.004 | −0.102 to −0.084 | <0.001 * | −0.142 | 0.006 | −0.154 to −0.131 | <0.001 * |
| Other_DAH#GHES | 0.016 | 0.004 | 0.009 to 0.023 | <0.001 * | 0.001 | 0.005 | −0.008 to 0.010 | 0.842 |
| U.S._DAH#Other_DAH#GHES | 0.0001 | 0.001 | −0.001 to 0.001 | 0.838 | 0.004 | 0.001 | 0.002 to 0.006 | <0.001 * |
| Physician density | 0.024 | 0.039 | −0.054 to 0.101 | 0.550 | −0.100 | 0.051 | −0.199 to −0.001 | 0.049 * |
| Gini coefficient | 0.468 | 0.154 | 0.167 to 0.770 | 0.002 * | 0.227 | 0.198 | −0.161 to 0.616 | 0.251 |
| SDI | −1.492 | 0.073 | −1.635 to −1.349 | <0.001 * | −1.755 | 0.094 | −1.940 to −1.571 | <0.001 * |
| Pre-paid private spending | −0.010 | 0.008 | −0.025 to 0.004 | 0.166 | 0.028 | 0.010 | 0.009 to 0.047 | 0.004 * |
| Out-of-pocket spending | 0.217 | 0.023 | 0.172 to 0.262 | <0.001 * | 0.243 | 0.030 | 0.185 to 0.301 | <0.001 * |
| Sex | ||||||||
| Female | Ref | - | - | |||||
| Male | −0.158 | 0.303 | −0.751 to 0.435 | 0.601 | −0.136 | 0.353 | −0.828 to 0.556 | 0.700 |
| Intercept | 4.638 | 0.947 | 2.782 to 6.495 | <0.001 * | 4.016 | 0.936 | 2.181 to 5.852 | <0.001 * |
| Random effects | ||||||||
| Sub-region level | ||||||||
| Intercept | 4.225 | 2.822 | 1.141 to 15.641 | 4.026 | 2.746 | 1.058 to 15.328 | ||
| Country level | ||||||||
| var(U.S._DAH) | 0.031 | 0.005 | 0.023 to 0.041 | 0.058 | 0.009 | 0.043 to 0.077 | ||
| var(intercept) | 2.466 | 0.346 | 1.873 to 3.247 | 3.420 | 0.483 | 2.594 to 4.510 | ||
| cov(U.S._DAH, intercept) | 0.001 | 0.034 | −0.064 to 0.067 | −0.062 | 0.053 | −0.166 to 0.042 | ||
| var(Residual) | 0.053 | 0.002 | 0.050 to 0.056 | 0.088 | 0.002 | 0.083 to 0.093 | ||
| Nsite | 5 | 5 | ||||||
| Nmix | 108 | 108 | ||||||
| ICC | 0.94 | 0.94 | ||||||
| N | 2700 | 2700 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nketia, R.; Atta-Nyarko, D.; Gyamfi, E.; Bessala, R.D.M.; Adotei, N.; Asare-Kyei, B.; Marfo, F.A.; Luri, P.T.; Limula, C.; Ahmed Farhan, A.; et al. The Cost of Cutbacks: How Reduction in Development Assistance for Health May Affect Progress Made in HIV/AIDS Control in Africa. Venereology 2025, 4, 8. https://doi.org/10.3390/venereology4020008
Nketia R, Atta-Nyarko D, Gyamfi E, Bessala RDM, Adotei N, Asare-Kyei B, Marfo FA, Luri PT, Limula C, Ahmed Farhan A, et al. The Cost of Cutbacks: How Reduction in Development Assistance for Health May Affect Progress Made in HIV/AIDS Control in Africa. Venereology. 2025; 4(2):8. https://doi.org/10.3390/venereology4020008
Chicago/Turabian StyleNketia, Richmond, Daniel Atta-Nyarko, Ebenezer Gyamfi, Rostand Dimitri Messanga Bessala, Naomi Adotei, Benjamin Asare-Kyei, Faustina Ameyaa Marfo, Prosper Tonwisi Luri, Charles Limula, Abubakr Ahmed Farhan, and et al. 2025. "The Cost of Cutbacks: How Reduction in Development Assistance for Health May Affect Progress Made in HIV/AIDS Control in Africa" Venereology 4, no. 2: 8. https://doi.org/10.3390/venereology4020008
APA StyleNketia, R., Atta-Nyarko, D., Gyamfi, E., Bessala, R. D. M., Adotei, N., Asare-Kyei, B., Marfo, F. A., Luri, P. T., Limula, C., Ahmed Farhan, A., Castelli, M., & Adobasom-Anane, A. G. (2025). The Cost of Cutbacks: How Reduction in Development Assistance for Health May Affect Progress Made in HIV/AIDS Control in Africa. Venereology, 4(2), 8. https://doi.org/10.3390/venereology4020008











