Abstract
Monkeypox, once a rare zoonotic disease, has been endemic to some African countries since its original identification among humans in 1970. Since then, cases in non-endemic regions have been linked to returning travelers or those who had contact with transported animals. The causative agent, Monkeypox virus, belongs to Orthopoxviruses, the same family as Variola—the causative organism for smallpox. Although most monkeypox outbreaks until recently were linked to zoonotic transmission, secondary human–human transmission in smallpox-unvaccinated individuals was observed in a small proportion of overall cases. Smallpox was declared to be eradicated in 1980, and since its eradication, Monkeypox virus has been the most significant poxvirus to cause human disease. The 2022 monkeypox outbreak marks a significant paradigm shift in the human and poxvirus association, with new modes of transmission and concerns of viral evolution and entrenchment as a sexually transmitted disease. Monkeypox clinically resembles smallpox but is far milder. At this time, there are no approved therapies for monkeypox, and antiviral agents effective against smallpox are being utilized. Additionally, preventive strategies being utilized include smallpox vaccinations such as JYNNEOS and ACAM2000. In this narrative review, we discuss the virology, epidemiology, transmission, clinical manifestations, diagnosis, management, and prevention strategies associated with monkeypox.
Keywords:
public health emergency; sexual health; monkeypox; smallpox; JYNNEOS; ACAM2000; tecovirimat; brincidofovir 1. History and Introduction
Monkeypox, once an uncommon largely “zoonotic” illness with a short chain of human to human transmission [1,2], is caused by the Monkeypox virus (MPV) of the Poxviridae family and the Orthopoxvirus genus. MPV was first isolated from cynomolgus monkeys exhibiting smallpox-like lesions. The disease name was coined in 1958 at the Statens Serum Institut research facility in Copenhagen, Denmark, housing monkeys for poliomyelitis vaccine research and production [3]. Subsequently, the earliest animal–human zoonotic transmission was described in 1970 in a 9-month-old male infant in the Democratic republic of Congo (DRC) [4]. In 1970, five more human cases of monkeypox were reported in Liberia and Sierra Leone (four in children aged 4–9 years and a fifth in a 24-year-old adult) [5]. A total of 59 cases in other sporadic outbreaks of monkeypox were reported between the 1970 and 1980s in Central and West African countries, mostly associated with wildlife contact [6,7,8].
Early human monkeypox outbreaks coincided with the last stages of the global smallpox eradication vaccination program. It was observed that vaccination and infection from one member of the Orthopoxvirus conferred cross-protection against another [5]. Since humans were not seen as a reservoir for MPV, the “global commission for eradication of smallpox” was prompted to consider monkeypox a non-threat to smallpox eradication, so it was decided to cease the vaccination program [8]. Epidemiological surveys of infections during this period indicated an increasing concern for rising human–human transmission of MPV in secondary cases of otherwise unvaccinated individuals, causing the commission to promote continuous monkeypox disease surveillance [8]. Subsequently, serological surveys (utilizing the vaccinia hemagglutination-inhibiting (HI) antibody testing) of over 10,000 unvaccinated children in Cote d’lvoire, Sierra Leone, and the DRC indicated antibody positivity rates of 12.5–19.2%. Furthermore, at least 30% of sera-positive individuals did not recollect any clinical manifestations, indicating a subclinical presentation [8]. As the screening and surveys were intensified, a few hundred cases of monkeypox were reported in the DRC between 1981 and 1986, largely in children (86%)—291 in children under the age of 10 and 175 in children under the age of 5—and a significant number of remaining cases in smallpox-unvaccinated adults; the overall fatality rate was reportedly 9.8% [6,8]. In the DRC, during a significant prolonged outbreak between 1996 and 1997, the fatality rate was 3.7%, with similar epidemiology as earlier but increasing human–human transmission [8,9,10]. In all these outbreaks, monkeys, squirrels and other mammalian species were seen as the key participants in the MPV lifecycle and transmission to humans [6,10,11]. Significantly, the genomic sequence analysis of MPV was observed to be mostly unchanged and human–human transmission was considered to be low [10]. In another outbreak in the DRC in 2001, a total of 31 human cases was reported and monkeys were noted as one of the sources of primary infection, while secondary human–human transmitted infection was seen in smallpox-unvaccinated individuals [6]. From 1970 to 2017, MPV led to multiple monkeypox cases in sporadic outbreaks across the Central and West African belts considered endemic regions—including Cameroon, Central African Republic, Congo Brazzaville, Côte d’Ivoire, the DRC, Gabon, Liberia, Nigeria, Sierra Leone, and South Sudan [12].
