Abstract
Background: The sexual transmissibility of enteric pathogens, including Salmonella spp., has been described in men who have sex with men (MSM). However, the factors seen in MSM with Salmonella spp. are poorly understood. Method: We aimed to systematically review the literature to explore any factors seen in MSM with Salmonella spp. (MSM). We searched six databases—Medline, PubMed, CINAHL, Embase, Emcare, and Global Health—in April 2024 for manuscripts which contained primary peer-reviewed data in English and the measurement of any risk factors observed in MSM with Salmonella spp. This review was registered on PROSPERO (CRD42023472864). Results: Eleven manuscripts were included in the final review and highlighted demographic (living with HIV), behavioural (oral–anal sex, receptive and penetrative anal sex, hand licking to stimulate their partner, group sex, non-condom use), and biological (co-infection with CMV, Mycobacterium avium complex, Strongyloides stercoralis, Blastocystis hominis, Klebsiella spp. Herpes simplex virus, Cytomegalovirus, Cryptosporidium, Histoplasmosis, Shigella spp.; previous infection with Treponema pallidum, Neisseria gonorhoeae, Chlamydia trachomatis and hepatitis B; and antimicrobial treatment failure) factors seen in MSM with Salmonella spp. Conclusion: Despite a limited number of manuscripts and individuals, this review highlighted some potential demographic, behavioural, and biological factors implicated in the transmission of Salmonella spp. in MSM. These data will provide insights for future guidelines, public health control strategies, and research.
1. Introduction
Salmonella spp. are Gram-negative, rod-shaped bacteria; they are highly diverse zoonotic bacteria which occur in the intestinal tracts of animals, including birds and reptiles, and there are more than 2600 serovars described [1]. There are two broad species of Salmonella: Salmonella enterica and Salmonella bongori [2]. S. bongori are generally restricted to cold-blooded animals and can rarely affect humans. S. Enterica can be divided into typhoidal salmonella (e.g. Salmonella typhi, Salmonella paratyphi) and non-typhoidal salmonella (e.g. Salmonella typhimurium) which are exclusively human pathogens and can cause severe invasive disease; thus, we will refer to these human salmonella pathogens as Salmonella spp. [2]. According to the global burden of disease study of 2017, human Salmonella affected 14.3 million people and caused 135,000 deaths annually [3]. In contrast, there are thousands of non-typhoidal salmonella subtypes affecting human and non-human hosts; these generally cause diarrhoeal illness [2]. Salmonella can persist in the natural environment, including fresh and salty water, soil, dust, and on or inside plant material [4]. Transmission of Salmonella spp. is generally due to poor sanitation regulations and limited access to clean, drinkable water [1]. In high-income settings, transmission is increasingly being driven by the commercial production of meat, eggs, and processed food, including chocolate, jalapeno peppers, and peanuts [5]. In humans, Salmonella spp. infection ranges from asymptomatic disease to severe invasive systemic disease, particularly in the extremes of age and in immunosuppressed hosts. Symptomatic salmonellosis can present with abdominal pain, diarrhoea, fever, and rash [1]. The antimicrobial resistance to first- and second-line antimicrobial regimens for Salmonella spp. is a global public health threat and includes the transmission of multi-drug and extensively drug-resistant isolates in the UK [6].
The sexual transmissibility of enteric pathogens has been described in sexual networks of men who have sex with men (MSM) since the late 1960s [7]. These include bacterial infections (Shigella spp. Campylobacter spp., diarrhoeagenic E. coli, and Salmonella spp.), hepatitis A, and parasites (Giardia duodenalis, Entamoeba histolytica, and Cryptosporidium) [8]. Sexual behaviour in MSM, particularly oro-anal sex, can risk faecal contamination and subsequent transmission of faeco-oral pathogens [9]. Selma Dritz described an outbreak of Salmonella spp. causing typhoid fever in men who have sex with men in the San Francisco area in 1977, before the discovery of HIV [10]. There have been sporadic and persistent outbreaks of other enteric bacteria in MSM, including extensively drug-resistant Shigella spp. in high-income settings [11]. Unlike Shigella spp., little is known about the transmission of Salmonella spp. in MSM [9]. Understanding the factors associated with Salmonella spp. in MSM may provide important insights for the design of future targeted interventions for Salmonella control, clinical guidelines, and research. This systematic review aimed to explore the published literature for the factors seen in MSM with Salmonella spp.
