Abstract
The World Health Organization (WHO) recommended the use of oral pre-exposure prophylaxis (PrEP) for HIV prevention in 2015. Although the number of countries with national PrEP recommendations increased from 2 in 2015 to 121 in 2019, there has been slow progress in Africa. The reason for this slow progress is that developing countries have budgetary constraints. Budget impact analysis (BIA) is an economic analysis that can help explore the affordability of oral PrEP. A rapid review was conducted to summarise the existing literature on the budget impact of adopting oral PrEP for HIV prevention. PubMed and Google Scholar databases were searched for relevant studies. Studies included in the review utilised primary data. Eleven studies met the inclusion criteria. This review reveals that most studies took a healthcare service provider perspective, targeted men who have sex with men (MSM), had time horizons of more than 10 years, used macro-costing, and performed univariate sensitivity analysis and discounting. If countries wish to perform a budget impact analysis of adopting oral PrEP for HIV prevention, we recommend that they select a target population that is most at risk of acquiring HIV. In addition, a time horizon of ten years or more should be used, and accurate values of the cost of oral PrEP and antiretroviral therapy (ART) and adherence to oral PrEP should be researched. Furthermore, deterministic sensitivity analysis should be carried out instead of probabilistic sensitivity analysis (PSA), as the degree of variability and the extent of the correlation among the parameters may not be known.
1. Introduction
In 2022, about 39 million people were living with Human Immunodeficiency Virus (HIV), while 630,000 people died of Acquired Immunodeficiency Syndrome (AIDS)-related illnesses. Eastern and Southern Africa had 20.8 million people living with HIV (PLHIV). In 2022 alone, about 1.3 million people became newly infected with HIV, and almost 40% of these were in Eastern and Southern Africa [1]. The World Health Organization (WHO) recommends a combination of HIV prevention interventions to reduce HIV transmission. The interventions can be divided into behavioural, biomedical, and structural interventions. Behavioural interventions include the use of condoms, delaying first sexual intercourse among youths, being faithful to one partner, and educating people using drugs to use clean needles and not share them [2]. Biomedical interventions include voluntary medical male circumcision (VMMC), antiretroviral treatment as prevention (TasP), prevention of mother-to-child transmission (PMTCT), pre-exposure prophylaxis (PrEP), and post-exposure prophylaxis (PEP) [3]. Structural interventions are essential where the social, political, legal, and economic situations of communities make people vulnerable to HIV infection. This is common in sub-Saharan Africa, where most people live in poverty and laws against sex work, drug use, and homosexuality still exist. This stigma and discrimination make some people more vulnerable to HIV infection, as they struggle to access prevention services [4].
The WHO recommended the use of oral PrEP for HIV prevention in 2015. The basis of this recommendation was the results of the Preexposure Prophylaxis Initiative (iPrEx) trial, which showed a 44% reduction in HIV infection in those who took tenofovir/emtricitabine over a variable period of up to 2.8 years. Additional evidence from the pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD) and the On-Demand Antiretroviral Pre-exposure Prophylaxis for HIV Infection in Men Who Have Sex With Men (IPERGAY) studies reported an 86% reduction in risk of HIV transmission in people receiving tenofovir/emtricitabine. The iPrEx study showed that, with full adherence, HIV transmission can be reduced by up to 99%. The WHO recommended that oral PrEP should be considered for any individual who is at risk of becoming infected with HIV [5]. Although the number of countries with national PrEP recommendations increased from 2 in 2015 to 121 in 2019, there has been slow progress in Africa. A few countries that are scaling up oral PrEP in Africa, like Lesotho, Kenya, South Africa, and Zimbabwe, have been targeting key populations like adolescent girls, young women, and sex workers [6]. The reason for the slow progress in implementing combination HIV interventions is that developing countries are finding it difficult to finance all of them at once due to budgetary constraints [7]. Budget impact analysis (BIA) is an economic analysis that can help explore the affordability of oral PrEP. It is defined as an economic evaluation method used to assess whether a new healthcare intervention is affordable to a budget holder, given its available resources [8]. It addresses the anticipated changes in the expenditure of a healthcare system after adopting a new healthcare intervention [9]. While BIA is a well-established concept in health economics, its application to specific interventions, such as oral pre-exposure prophylaxis (PrEP) for HIV prevention, has gained increased attention in recent years due to the growing need for evidence on the affordability of such interventions.