Interestingly, pet prairie dogs, transported with other infected small exotic mammals from Ghana, were associated with the “first non-endemic outbreak” of human monkeypox occurring in the United States (US), with 47 confirmed and probable cases but no community transmission [13,14]. Subsequently, a few cases of travel-associated monkeypox, outside of the endemic African regions, were encountered in in Israel in September 2018; in the United Kingdom (UK) in September 2018, December 2019, May 2021, and May 2022; in Singapore during May 2019; and in the US in July and November 2021; all cases were associated with limited secondary spread [15,16,17,18]. These cases, in travelers returning from Nigeria, coincided with the Nigerian outbreak of human monkeypox in 2017–2018, infecting 122 individuals with a fatality rate of 6% [12].
In May 2022, beginning with a cluster of cases detected in the UK and subsequently through the European Union, several countries in historically non-endemic regions reported multiple cases of monkeypox associated with significant human–human transmission, now spread across 92 non-endemic and 7 historically endemic countries [19]. Monkeypox has recently been declared a “public health emergency of international concern” by the World Health Organization (WHO), a critical designation concurrently shared with COVID-19 and poliomyelitis. Globally, significant concerns are being raised due to the presentation of newer modes of transmission, potential entrenchment as a sexually transmitted disease, and the evolution of the virus, among others [20].
With an aim to decipher current circumstances, understanding the virology of MPV has become the key first step towards understanding monkeypox.
2. Virology and Pathogenesis
MPV, a double-stranded DNA (dsDNA) virus, is similar in structure to other Orthopoxviruses, i.e., Variola, Vaccinia, and Cowpox virus. MPV, like other poxviruses, has a brick-like, oval structure measuring 200–250 nm. The viruses are characterized by a dumbbell-shaped nucleus housing the linear double-stranded deoxyribonucleic acid (dsDNA) of ~197 kb, surrounded by lateral bodies [21]. The virions are enclosed by a lipoprotein outer membrane with surface tubules. Palindromic hairpins covalently combine the ends of the DNA strands, while inverted terminal repeats, which hold the origins of replication for DNA viruses, comprise a hairpin loop, tandem repeats, and some open reading frames (ORFs). DNA synthesis is initiated at one end of the inverted terminal repeats and continues to the other end [22]. Similar to other Orthopoxviruses, the MPV genome consists of 190 largely non-overlapping ORFs of ≥60 amino acid residues, as well as structural features [21]. Additionally, the central part of the MPV genome consists of highly conserved genes, seen across Orthopoxviruses. Uniquely to MPV, significant lengths of its right side genome contain duplications of the four left terminal ORFs as a part of terminal inverted repeat. These terminal regions exhibit considerable variations as a result of ORF truncations and deletions. There is an approximate 84.5–84.6% overlap of genome nucleotide sequences between MPV and the Variola virus [21].
The lifecycle of MPV in the host-cell cytoplasm is largely similar to other Orthopoxviruses [22,23,24,25]. The entry of the virus into the host cell is mediated by the fusion of viral proteins (glycoproteins B5 and A34) to host-cell membrane glycosaminoglycans [26]. This triggers the virion to release its contents into the host-cell cytoplasm. In an immediate next phase, as early as 30 min post-infection, viral RNA-polymerase transcribes the early expression of viral proteins and causes the uncoating of its entire genome [23,27]. The next intermediate phase, taking place approximately 100 min post-infection, involves the expression of a series of genes orchestrating the recruitment of viral DNA-polymerase for replication. In the subsequent late phase, 2–48 h post infection, structural proteins are produced through the transcription of late genes, the recruitment of the host-cell endoplasmic reticulum and Golgi apparatus, and the assembly of spherical proto-virions in host cytoplasmic viral factories [23,27,28]. Mature virions can be released from the host cell via lysis as a non-enveloped intracellular mature virion (IMV) or bud out as an external enveloped virion (EEV) having acquired a double membrane from the Golgi apparatus. The EEV further sets up a cellular microtubule transport that fuses to the host-cell membrane lipoprotein before being released outside [22,23,27]. Interestingly, these two forms of mature virions are believed to exhibit variable antigenic properties and host-cell attachment sites. These variations modulate the host immune response and infectivity of progeny virions [27]. Additionally, one study showed that the EEV plays a key role in viral dissemination while the IMV plays a predominant role in host–host transmission [29]. These factors of virion replication and egress need to be looked at carefully and may hold insights into the new transmission modes associated with the 2022 global monkeypox outbreak. Recent studies have shown evidence of microevolution within the MPV genome sequence that might be indicative of the changes observed in the transmission chain [30,31].