2. Materials and Methods
We followed PRISMA guidelines to perform a systematic review of the published literature in April 2024 [12]. We searched 6 bibliographical databases (PubMed, MEDLINE, EMBASE, CINAHL, EMCARE, and Global Health). We used the following search terms ((“Salmonella” OR “typhoid fever”) AND (“MSM” OR “Men who have sex with men” OR “gay” OR “homosexual” OR “bisexual”)). We only included manuscripts containing primary data exploring factors seen in MSM with Salmonella spp., written in English. For manuscripts that contained mixed populations (MSM and other non-MSM groups), or mixed pathogens, we only extracted and analysed the MSM data. There was no restriction on the year the studies were completed. Grey literature, non-peer-reviewed manuscripts, and conference abstracts were excluded.
Study Selection and Data Synthesis
We used a sequential step process to select the manuscripts in this review. In the initial step, the primary researcher (VS) reviewed the citations and abstracts and removed any duplicates. The primary author and a second researcher (NW) independently reviewed full-text manuscripts for eligibility. Any discrepancy was discussed with the research team to make a final decision. The quality and the bias risk were assessed independently by VS and NW using the Joanna Briggs Institute critical appraisal tools, and each manuscript was rated as having either a low, medium, or high risk of bias [13]. Any discrepancy was discussed with the research team to make a final decision. The final manuscript reference lists were hand-searched for any manuscripts which were not included in the initial search for eligibility review. We synthesised the narrative data into a table (Table 1). The review was registered on PROSPERO (CRD42023472864).

Table 1.
Factors associated with Salmonella spp. in men who have sex with men.
3. Results
The flowchart in Figure 1 demonstrates the screening process for the final manuscripts included in this review. Our initial search found 291 citations and 128 duplicates that were removed. There were 163 abstracts screened, of which 126 were excluded. Thirty-nine full-text manuscripts were reviewed by the two researchers, and thirty-two were excluded. Eight additional manuscripts were found from handsearching, and four of these were eligible for inclusion. Eleven manuscripts published between 1977 and 2024 were included in the final review [7,14,15,16,17,18,19,20,21,22,23]. The manuscripts consisted of two case reports, three cases series, and six cross-sectional studies from the USA (n = 7), the UK (n = 3), and Australia (n = 1), with a total of 77 MSM with Salmonella spp. Risk of bias was assessed as medium in six manuscripts and high in four manuscripts (Appendix A).
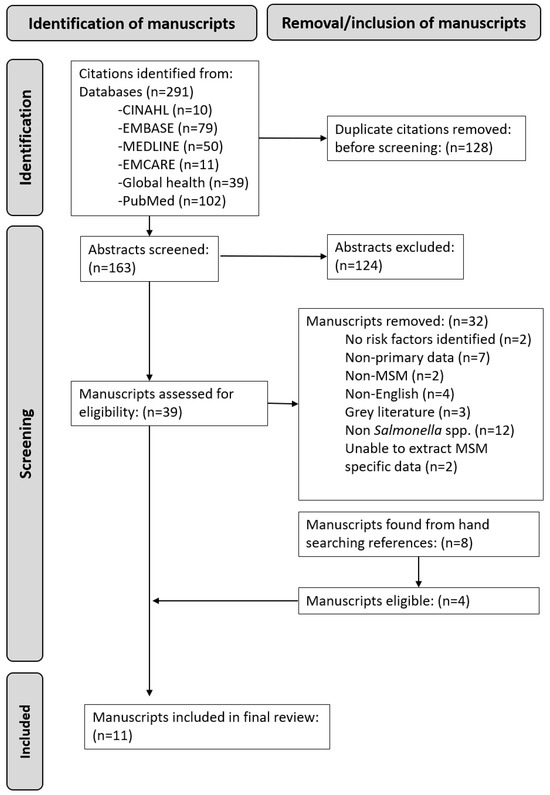
Figure 1.
Prisma flowchart of manuscript reviewing process.
- Demographic factors
We found that living with HIV was a factor observed in MSM with Salmonella spp. in MSM [14,15,16,17,18,19,20,21] (Table 2).

Table 2.
Risk factors seen in MSM with Salmonella spp.