This rapid review aims to map the available literature on what is known about the budget impact analysis of adopting oral PrEP. This might empower decisionmakers and policymakers to make informed decisions on how to carry out the BIA of oral PrEP for HIV prevention. Such information might be used to inform their finance ministries and parliaments whether or not the programme is worth adopting. The information might also be used to source funds for the programme from international partners.
2. Methodology
2.1. Information Sources and Literature Search
We searched PubMed and Google Scholar databases for relevant studies. The key search terms included budget impact, PrEP, tenofovir/emtricitabine, cost, expenditure, and HIV. All database searches were initially conducted from 1 to 30 October 2021. We then conducted a second search in December 2024 to retrieve new articles published since the last search.
2.2. Participants
We included studies conducted anywhere in the world. The included studies were conducted at the national level on budget impact analysis of adopting oral PrEP for HIV prevention.
2.3. Study Selection and Inclusion Criteria
We searched for primary studies. Peer-reviewed articles that were less than 10 years old from the publication date and written in English were selected. Systematic reviews, case reports, review articles, editorials, and letters to the editor were excluded.
2.4. Screening and Data Abstraction Process
We developed a screening criterion a priori for each of the three stages: title, abstract, and full text. Two reviewers conducted data extraction from the identified studies. A data extraction form was developed a priori. The following study characteristics were documented from each of the selected articles: author(s) and country, year of publication, study setting, study perspective, target population, time horizon, costing and resource utilisation, sensitivity analysis, discounting, and key findings on the budget impact of adopting PrEP for HIV prevention.
2.5. Quality of the Selected Studies
The quality of the studies selected for this review was assessed using the BIA checklist proposed in one study [10]. The results are shown in Table 1. The checklist used for this study had 13 items. Each checklist item had a maximum score of one and a minimum score of zero [10]. The overall quality scores ranged from 11 to 13, and the average quality score was 12.2. Therefore, the quality of the chosen studies was good.

Table 1.
Quality of selected studies.
2.6. Data Synthesis
All of the study characteristics were collected and tabulated for each study. Thereafter, collating, summarising, and reporting the findings were performed. The findings of this study are reported in a narrative synthesis. The reporting of the results follow the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) checklist.
3. Results
Based on the search terms, 63 articles were identified from the two databases. Following title screening, all the 63 articles were eligible for abstract screening. A total of 34 studies were excluded at the abstract screening stage, leaving 29 studies for full-text screening. Eighteen studies were excluded at the full-text screening stage. The two articles excluded from the review focused only on the cost-effectiveness analysis (CEA) of PrEP or other HIV prevention methods and did not include the BIA of oral PrEP, thirteen were not primary studies, and three were not published in English. A total of 11 studies were included in this review. More details are presented in Figure 1: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
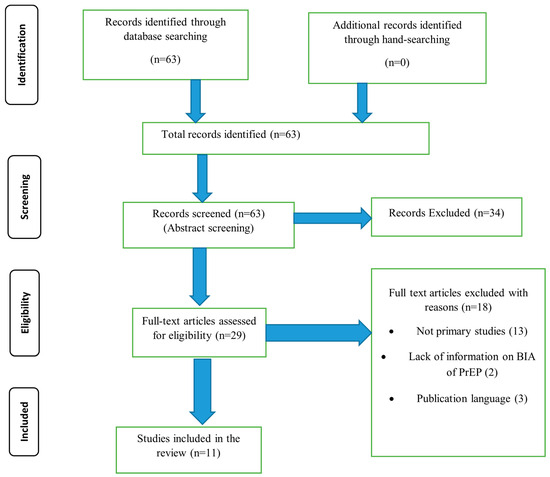
Figure 1.
PRISMA flow diagram.
3.1. Characteristics of the Included Studies
Among the studies selected, five were conducted in Europe. Of the five studies conducted in Europe, two were conducted in the United Kingdom [13,14], one in Germany [11], one in Belgium [12], and one in Spain [17]. The study conducted in Asia was conducted in India [16], while in South America was conducted in Colombia [15]. The two studies conducted in Africa were conducted in South Africa [18] and Namibia [21], only one in Australia [20], and only one in the United States of America [19].