The infected cell hosts most of the viral DNA replication, transcription, assembly, and release, as well as all housekeeping genes present on the conserved central region of the genome [22]. Significantly, there is an 83.5–93.6% commonality between the Variola and MPV putative virulence and immunomodulatory amino acid sequences. Alongside these significant similarities, Vaccinia virus vaccination can elicit cross-reactive immunity against similar targets on MPV for neutralizing antibody and T-cell activity [32]. Furthermore, previous studies have shown that certain mutations in genes for two interferon (IFN) resistance-encoding intracellular proteins (causing IFN sensitivity) could have been key in making the human–human transmission of MPV less efficient in earlier outbreaks, as these genes were intact in other Orthopoxviruses such as Variola [21]. Other differences in complement-binding proteins compared to Variola and the presence of an L-1β-binding protein in MPV may contribute to a less pronounced clinical disease, while the absence of the latter in Variola has been associated with significant pathogenicity and fever [21]. The phylogenetic analysis of Orthopoxviruses have shown that MPV is slightly distant from the Variola and Vaccinia viruses while the Cowpox virus may be a progenitor [21,33]. These and other discrete differences in the MPV genome, compared with other Orthopoxviruses, require attention and may hold answers regarding its unique virulence, pathogenesis and transmission, particularly as the 2022 monkeypox global outbreak exhibits differences in these aspects from previous outbreaks.
Phylogenetically, MPV has been grouped into two genetic clades based on geography, disease severity, and sequence homology (the Central African/Congo Basin (CB) and West African (WA) clades), and reports suggest that the latter has been associated with a milder form of disease [34,35]. The human–human transmissibility, disease-associated morbidity, mortality, and viraemia are more severe with the CB clade [34]. The most significant difference between the two clades is associated with the DNA sequence diversity at the terminal region for genes that encode the host-response modulation proteins [34,35,36]. Prior epidemiological surveys assessing monkeypox showed that infection with either clade has a similar serological response in smallpox-unvaccinated individuals but is associated with higher mortality rates in the CB-clade-infected patients [36,37].
The origins of the 2022 global monkeypox outbreak remain to be determined, and most cases have lacked an obvious epidemiological link to Africa. Viral genomic analysis has recently been performed to understand possible links between global cases, origins of infection, and transmission dynamics. An MPV sequence comparison with a 2021 US case, associated with travel from Nigeria, showed a high similarity to the 2022 outbreak sequences, suggesting that they belong to the WA clade [31,38].
3. Epidemiology—Current Status
Between 1 January 2022 and 30 August 2022, a total of 49,974 cases of monkeypox were reported among 99 countries (of which 92 countries had not previously reported cases) including 15 deaths [19,39]. The vast majority of the cases have been reported outside of the European region, with the most affected countries being the US, Spain, UK, Germany, France, Brazil, Netherlands, Canada, Portugal and Italy. Per recent WHO data, these countries contain 88.9% of all the cases reported globally [40]. On 17 May 2022, the first confirmed case of monkeypox in the US was reported in the state of Massachusetts [41]. US case counts at the time of this writing are 18,417, with New York being the most affected state (3273 cases as of 30 August 2022). Coastal states with higher population densities have reported more cases than others, namely New York and California [42].