- Behavioural factors
Oral–anal sex, receptive and penetrative anal sex, hand licking to stimulate their partner, group sex, and non-condom use was seen in MSM with Salmonella spp. [21,22,23]. Attending sex-on-premises venues (Turkish baths) was seen in MSM with Salmonella spp. [16].
- Biological factors
Co-infection with Mycobacterium avium complex, Strongyloides stercoralis, Blastocystis hominis, Klebsiella spp. Herpes simplex virus, Cytomegalovirus, Cryptosporidium, Histoplasmosis, and Shigella spp. was seen in MSM with Salmonella spp. [14,18,19,20]. Previous infection with Treponema pallidum, Neisseria gonorrhoeae, Chlamydia trachomatis, and hepatitis B was seen in MSM with Salmonella spp. [16,23]. Antimicrobial treatment failure was observed in MSM with Salmonella spp. [7,15,17,20].
4. Discussion
This review highlighted an association between MSM living with HIV and being diagnosed with gastrointestinal Salmonella spp. In the pre-antiretroviral era of HIV, it was not uncommon to see MSM living with HIV affected by enteric infections, most commonly Shigella spp., Entamoeba histolytica, and Campylobacter spp. [8,19]. It is unclear whether the risk of Salmonella spp. in MSM living with HIV is biological, as there is little evidence to support this [16,18,20,21]. Furthermore, the mode of transmission of Salmonella spp. in individuals living with HIV is not always clear. Some MSM living with HIV or advanced HIV, who lack access to antiretrovirals or experience poor adherence to antiretroviral treatment, also experience other poor determinants of health that may impact their access to interventions for STI prevention and increased rates of STIs and Salmonella spp. transmission [16,18,20,24]. In the era of HIV pre-exposure prophylaxis, it is likely that the discrete sexual networks of MSM living with HIV and HIV-negative MSM will merge, and the effect of living with HIV be less important in the transmission of enteric pathogens [25].
This review has highlighted some specific sexual behaviours associated with Salmonella spp. in MSM [16,21,22,23]. These sexual behaviours, which increase the risk of faecal contamination during sexual activity, are associated with the transmission of other enteric infections in MSM, including Shigella spp. [9]. An interesting association was hand licking after sexually stimulating a partner, highlighting how hygiene practices, particularly after faecal contamination during sex, may impact the transmission of Salmonella spp. and other sexually transmissible enteric infections [21]. An Australian study showed that poor hygiene practice in a sex-on-premises venue in Sydney was associated with the transmission of Shigella spp. in MSM [26]. Our review describes a case of an individual living with advanced HIV who acquired Salmonella spp. following a sexual encounter in a bathhouse [16].
This review has highlighted co-infection with other pathogens, including STIs and enteric pathogens, observed in MSM with Salmonella spp. (Mycobacterium avium complex, Strongyloides stercoralis, Blastocystis hominis, Klebsiella spp. Herpes simplex virus, Cytomegalovirus, Cryptosporidium, Histoplasmosis, Shigella spp. Treponema pallidum, Neisseria gonorrhoeae, Chlamydia trachomatis, and hepatitis B).
The incidence of STIs, including antimicrobial-resistant enteric infections, has increased over the past 3 decades [11,27,28,29]. Antimicrobial-resistant Salmonella spp. leads to limited treatment options for individuals affected and the further transmission of resistant organisms [30]. Some individuals with antimicrobial-resistant Salmonella spp. may end up becoming asymptomatic carriers, therefore risking transmission of resistant Salmonella spp. to other MSM in their sexual networks [30]. This is well described in other studies of Shigella spp. in MSM in the UK and Australia, where circulating strains have become extensively antimicrobial-resistant [11,31]. This review has shown some antimicrobial treatment failure, suggesting that some Salmonella spp. isolates in MSM have antimicrobial drug resistance [7,17,20]. There have also been reports of multi-drug antimicrobial-resistant strains of Salmonella spp. identified in Pakistan [32].
Sexual behaviours, such as attending sex-on-premises venues (such as bath houses), having multiple sexual partners, and condomless sex, also have a part to play in the transmission of antimicrobial-resistant strains of enteric pathogens [9,26]. Climate change, environmental changes, the increasing use of antimicrobials, and access to antimicrobials are likely to result in the transmission of antimicrobial-resistant STIs and sexually transmissible enteric pathogens, including Salmonella spp. [33,34]. Enteric pathogens remain neglected diseases and globally affect disadvantaged people living in poverty with poor access to sanitation and healthcare [35].