3.2. Study Findings
3.2.1. Study Perspective
The studies selected for the literature review had different perspectives. Four of them took a healthcare service provider perspective [13,14,17,20], two took a societal perspective [16,19], one took a pharmaceutical budget holder perspective [12], one took a healthcare payer perspective [11], one took a health system perspective [15], and two took a health ministry perspective [18,21].
3.2.2. Target Population
All of the studies selected for this review used mathematical modelling, country-specific data, and the published literature data to develop the target population for oral PrEP. Six studies had a target population of men who have sex with men (MSM) [11,13,14,17,19,20]; one used the whole population [12]; one used all people at risk of acquiring HIV [21]; one used heterosexual women [12]; one used MSM and transgender women (TGW) [15]; and another one used MSM and people who inject drugs (PWID) [16].
3.2.3. Time Horizon
The time horizons for the studies in this review ranged from one year [17] to eighty years [13]. One of the studies used a time horizon of 3 years [15,21], two used a time horizon of 10 years [14,20], two used a time horizon of 15 years [12,16], two used a time horizon of 20 years [18,20], and one used a time horizon of 40 years [11].
3.2.4. Costing and Resource Utilisation
The type of costing used in the studies used in this review was also different. Only one study used micro-costing [11], and the rest used macro-costing [12,13,14,15,16,17,18,19,20,21]. The costs considered in some of the studies in this review include the price of PrEP drugs, antiretroviral therapy (ART) drugs, laboratory tests, counselling, consultation, treatment of side effects, and other oral PrEP programme costs [11,13,14]. However, because one study took a pharmaceutical budget holder perspective [12], the analysis did not include testing, diagnosis, and consultation fees.
3.2.5. Sensitivity Analysis
All of the studies selected for this review conducted sensitivity analysis. All of the studies conducted a univariate sensitivity analysis [11,12,13,14,15,16,17,18,19,20,21], four conducted a multivariate analysis [11,18,19,21], three conducted a scenario analysis [14,15,21], and another one conducted a Probabilistic sensitivity analysis (PSA) [13]. The analyses were sensitive to the efficacy of oral PrEP [14,18,21, the price of oral PrEP [11,12,14,15,17,18,19,20,21], the price of antiretroviral drugs [11,12,20,21], and the coverage of PrEP [18,20].
3.2.6. Discounting
Seventy percent of the studies in this review performed discounting on costs. The discounting rate ranged from 3% [11,16,18,19,20] to 3.5% [21]. Four studies did not perform discounting [12,15,17,21].
3.2.7. Summary of Conclusions from the Studies
The conclusions from the selected studies were different (Table 2). Although 40% of the studies demonstrated that adopting oral PrEP might be cost-saving, this was after a variable investment period. In one study, higher uptake of oral PrEP in the first decade of adopting it might result in higher expenses, but cost savings might only be realised after ten years. The cost of HIV care was expected to reduce by EUR 5.1 billion over 40 years [11]. In another study, the breakeven of the initial investment was expected after 10 years. However, by 2030, the expected budgetary savings may reach EUR 33.7 million [12]. Another study showed that the overall costs of HIV care were expected to increase for 20 years. However, cost savings of GDP 1 billion may be reached in 80 years [13]. A study conducted in the United Kingdom showed that a breakeven situation may be reached after 23 years if the oral PrEP is 86% effective. However, if the oral PrEP price is reduced by 90%, the breakeven point may be reached by the fifth year [14]. A study conducted in Colombia showed that oral PrEP may be cost-saving by the third year [15]. A study conducted in Spain revealed that oral PrEP may be cost-saving in twenty years of adoption if the price of the oral PrEP is reduced by 80% [17]. A study conducted in South Africa showed that achieving coverage for half of the women in South Africa would require USD 6 billion over 5 years. They concluded that targeting women at highest risk and/or those most likely to adhere to oral PrEP might pay the highest returns on investment and might even be cost-saving [18]. A study conducted in the United States of America concluded that adopting oral PrEP might not be cost-saving and, therefore, unaffordable over 20 years since only USD 3 billion may be saved on HIV care after an investment of USD 98 billion [19]. A study which was conducted in Australia concluded that PrEP might be affordable if only 15% of MSM in discordant regular relationships were targeted [20]. A study which was conducted in India also concluded that oral PrEP might not be affordable [16]. A Namibian study revealed that cost savings could be realised from the first year of adoption. The study also reported that cost savings could increase by one percent from the second to the third year [21].