The 2022 monkeypox outbreak has predominantly affected men who have sex with men (MSM) (95.2%) and bisexual men. The bulk of the global cases have been reported among young males (78.1%), with a median age of 36 years (interquartile range: 18–44 years). Notably, 134 cases reported to date in the US have occurred among those in the 16–20 years age group [43]. Human immunodeficiency virus (HIV) infection has been reported among 44.6% of those with monkeypox and known HIV status [40]. There have been 277 cases among health workers, and community versus occupational exposure remains subject to ongoing investigation. While direct contact seems to be the most likely mode of transmission, a novel finding amid this outbreak is the report of a sexual encounter as a transmission event, specifically large events with sexual contacts [40]. Demographic findings from US data are concordant with global reports published by the WHO and indicate that young MSM comprise the majority of cases (median age: 36 years). Based on available data regarding race and ethnicity (as of 30 August 2022), about 30.6% have been reported to be Caucasian/non-Hispanic, 33.2% have been reported to be African American, and 31.8% have been reported to be Hispanic [41]. For the first time, monkeypox deaths have also been reported in non-endemic countries, outside Central and West African countries. The deaths have been linked to viral encephalitis and underlying immunocompromising conditions and comorbidities [40].
The US CDC has provided case definitions for suspected, probable, and confirmed cases of monkeypox [44]. Additionally, guidance on epidemiological and exclusion criteria have also been provided as a part of the 2022 monkeypox response. Suspected cases include those with a new characteristic rash or cases that meet epidemiologic criteria and have a high clinical suspicion. Probable cases have been defined as those without suspicion of other recent Orthopoxvirus exposure, the demonstration of Orthopoxvirus DNA by PCR, or the demonstration of Orthopoxvirus via the immunohistochemistry or electron microscopy analysis of a clinical specimen. Suspected cases could also be established by the demonstration of anti-Orthopoxvirus IgM antibodies from 4 to 56 days after rash onset. Confirmed cases include those with MPV DNA presence, established from clinical specimens by PCR or next-generation sequencing (NGS), or those where MPV has been isolated in culture.
The epidemiologic criteria are, within 21 days of onset of symptoms, as follows:
- Contact with one or more individuals with a similar rash who received a probable or confirmed monkeypox diagnosis.
- Close or intimate personal contact with individuals in a social network where monkeypox is present, including MSM who meet partners on the web or on mobile applications or at social events including parties.
- Travel outside the US to a country with confirmed monkeypox cases or to a monkeypox-endemic country.
- Contact with a live or dead wild animal or exotic pet that is an African-endemic species or the use of a product derived from such animals, including lotions, cream, powder, and game meat.
Exclusion criteria are an alternate diagnosis, a lack of rash within 5 days of the onset of illness in an individual with symptoms consistent with monkeypox, or the inability to demonstrate Orthopoxvirus, Monkeypox virus, or antibodies to Orthopoxvirus despite high-quality clinical specimens. More recent monkeypox case fatality rates were 3–6% according to the WHO, with historic rates ranging from 0 to 11% among the general population [18].
4. Transmission
MPV transmission has been observed through two routes—animal–human and human–human. While humans are the sole reservoir for Variola, the causative virus of smallpox, rodents are considered to be reservoirs of MPV, though this has yet to be confirmed. Trapping studies to understand monkeypox seroprevalence in small African mammals and non-human primates suggested an abundance of seropositivity, isolation of MPV, and presence of sparse rash lesions in one small mammalian species—rope squirrels (Funisciuris), suggesting that it could be a likely reservoir of MPV [37,45]. Animal–human transmission may occur through direct contact with infected animal skin lesions, bodily fluids, and animal bites and scratches, as well as the handling and consumption of infected animal meat. Human–human transmission may occur through direct personal contact with lesions and bodily fluids, prolonged face to face contact, or indirect contact with fomites such as clothing and bedding. Contact with respiratory secretions may also contribute to transmission, though airborne transmission has not been reported as of this writing [1,2,37,46]. Nosocomial transmission has been observed in previous outbreaks and it associated with both clades of MPV [1,12,47].