The demographic, behavioural, and biological factors seen in MSM with Salmonella spp. in this review are broadly similar to what has been observed in MSM with other enteric bacteria (Shigella spp. Camplylobacter spp.) and parasites (Entamoeba histolytica, Giardia duodenalis), suggesting a similar profile of MSM or similar sexual networks [9,25,35,36]. Understanding these factors can contribute to effective surveillance and design, the development and delivery of infection control measures, and other interventions.
This review suggests that there is the potential for limited sexual transmission of Salmonella spp. in sexual networks of MSM and attempts to raise awareness of the importance of testing for Salmonella in sporadic cases or outbreaks of diarrhoeal illness in networks of MSM. Lack of appropriate testing, including antimicrobial sensitivity testing, could lead to significant uncontrolled outbreaks and morbidity. It is curious that some bacterial pathogens appear to be readily transmitted amongst sexual networks of MSM, such as Shigella spp.; however, others are rarely reported (such as Salmonella spp.), which may be a result of the inoculum dose required for transmission [30,37,38,39].
There are several limitations to this review, including significant publication bias. All of the MSM included in this review were from high-income settings. There is a lack of data on sexually transmissible enteric infections in MSM from the global south and low-/middle-income settings. It is challenging to delineate the mode of transmission of enteric pathogens where surveillance mechanisms are unable to distinguish between food-/waterborne infections and possible sexual transmissibility. There is significant reporting bias, as the data collected from the manuscripts were from MSM who presented to healthcare with symptoms, provided faecal and blood samples, and disclosed sexual behaviour. MSM with mild symptoms of Salmonella spp. may not have presented to health settings or provided a specimen of stool for analysis and therefore would not have been included in this review, and some MSM may not have participated in studies due to fear of stigma. The studies in this review did not report on the method used to detect and diagnose Salmonella spp.; we can assume that most of the older studies used traditional culture, which is limited by sensitivity. Stigma could also have affected data collected on sexual orientation and the types of sexual activities individuals engaged in. There was a broad heterogeneity of studies, including quality, sample size, and data collection in the manuscripts included (including case reports and case series, which are low in the hierarchy of evidence), making the interpretation of the findings in this review challenging. Some of the data included were for all enteric pathogens as it was not possible to extract Salmonella spp.-specific data, making the overall interpretation challenging.
5. Conclusions
Salmonella spp. and other enteric pathogens continue to be neglected diseases with a high risk of antimicrobial resistance, and they affect some of the most vulnerable people globally [1,35]. This review provides some evidence to suggest that Salmonella spp. can be sexually transmissible, albeit in small outbreaks. The magnitude of this relationship is inconclusive. Our understanding of the dynamics of the sexual transmission of Salmonella spp. remains unclear as there are some large datasets exploring the prevalence of enteric pathogens in MSM where no Salmonella spp. has been found [24,35,40]. Understanding the intersectionality of demographic, behavioural, and antimicrobial factors contributing to the transmission of Salmonella spp. and other sexually transmissible enteric pathogens in MSM can underpin the design and delivery of future public health control measures, including increasing awareness and the opportunities for research.