Table 2.
Main findings from included studies.
4. Discussion
In this review, the studies in countries without a single purchaser of health services, like the United States of America and India, took a societal perspective. This can be challenging as different budget holders are involved, making it difficult to determine the budget impact on each budget holder [22]. The different perspectives influence the costs that should be included in the BIA. This is very important as the perspective taken may determine whether the intervention is cost-saving. Some interventions may not be affordable from one perspective, but affordable from another [22]. Heterosexual women, MSM, PWID, and TGW were used as target populations in the studies used in this review. This is because these populations are considered to be at high risk of HIV transmission [12]. Using high-risk populations may result in a favourable budget impact compared to when the whole population is used [23]. The longer time horizons used by studies in this review may be because the BIA were conducted as part of CEA, and similar time horizons were chosen for both evaluations. The time horizon chosen for a BIA should be within the period most relevant to the decisionmakers. Such a time horizon should capture the period within which meaningful differences between costs and outcomes of the current intervention and the new intervention can be observed [5]. The time horizon chosen determines whether the BIA will capture meaningful resource changes, affecting its usefulness to decisionmakers [24]. Most of the studies in this review used macro-costing because it is cheap, less time-consuming, generalisable, and easier to apply when compared to micro-costing. However, the disadvantage is that it is less accurate, as costs cannot be directly traced to the individual who incurred the cost. There may also be a misallocation of some costs, while other costs may be excluded from the costing [25]. Although micro-costing is time-consuming and labour-intensive, it should be used because it allows for a more accurate assessment of healthcare interventions [26]. The findings of this review revealed that the costs considered for the BIA differed according to the perspective taken in the study. The costs usually cover the costs of drugs, clinical personnel, laboratory tests, fixed costs, overheads, and administrative and supervisory personnel. Drug costs include the purchase, central storage, and distribution costs. Fixed costs may include centralised start-up costs for microplanning and training and capital like equipment and furniture. Overheads may include the cost of buildings, transport, and communication. Costs of oral PrEP may be reduced if the oral PrEP programme is integrated into other existing services, such as maternal and child health and family planning, because some start-up costs, capital costs, and some overheads may be avoided or reduced [27]. Most studies in this review performed discounting. These findings are understandable, as most studies had a long-term time horizon. Long-term costs should be discounted in economic evaluations because individuals and society prefer to have money or resources now rather than later. Costs committed to health at the present moment can be invested elsewhere, resulting in a positive rate of return. Money available now can generate more money that can be used to secure greater health in the future [28].
In this review, all studies selected performed univariate analysis, while only one performed PSA. PSA might not have been carried out in most of the studies reviewed due to a lack of information on the ranges of the parameters used in the BIA. In theory, all the different types of sensitivity analysis can be carried out in BIA. However, in practice, their usefulness depends on the amount of data available and the quality of the data. Probabilistic sensitivity analysis (PSA) is not recommended when the degree of variability and the extent of the correlation among parameters are unknown. Although deterministic sensitivity analysis is preferred to PSA, PSA has the potential to provide a more realistic assessment of parameter uncertainty [7]. This review reveals that the budget impact analysis of oral PrEP was sensitive to efficacy, price, coverage of oral PrEP, and price of ART. This shows that prices of oral PrEP drugs should be lower compared to ART drugs, and few high-risk populations who are likely to have high adherence to oral PrEP should be covered for the budget impact to be favourable. The review also revealed that the period required for the budget impact of oral PrEP to be favourable varied by study. Higher expenses were expected in the first few years after the adoption of oral PrEP. This shows that governments should be prepared to invest a large sum of money before they realise the cost savings of adopting oral PrEP.