The incubation period of monkeypox is usually 6–13 days (range: 5–21 days) [18], but shorter incubation periods have been reported in the 2022 cases, with a median incubation period of 7 days (range: 3–20 days) [48]. In the current global monkeypox outbreak, human to human transmission seems to be predominant. While initial US cases reported international travel within 21 days of symptom onset to countries experiencing monkeypox outbreaks, more recent cases seem to show signals of community transmission [41]. Close sexual contact has also been observed as a key factor in disease transmission. The replication of competent MPV isolation from the seminal fluid of a male patient with monkeypox has been reported and speculated to be a result of passive viral diffusion from blood or exfoliated genital epithelium. However, it remains unclear whether MPV can replicate in the seminiferous tubules or the remainder of the genital tract. Interestingly, this patient had received a smallpox vaccination during childhood [49]. Although the precise transmission patterns of the 2022 global monkeypox outbreak are yet to be fully understood, evidence supporting transmission as a result of sexual activity continues to accumulate [48]. A lack of or waning herd immunity from the cessation of smallpox vaccination prevents cross-protection offered against monkeypox by smallpox vaccination [50].
The vertical transmission of MPV was observed in a still-born fetus birthed through a second-trimester loss to a monkeypox-infected mother. The case demonstrated generalized skin rashes with the detection of viral agents in the fetal tissue, umbilical cord, and placenta [51]. Previous outbreaks have shown that monkeypox, like smallpox, can have a high risk for pregnancy loss, severe congenital infection, and severe maternal morbidity and mortality; however, the impact of MPV on maternal and fetal health during the current outbreak remains unknown. Recent guidelines strongly recommend close observation, reporting, management, and prevention mechanisms to aid maternal and fetal health [52].
5. Clinical Manifestations and Diagnosis
Early diagnosis and robust management are key to curtailing the extent of the ongoing monkeypox outbreak as transmission continues. The diagnosis of monkeypox is based on patient history, clinical presentation, and diagnostic testing. Gathering a detailed travel and sexual history are of utmost importance given evolving transmission patterns and disease spread beyond its endemic distribution. The clinical features of monkeypox traditionally include prodromal symptoms such as fever, malaise, chills, lymphadenopathy, myalgia, or headache, along with a pleomorphic, often umbilicated skin rash. The lesions have been described as firm, deep-seated, well-circumscribed, and potentially affecting palms and soles [53]. Macules, papules, vesicles and pustules progress to scabs and eventually desquamate. An individual is considered infectious until all skin lesions have completely re-epithelialized. Pitted scars or skin with variable pigmentation may result at the sites of prior skin lesions. Monkeypox is generally a self-limited disease that resolves over 2–4 weeks. Severe cases can occasionally occur and are more common among children, and worse outcomes remain a possibility among immunocompromised hosts. The features of severe disease include encephalitis, sepsis, hemorrhage, confluent skin lesions, and other complications resulting in hospitalization.
The potential complications of monkeypox include encephalitis, corneal ulceration and scarring resulting in a loss of vision, the secondary bacterial infection of skin lesions, and respiratory tract infections such as bronchopneumonia and sepsis [18]. Disease prognosis depends on several factors including age, prior smallpox vaccination status, medical comorbidities, use of immunosuppressive drugs, and the severity of the illness.
Although the true extent of asymptomatic monkeypox remains unknown, fresh reports of this phenomenon have emerged this year. Three Belgian male attendees of a sexual health clinic were identified to be MPV PCR-positive on samples collected from the anorectal region, with eventual spontaneous viral clearance [54].
The salient clinical features of the 2022 monkeypox cases include dermal lesions over the external genitalia, anal region, and oral mucosa, which are most likely portals of exposure and viral entry. Thornhill et al. described similar clinical presentations among those with and without HIV [48]. Sore throat, penile edema, rectal pain have also been observed among patients from London, UK with PCR-confirmed monkeypox [55]. Patel et al. also reported a variable timeline of systemic and mucocutaneous symptoms among cases and described several cases where patients solely presented with dermatological symptoms without systemic features of the disease. Novel clinical findings from the 2022 cases also include mucocutaneous ulcers and fewer skin lesions, less disseminated disease, and proctitis causing anorectal pain [48].