Author Contributions
Conceptualization, D.R. and C.L.; methodology, D.R., C.L. and V.S.; formal analysis, V.S. and D.R.; investigation, V.S., N.W. and V.D.; data curation, V.S., N.W. and V.D.; writing—original draft preparation, D.R.; writing—review and editing, D.R. and C.L.; supervision, D.R. and C.L.; project administration, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The protocol was registered on PROSPERO ID: CRD42023472864.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data in this systematic review are available in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Risk of Bias Assessment
| Study | Type | Reasons for Bias | Risk of Bias |
| Dritz et al., 1977 [7] | Case series | The demographics of the participants were not clear enough; age and ethnicity were the only identifiable indicators of the patients. There were no inclusion or exclusion criteria; so, we do not know if all available participants were collected here. There was no clear reporting of follow-up, such as what other investigations were completed; only the stool and urine samples of 2 were stated. | High |
| Bottone EJ et al., 1984 [14] | Case series | Some details of the patient demographics were described, such as age, ethnicity, and sexuality. However, there was minimal information regarding the patients’ presentations, the tests they underwent to obtain the Salmonella diagnosis, and the treatment they received. Minimal follow up details were displayed other than that one patient had died and the other underwent further follow-up for his advanced HIV. | High |
| Whimbey E et al., 1986 [15] | Cross-sectional | The site and subjects of the study were clearly cited; however, how they individually diagnosed each type of enteric bacteria and fungi was not described. Confounding factors were not discussed or identified; therefore, the reliability of the results is questionable. | High |
| Jarrett et al., 1986 [16] | Case report | The adverse events or any unanticipated events were not identified or described. | Medium |
| Smith PD et al., 1988 [17] | Case series | There were no key features of the patients included in the study; the only key feature discussed was the patients’ advanced HIV status. However, it did have solid follow-up, diagnostic, treatments, and monitoring criteria. The reduction in symptoms was deemed a resolution of conditions, which is not accurate as patients could be asymptomatic carriers or treatment may have failed. | Medium |
| Antony et al., 1988 [18] | Cross-sectional | Confounding factors were not stated nor explored; the sample of the study was mixed between heterosexual men and MSM, with the causes of advanced HIV being stated and differentiated. | Medium |
| Crowley S et al., 1992 [19] | Case report | The patients’ demographics, presentation, interventions, and follow-up were all discussed in detail. But due to it being a case report study, it is subject to high bias. | Medium |
| Nelson et al., 1992 [20] | Cross-sectional | Confounding factors were not identified or explored; hence, we are unable to conclude that the risk factor discussed caused the salmonella transmission. | Medium |
| Reller et al., 2003 [21] | Case series | It was unclear whether there was consecutive inclusion of the participants, due to the fact that there was very little information regarding all but one participant. The follow-ups regarding the participants were very minimal and unclear as to what was done, what parameters were used, etc. | Medium |
| Williamson DA. et al., 2019 [22] | Cross-sectional | This study used a large sample of asymptomatic MSM, who underwent questioning regarding all types of sexual practices. They all underwent the same testing for HIV status as well enteric bacteria. However, it is unclear whether confounding factors were identified and whether there was a strategy in place to deal with them. There were no further follow-ups since all the participants were asymptomatic | Medium |
| Miller et al., 2024 [23] | Cross-sectional | Confounding factors were not accounted for during the study. The sample size for salmonella was small. The degree of factors such as the length of living with HIV, CD4 count, and other factors was not measured. | High |
References