In addition to oral PrEP, there have been recent developments in alternative forms of pre-exposure prophylaxis, such as lenacapavir, an injectable option that has shown promise in clinical trials [29]. While lenacapavir represents a significant advancement in HIV prevention, it comes with a higher cost [30], which may present challenges for resource-constrained settings. Its adoption could affect the budget impact for HIV prevention, and future BIAs should consider both oral and non-oral PrEP options in their analyses. Given its higher cost, lenacapavir may be more suitable for specific high-risk populations, such as those with adherence challenges to oral PrEP. However, the affordability and budget impact of incorporating such new interventions into public health programmes will need to be carefully evaluated, especially in countries with limited resources
This review has several limitations. One of the limitations is that two databases were used in this review. Using only two databases might have resulted in some of the relevant literature being missed. Another limitation is that it included only articles published in English. This might have resulted in non-English studies being missed. Furthermore, the review did not follow all the steps of a systematic review. However, we believe that the results of this review produced important findings that might inform policymakers on what to consider when deciding to conduct a BIA of adopting oral PrEP for HIV prevention.
5. Conclusions
A BIA of oral PrEP for HIV prevention can take different perspectives and use different target populations and time horizons, depending on the needs of the decisionmakers and policymakers. The chosen perspective usually determines the resources included in the BIA. Although discounting may not be performed in a BIA, which takes a short time horizon, a medium- to long-time-horizon BIAs should perform discounting to account for the net present value of future amounts. Different types of sensitivity analyses should be performed in BIAs to address uncertainty. However, the type of sensitivity analysis chosen depends on data availability for the key parameters. Cost-effectiveness is an important consideration in the budget impact of oral PrEP. Our findings suggest that when PrEP is targeted towards high-risk populations, such as MSM, PWID, and TGW, the budget impact is more favourable and the return on investment is higher. This highlights the potential financial benefits of PrEP interventions in reducing long-term HIV treatment costs and preventing new infections. If countries wish to perform a BIA of adopting oral PrEP for HIV prevention, we recommend that they select a target population that is most at risk of acquiring HIV, since this has been found to have better investment returns. In addition, a time horizon of ten years or more should be used, and accurate values of the cost of oral PrEP and ART and adherence to oral PrEP should be researched. Furthermore, deterministic sensitivity analysis should be preferred over PSA, as the degree of variability and the extent of the correlation among parameters may not be known.
Author Contributions
Conceptualisation: E.M. and L.B.; Methodology: E.M., P.M., and L.B.; Formal analysis: E.M., D.M., and M.M.; Writing—original draft preparation: E.M. and P.M.; Writing—review and editing: L.B., D.M., M.M., and T.D.; Supervision: L.B. and T.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
There is no dataset related to this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The research was conducted without commercial or financial relationships that could be construed as a potential conflict of interest.
References
- The Joint United Nations Programme on HIV/AIDS (UNAIDS). The Path That Ends AIDS: Unaids Global AIDS Update 2023. 2023. Available online: https://www.unaids.org/en/resources/documents/2023/global-aids-update-2023 (accessed on 5 October 2023).
- WHO. Consolidated Guidelines on HIV Prevention, Diagnosis, and Care for Key Populations. 2016. Available online: http://apps.who.int/iris/bitstream/handle/10665/246200/9789241511124-eng.pdf?sequence=8 (accessed on 10 September 2023).
- Bekker, L.G.; Beyrer, C.; Quinn, T.C. Behavioral and Biomedical Combination Strategies for HIV Prevention. Cold Spring Harb. Perspect. Med. 2012, 2, a007435. [Google Scholar] [CrossRef]
- Sipe, T.A.; Barham, T.L.; Johnson, W.D.; Joseph, H.A.; Tungol-Ashmon, M.L.; O’leary, A. Structural Interventions in HIV Prevention: A Taxonomy and Descriptive Systematic Review. AIDS Behav. 2017, 21, 3366–3430. [Google Scholar] [CrossRef]
- Hodges-Mameletzis, I.; Dalal, S.; Msimanga-Radebe, B.; Rodolph, M.; Baggaley, R. Going global: The adoption of the World Health Organization’s enabling recommendation on oral pre-exposure prophylaxis for HIV. Sex Health 2018, 15, 489–500. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global PrEP Network Highlight-March 2021. 2021. Available online: https://www.who.int/news-room/feature-stories/detail/global-data-shows-increasing-prep-use-and-widespread-adoption-of-who-prep-recommendations (accessed on 26 December 2024).