Confirmatory laboratory tests—immunohistochemistry, enzyme-linked immunosorbent assay (ELISA), viral culture, and polymerized chain reaction (PCR)—are performed using scab material, lesion fluid, vesicular fluid, or biopsy specimens from an individual suspected to have monkeypox to confirm diagnosis and rule out other diseases [18,56]. MPV protein assessment using gel-electrophoresis can also confirm diagnosis, but it may not be appropriate as a first-line diagnostic in the clinical setting [57]. Real-time PCR, based on nucleic acid amplification, is considered the gold standard, first-line diagnostic test due to its accuracy and high sensitivity. Other tests are recommended when a suspected case tests negative with PCR [58]. RT-PCR testing targets regions of the DNA polymerase gene, E9L, F3L, extracellular-envelope protein gene (B6R), and DNA-dependent RNA polymerase subunit 18, among others [12,59]. On the other hand, whole-genome sequencing using NGS is a comprehensive and insightful testing methodology that is seldom used and reserved for use downstream due its laborious, expensive, and time-consuming nature. RT-PCR test results can take 24–72 h and are currently only conducted by public health laboratories and five commercial labs (Aegis Science, LabCorp, Mayo Clinic Laboratories, Quest Diagnostics, and Sonic Healthcare) in the US [60]. The US CDC has established the diagnostic process for MPV testing [61]. Testing for non-Variola Orthopoxvirus PCR can be set up after initial patient assessment. Healthcare providers are responsible for establishing contact with public health authorities at the local or state level. If Orthopoxvirus testing (PCR) results are positive, MPV characterization is forwarded to the CDC.
Importantly, when possible, vaccinated individuals (smallpox vaccinated within the last 3 years) should handle monkeypox-suspected specimens in BSL-2 containment using BSL-3 practices [61]. The laboratory testing of MPV in the US has suggested that cases in the current outbreak are associated with the West African clade [62].
6. Management
The majority of monkeypox cases tend to have a mild clinical course, with self-resolution even without treatment. There is no current US Food and Drug Administration (FDA)-approved antiviral labeled for use specifically against MPV. The antivirals available for use include repurposed agents effective against smallpox, namely, tecovirimat (TPOXX/ST-246) and brincidofovir. Cidofovir, an antiviral approved for use against cytomegalovirus (CMV), has also shown efficacy against Orthopoxviruses in vitro.
6.1. Tecovirimat
Tecovirimat (TPOXX) was approved by the FDA in 2018 for the treatment of smallpox in adults and children. It inhibits VP37, a viral envelope wrapping protein, and disrupts viral replication and release. It is currently available for use in the US under an expanded access investigational new drug protocol at no cost (EA-IND) [63]. Tecovirimat is available in oral and intravenous formulations. While efficacy data on the use of TPOXX against monkeypox are lacking, a favorable safety profile with common adverse effects such as headache, nausea, vomiting and abdominal pain has been reported. Neutropenia was also reported for use with one trial participant [64]. Intravenous formulation use may result in infusion site erythema, pain and swelling [63]. Thornhill et al. reported on the treatment of recent monkeypox cases with TPOXX [48]. Adler et al. described the management of a human monkeypox case with TPOXX with a favorable outcome [65]. Monkeypox in a returning traveler from Nigeria to the US, treated with TPOXX, was also recently described [66].
6.2. Brincidofovir
Brincidofovir was approved by the FDA for use against smallpox in adults and pediatric patients in June 2021. It is a prodrug of cidofovir and contains a lipid conjugate. Intracellularly, it is converted to cidofovir and eventually its active metabolite, cidofovir diphosphate (CDP), which incorporates into viral DNA and inhibits viral DNA polymerase, thereby inhibiting viral replication. Large-scale human data on the use of brincidofovir against MPV are lacking, but an animal model showed trends of protection against lethal monkeypox, with 29–57% survival rates among infected prairie dogs depending on the time of treatment initiation [67].
Adler et al. also described three human cases of monkeypox treated with brincidofovir. Treatment cessation occurred due to the elevation of liver enzymes [65]. Brincidofovir is available as an oral formulation (tablet and oral suspension) and has a better renal safety profile than cidofovir [65].