- Basnyat, B.; Qamar, F.N.; Rupali, P.; Ahmed, T.; Parry, C.M. Enteric fever. BMJ 2021, 372, n437. [Google Scholar] [CrossRef] [PubMed]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef] [PubMed]
- Stanaway, J.D.; Reiner, R.C.; Blacker, B.F.; Goldberg, E.M.; Khalil, I.A.; Troeger, C.E.; Andrews, J.R.; Bhutta, Z.A.; Crump, J.A.; Im, J.; et al. The global burden of typhoid and paratyphoid fevers: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.W.; Bell, R.; Zhang, G.; Timme, R.; Zheng, J.; Hammack, T.S.; Allard, M.W. Salmonella Genomics in Public Health and Food Safety. EcoSal Plus 2021, 9, eESP00082020. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Chattaway, M.A.; Gentle, A.; Nair, S.; Tingley, L.; Day, M.; Mohamed, I.; Jenkins, C.; Godbole, G. Phylogenomics and antimicrobial resistance of Salmonella Typhi and Paratyphi A, B and C in England, 2016–2019. Microb. Genom. 2021, 7, 633. [Google Scholar] [CrossRef]
- Dritz, S.K.; Ainsworth, T.E.; Back, A.; Boucher, L.A.; Garrard, W.F.; Palmer, R.D.; River, E. Patterns of sexually transmitted enteric diseases in a city. Lancet 1977, 2, 3–4. [Google Scholar] [CrossRef]
- McNeil, C.J.; Kirkcaldy, R.D.; Workowski, K. Enteric Infections in Men Who Have Sex With Men. Clin. Infect. Dis. 2022, 74, S169–S178. [Google Scholar] [CrossRef]
- Siddiq, M.; O’Flanagan, H.; Richardson, D.; Llewellyn, C.D. Factors associated with sexually transmitted shigella in men who have sex with men: A systematic review. Sex. Transm. Infect. 2022, 99, 58–63. [Google Scholar] [CrossRef]
- Dritz, S.K.; Back, A.F. Letter: Shigella enteritis venereally transmitted. N. Engl. J. Med. 1974, 291, 1194. [Google Scholar]
- O’Flanagan, H.; Siddiq, M.; Llewellyn, C.; Richardson, D. Antimicrobial resistance in sexually transmitted Shigella in men who have sex with men: A systematic review. Int. J. STD AIDS 2023, 34, 374–384. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Sears, K.; Sfetcu, R.; Currie, M.; Lisy, K.; Tufanaru, C.; Qureshi, R.; Mattis, P.; Mu, P. Conducting systematic reviews of association (etiology): The Joanna Briggs Institute’s approach. Int. J. Evid. Based Health 2015, 13, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J.; Wormser, G.P.; Duncanson, F.P. Nontyphoidal Salmonella bacteremia as an early infection in acquired immunodeficiency syndrome. Diagn. Microbiol. Infect. Dis. 1984, 2, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Whimbey, E.; Gold, J.W.; Polsky, B.; Dryjanski, J.; Hawkins, C.; Blevins, A.; Brannon, P.; Kiehn, T.E.; Brown, A.E.; Armstrong, D. Bacteremia and fungemia in patients with the acquired immunodeficiency syndrome. Ann. Intern. Med. 1986, 104, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, D.R.; Zeegen, R. Recurrent typhoid in an HTLV-III antibody positive man. Gut 1986, 27, 587–588. [Google Scholar] [CrossRef][Green Version]
- Smith, P.D.; Lane, H.C.; Gill, V.J.; Manischewitz, J.F.; Quinnan, G.V.; Fauci, A.S.; Masur, H. Intestinal infections in patients with the acquired immunodeficiency syndrome (AIDS). Etiology and response to therapy. Ann. Intern. Med. 1988, 108, 328–333. [Google Scholar] [CrossRef]
- Antony, M.A.; Brandt, L.J.; Klein, R.S.; Bernstein, L.H. Infectious diarrhea in patients with AIDS. Dig. Dis. Sci. 1988, 33, 1141–1146. [Google Scholar] [CrossRef]
- Crowley, S.; Coker, R.J.; Murphy, S.M. Concurrent salmonellosis and histoplasmosis in AIDS: An unusual co-existence in Britain. Sex. Transm. Infect. 1992, 68, 258–259. [Google Scholar] [CrossRef][Green Version]
- Nelson, M.R.; Shanson, D.C.; Hawkins, D.A.; Gazzard, B.G. Salmonella, Campylobacter and Shigella in HIV-seropositive patients. Aids 1992, 6, 1495–1498. [Google Scholar] [CrossRef]
- Reller, M.E.; Olsen, S.J.; Kressel, A.B.; Moon, T.D.; Kubota, K.A.; Adcock, M.P.; Nowicki, S.F.; Mintz, E.D. Sexual transmission of typhoid fever: A multistate outbreak among men who have sex with men. Clin. Infect. Dis. 2003, 37, 141–144. [Google Scholar] [CrossRef]
- Williamson, D.A.; Chow, E.P.F.; Lee, D.; Maddaford, K.; Sait, M.; Easton, M.; Ingle, D.; Wigan, R.; De Petra, V.; Howden, B.P.; et al. Risk Factors for Asymptomatic Enteric Pathogen Detection among Men Who Have Sex with Men. Open Forum Infect. Dis. 2019, 6, ofz326. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.I.; Banning, S.; Lieberman, J.A. Risk factors and provider awareness of sexually transmitted enteric pathogens among men who have sex with men. Microbiol. Spectr. 2024, 12, e0357723. [Google Scholar] [CrossRef]