- Pretorius, C.; Schnure, M.; Dent, J.; Glaubius, R.; Mahiane, G.; Hamilton, M.; Reidy, M.; Matse, S.; Njeuhmeli, E.; Castor, D.; et al. Modelling impact and cost-effectiveness of oral pre-exposure prophylaxis in 13 low-resource countries. J. Int. AIDS Soc. 2020, 23, e25451. [Google Scholar] [CrossRef] [PubMed]
- Leelahavarong, P. Budget impact analysis. J. Med. Assoc. Thail. 2014, 97 (Suppl. S5), S65–S71. [Google Scholar]
- Garattini, L.; van de Vooren, K. Budget impact analysis in economic evaluation: A proposal for a clearer definition. Eur. J. Health Econ. 2011, 12, 499–502. [Google Scholar] [CrossRef]
- Carvalho, N.; Jit, M.; Cox, S.; Yoong, J.; Hutubessy, R.C.W. Capturing Budget Impact Considerations Within Economic Evaluations: A Systematic Review of Economic Evaluations of Rotavirus Vaccine in Low- and Middle-Income Countries and a Proposed Assessment Framework. Pharmacoeconomics 2018, 36, 79–90. [Google Scholar] [CrossRef]
- van de Vijver, D.A.M.C.; Richter, A.-K.; Boucher, C.A.B.; Gunsenheimer-Bartmeyer, B.; Kollan, C.; E Nichols, B.; Spinner, C.D.; Wasem, J.; Schewe, K.; Neumann, A. Cost-effectiveness and budget effect of pre-exposure prophylaxis for HIV-1 prevention in Germany from 2018 to 2058. Eurosurveillance 2019, 24, 1800398. [Google Scholar] [PubMed]
- Vermeersch, S.; Callens, S.; De Wit, S.; Goffard, J.-C.; Laga, M.; Van Beckhoven, D.; Annemans, L. Health and budget impact of combined HIV prevention—First results of the BELHIVPREV model. Acta Clin. Belg. 2018, 73, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Cambiano, V.; Miners, A.; Dunn, D.; McCormack, S.; Ong, K.J.; Gill, O.N.; Nardone, A.; Desai, M.; Field, N.; Hart, G.; et al. Cost-effectiveness of pre-exposure prophylaxis for HIV prevention in men who have sex with men in the UK: A modelling study and health economic evaluation. Lancet Infect. Dis. 2018, 18, 85–94. [Google Scholar] [CrossRef]
- Ong, K.J.; Desai, S.; Field, N.; Desai, M.; Nardone, A.; van Hoek, A.J.; Gill, O.N. Economic evaluation of HIV pre-exposure prophylaxis among men-who-have-sex-with-men in England in 2016. Eurosurveillance 2017, 22, 15–24. [Google Scholar] [CrossRef]
- Alvis-Zakzuk, N.; Tolosa-Pérez, N.; Buitrago, G.; Moreno, C.A.; Sotomayor, M.C.; De La Hoz, F. Budget impact of pre-exposure prophylaxis (PrEP) strategy for the prevention of HIV in Colombia, 2019–2020. Value Health 2020, 23 (Suppl. S1), S174. [Google Scholar]
- Kazemian, P.; Costantini, S.; Kumarasamy, N.; Paltiel, A.D.; Mayer, K.H.; Chandhiok, N.; Walensky, R.P.; A Freedberg, K. The Cost-effectiveness of Human Immunodeficiency Virus (HIV) Preexposure Prophylaxis and HIV Testing Strategies in High-risk Groups in India. Clin. Infect. Dis. 2020, 70, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Urueña, J.; Campbell, C.; Diez, E.; Ortún, V.; Casabona, J. Can we afford to offer pre-exposure prophylaxis to MSM in Catalonia? Cost-effectiveness analysis and budget impact assessment. AIDS Care 2017, 30, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Walensky, R.P.; Park, J.-E.; Wood, R.; Freedberg, K.A.; Scott, C.A.; Bekker, L.-G.; Losina, E.; Mayer, K.H.; Seage, G.R.; Paltiel, A.D. The Cost-effectiveness of Pre-Exposure Prophylaxis for HIV Infection in South African Women. Clin. Infect. Dis. 2012, 54, 1504–1513. [Google Scholar] [CrossRef]