6.3. Cidofovir
Cidofovir has the same mechanism of action as its prodrug, brincidofovir. Large-scale human data on efficacy of cidofovir against monkeypox are lacking. However, animal data on its use against Orthopoxviruses including cowpox, vaccinia, ectromelia and rabbitpox exist [68].
Thornhill et al. reported cases amid the 2022 monkeypox outbreak that were treated with cidofovir. It is only available as an intravenous formulation and can have significant renal toxicity [48].
6.4. Vaccinia Immune Globulin Intravenous (VIGIV)
VIGIV is FDA-licensed for the treatment of complications after Vaccinia vaccination, including vaccinia (progressive or severe generalized), eczema vaccinatum, and aberrant infections due to Vaccinia virus. It can also be used for vaccinia infections in those with certain skin conditions [69]. Data for its use against monkeypox are lacking, but it is available in the US as a response measure in the event of Orthopoxvirus outbreaks under an EA-IND.
Figure 1 is an illustration of mechanisms of action of available therapies against monkeypox, along with its clinical symptoms.
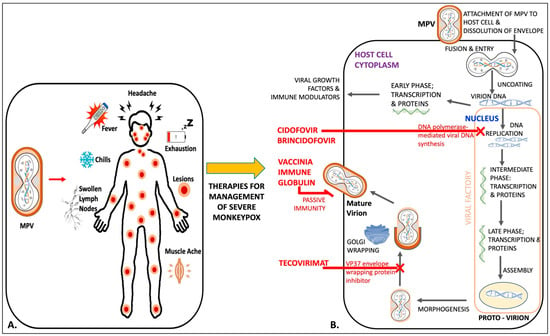
Figure 1.
An illustration of the symptoms of monkeypox (A) and the life cycle of MPV inside the host-cell cytoplasm to elicit the mechanism of action of four different antiviral therapies: cidofovir, brincidofovir, vaccinia immune globulin, and tecovirimat (B).
Interim guidance for the treatment of monkeypox has been provided by the US CDC [69]. Antiviral use for those with the clinical features of aberrant disease in atypical locations, severe disease, complications, and those at high risk for severe disease has been suggested. Risk factors for severe disease include age < 8 years, atopic or other exfoliative dermatitis, pregnancy, lactation, and immunocompromised status. Immunocompromising conditions include uncontrolled HIV, acquired immunodeficiency syndrome, leukemia, lymphoma, other malignancies, radiation, solid organ transplantation, hematopoietic stem cell transplantation < 24 months post-transplant or >24 months post-transplant with graft-versus-host disease or disease relapse, autoimmune disease with immunodeficiency, and iatrogenic immunosuppression as a result of the use of alkylating agents, antimetabolites, tumor necrosis factor inhibitors, or high doses of corticosteroids.
Additionally, symptomatic and supportive care, along with effective pain management, is paramount [69].
7. Prevention
Some measures can help prevent the spread of MPV. Direct-contact-based prevention strategies include avoiding close and direct contact including hugging and kissing with people who have skin lesions resembling monkeypox, avoiding touching the rash and scabs, avoiding sexual contact with infected individuals, and avoiding contact with animals that exhibit monkeypox-like symptoms [70]. Other prevention measures include avoiding sharing utensils with a person who has monkeypox and avoiding touching items that have been in contact with a person infected with monkeypox such as bedding and clothes [70]. Frequently washing hands with soap and water or using an alcohol-based hand sanitizer can be very effective. Healthcare professionals caring for patients should wear proper personal protective equipment, cover their entire body with a water-resistant gown, be double-gloved, and use N-95 masks. Patients should be placed under contact isolation in single-patient rooms until all lesions have crusted and fully re-epithelialized [70].