- Mitchell, H.; Hughes, G. Recent epidemiology of sexually transmissible enteric infections in men who have sex with men. Curr. Opin. Infect. Dis. 2018, 31, 50–56. [Google Scholar] [CrossRef]
- Wahab, N.; Dubey, V.; Sivachandran, V.; Llewellyn, C.; Richardson, D. Campylobacter spp. in men who have sex with men: A systematic review. Int. J. STD AIDS 2024, 09564624241280739. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Delpech, V.; Pontivivo, G.; Karagiannis, T.; Marriott, D.; Harkness, J.; McAnulty, J.M. Shigellosis linked to sex venues, Australia. Emerg. Infect. Dis. 2002, 8, 862–864. [Google Scholar] [CrossRef]
- Richardson, D.; Pakianathan, M.; Ewens, M.; Mitchell, H.; Mohammed, H.; Evans, A. The new 2023 BASHH sexually transmitted enteric infections guideline. Sex. Transm. Infect. 2023, 99, 363–364. [Google Scholar] [CrossRef]
- Cresswell, F.V.; Ross, S.; Booth, T.; Pinto-Sander, N.; Alexander, E.; Bradley, J.; Paul, J.; Richardson, D. Shigella flexneri: A Cause of Significant Morbidity and Associated with Sexually Transmitted Infections in Men Who Have Sex with Men. Sex. Transm. Dis. 2015, 42, 344. [Google Scholar] [CrossRef]
- Richardson, D.; Devlin, J.; Fitzpatrick, C.; Pinto-Sander, N. Sexually transmitted Shigella flexneri and Shigella sonnei in men who have sex with men. Sex. Transm. Infect. 2021, 97, 244. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Savary-Trathen, A.; Fitzpatrick, C.; Williams, D. Estimated prevalence and associations of sexually transmissible bacterial enteric pathogens in asymptomatic men who have sex with men: A systematic review and meta-analysis. Sex. Transm. Infect. 2024. [CrossRef] [PubMed]
- Ingle, D.J.; Andersson, P.; Valcanis, M.; Barnden, J.; da Silva, A.G.; Horan, K.A.; Seemann, T.; Easton, M.; Williamson, D.A.; Sherry, N.L.; et al. Prolonged Outbreak of Multidrug-Resistant Shigella sonnei Harboring bla(CTX-M-27) in Victoria, Australia. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef]
- Hussain, A.; Satti, L.; Hanif, F.; Zehra, N.M.; Nadeem, S.; Bangash, T.M.; Peter, A. Typhoidal Salmonella strains in Pakistan: An impending threat of extensively drug-resistant Salmonella Typhi. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Cornelisse, V.J.; Chow, E.P.; Chen, M.Y.; Bradshaw, C.S.; Fairley, C.K. Summer heat: A cross-sectional analysis of seasonal differences in sexual behaviour and sexually transmissible diseases in Melbourne, Australia. Sex. Transm. Infect. 2016, 92, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Cherrie, M.P.C.; Nichols, G.; Iacono, G.L.; Sarran, C.; Hajat, S.; Fleming, L.E. Pathogen seasonality and links with weather in England and Wales: A big data time series analysis. BMC Public Health 2018, 18, 1067. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Richardson, D.; Fitzpatrick, C. Factors associated with Entamoeba histolytica proctocolitis in men who have sex with men: A systematic review. Frontline Gastroenterol. 2024, 15, 321–327. [Google Scholar] [CrossRef]
- Dubey, V.; Sivachandran, V.; Wahab, N.; Llewellyn, C.; Richardson, D. Giardia duodenalis in men who have sex with men: A systematic review. Frontline Gastroenterol. 2024, 15, 417–423. [Google Scholar] [CrossRef]
- DuPont, H.L.; Levine, M.M.; Hornick, R.B.; Formal, S.B. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 1989, 159, 1126–1128. [Google Scholar] [CrossRef]
- Charles, H.; Prochazka, M.; Thorley, K.; Crewdson, A.; Greig, D.R.; Jenkins, C.; Painset, A.; Fifer, H.; Browning, L.; Cabrey, P.; et al. Outbreak of sexually transmitted, extensively drug-resistant Shigella sonnei in the UK, 2021–2022: A descriptive epidemiological study. Lancet Infect. Dis. 2022, 22, 1503–1510. [Google Scholar] [CrossRef]
- Blaser, M.J.; Newman, L.S. A review of human salmonellosis: I. Infective dose. Rev. Infect. Dis. 1982, 4, 1096–1106. [Google Scholar] [CrossRef]
- Braam, J.F.; Bruisten, S.M.; Hoogeland, M.; de Vries, H.J.C.; van der Loeff, M.F.S.; van Dam, A.P. Shigella is common in symptomatic and asymptomatic men who have sex with men visiting a sexual health clinic in Amsterdam. Sex. Transm. Infect. 2022, 98, 564–569. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).