- Juusola, J.; Brandeau, M.; Owens, D.; Bendavid, E. The Cost-Effectiveness of Preexposure Prophylaxis for HIV Prevention in Men Who Have Sex with Men in the United States. Ann. Intern. Med. 2012, 156, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.; Gray, R.T.; Wilson, D.P. A cost-effectiveness analysis of HIV preexposure prophylaxis for men who have sex with men in Australia. Clin. Infect. Dis. 2014, 58, 1027–1034. [Google Scholar] [CrossRef]
- Moyo, E.; Barham, L.; Mhango, M.; Musuka, G.; Dzinamarira, T. Estimating the budget impact of adopting tenofovir/emtricitabine for pre-exposure prophylaxis of HIV in the public health sector in Namibia (2021–2023). J. Infect. Public Health 2022, 15, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Sittimart, M.; Rattanavipapong, W.; Mirelman, A.J.; Hung, T.M.; Dabak, S.; Downey, L.E.; Jit, M.; Teerawattananon, Y.; Turner, H.C. An overview of the perspectives used in health economic evaluations. Cost Eff. Resour. Alloc. 2024, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Gomez, G.B.; Borquez, A.; Case, K.K.; Wheelock, A.; Vassall, A.; Hankins, C. The Cost and Impact of Scaling Up Pre-exposure Prophylaxis for HIV Prevention: A Systematic Review of Cost-Effectiveness Modelling Studies. PLoS Med. 2013, 10, e1001401. [Google Scholar] [CrossRef]
- Sullivan, S.D.; Mauskopf, J.A.; Augustovski, F.; Caro, J.J.; Lee, K.M.; Minchin, M.; Orlewska, E.; Penna, P.; Barrios, J.-M.R.; Shau, W.-Y. Budget impact analysis-principles of good practice: Report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health 2014, 17, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Cunnama, L.; Sinanovic, E.; Ramma, L.; Foster, N.; Berrie, L.; Stevens, W.; Molapo, S.; Marokane, P.; McCarthy, K.; Churchyard, G.; et al. Using Top-down and Bottom-up Costing Approaches in LMICs: The Case for Using Both to Assess the Incremental Costs of New Technologies at Scale. Health Econ. 2016, 25 (Suppl. S1), 53–66. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.; Davies, C.; Davies, G.; Rice, C.; Hollingworth, W. The use of micro-costing in economic analyses of surgical interventions: A systematic review. Health Econ Rev. 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Barnabas, R.V.; Abuna, F.; Lagat, H.; Kinuthia, J.; Pintye, J.; Bochner, A.F.; Forsythe, S.; Gomez, G.B.; Baeten, J.M.; et al. The role of costing in the introduction and scale-up of HIV pre-exposure prophylaxis: Evidence from integrating PrEP into routine maternal and child health and family planning clinics in western Kenya. J. Int. AIDS Soc. 2019, 22, e25296. [Google Scholar] [CrossRef] [PubMed]
- Attema, A.E.; Brouwer, W.B.F.; Claxton, K. Discounting in Economic Evaluations. Pharmacoeconomics 2018, 36, 745–758. [Google Scholar] [CrossRef]
- Kelley, C.F.; Acevedo-Quiñones, M.; Agwu, A.L.; Avihingsanon, A.; Benson, P.; Blumenthal, J.; Brinson, C.; Brites, C.; Cahn, P.; Cantos, V.D.; et al. Twice-Yearly Lenacapavir for HIV Prevention in Men and Gender-Diverse Persons. N. Engl. J. Med. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yamey, G.; Machingaidze, S. Lenacapavir: A giant step forward in HIV prevention-but a missed opportunity for achieving equity and access. BMJ 2024, 387, q2254. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).