Studies have shown that the smallpox vaccine provides significant protection against monkeypox and may improve disease outcomes [8,71]. The two US FDA-approved vaccines—JYNNEOS and ACAM2000—can be useful in prevention strategies for monkeypox. JYNNEOS contains a live Vaccinia virus that is not replication-competent in human cells and is administered as two subcutaneous doses, 28 days apart, with full protection being afforded 14 days after the completion of the vaccine series. JYNNEOS is licensed by the FDA for adults 18 years and older against smallpox and monkeypox. Unlike ACAM2000, JYNNEOS can be given to those with HIV and atopic or other exfoliative dermatitis. ACAM2000, on the other hand, contains live replication-competent Vaccinia virus, and it is given a single percutaneous dose via the multiple puncture technique. After inoculation, a lesion (also called “take”) develops at the injection site and may take up to 6 weeks to heal. Protective immunity is achieved at least 4 weeks after vaccination. ACAM2000 is licensed for use against smallpox and can be used against monkeypox with EA-IND. Since this is a live viral vaccine, it should not be given to certain individuals. ACAM2000 has been linked to cases of vaccination-induced myocarditis and pericarditis [72].
The US CDC’s recommendation on “post-exposure prophylaxis” (PEP), coupled with isolation and other preventive methods, is vaccinating individuals 4 days after monkeypox exposure to prevent disease, and administration between 4 and 14 days can improve disease outcome [72]. PEP plus plus (PEP++) is an expanded approach aimed to reach people with certain risk factors even if they have not had documented exposure to confirmed monkeypox cases, with the objective of flattening the epidemiological curve and slowing disease spread in areas with high levels of transmission [72]. These strategies can help prevent transmission and further control disease outbreak. Additionally, the pre-exposure prophylaxis (PrEP) guidelines by the CDC suggest vaccinating individuals at a high risk for monkeypox [72]. While these vaccines have been tested for efficacy against monkeypox in animal studies (JYNNEOS) or allowed for clinical use under the FDA’s EA-IND, there are currently no data on their efficacy for PEP, PEP++, or PrEP for the current global outbreak [72,73,74,75]. Additionally, the administration of ACAM2000 is contraindicated in individuals with immunosuppression or immunocompromised status, atopic dermatitis, eczema, pregnancy or breastfeeding activity, underlying heart disease, and major cardiac risk-factors, as well as those of infant age [72,74].
8. Conclusions
The 2022 monkeypox outbreak is a new paradigm in the human and poxvirus interaction, and it marks a historic time since the eradication of smallpox in 1980. Once considered a rare disease, monkeypox spread across the Central and West African countries through sporadic outbreaks in the four decades since the first case was reported in 1970. Importantly, the transmission mode of monkeypox has seen a significant shift over time. While early reports described a primarily zoonotic spread, the hallmark of the 2022 global monkeypox outbreak is human–human sustained chain of transmission, most prominently affecting the MSM population. This infectious disease is the latest to be added to the list of public health emergencies of international concern, with a worry that the disease might not only become entrenched as a sexually transmitted infection but also have the ability to cause significant morbidity among a vulnerable global population that lacks immunity as a result of smallpox vaccination cessation. A lack of robust engagement in MPV research and the potential underestimation of its pathogenicity have led to repurposing of antivirals and vaccines for use against MPV. While most individuals infected with MPV experience a mild, self-limiting disease, the 2022 cases have exhibited unique clinical features including fewer lesions located on the external genitalia and rectal area, notably accompanied with pain. The global emergence of monkeypox has demonstrated key needs to refocus the understanding of MPV as a significant member of Orthopoxviruses and to develop specific interventions to prevent and manage the disease.
Funding
This work did not involve any form of funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Learned, L.A.; Reynolds, M.G.; Wassa, D.W.; Li, Y.; Olson, V.A.; Karem, K.; Stempora, L.L.; Braden, Z.H.; Kline, R.; Likos, A.; et al. Extended Interhuman Transmission of Monkeypox in a Hospital Community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005, 73, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Osadebe, L.; Katomba, J.; Likofata, J.; Mukadi, D.; Monroe, B.; Doty, J.; Hughes, C.M.; Kabamba, J.; Malekani, J.; et al. Extended Human-to-Human Transmission during a Monkeypox Outbreak in the Democratic Republic of the Congo. Emerg. Infect. Dis. 2016, 22, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- von Magnus, P.; Andersen, E.K.; Petersen, K.B.; Birch-Andersen, A. A Pox-Like Disease in Cynomolgus Monkeys. Acta Pathol. Microbiol. Scand. 1959, 46, 156–176. [Google Scholar] [CrossRef]
- Ladnyj, I.D.; Ziegler, P.; Kima, E. A Human Infection Caused by Monkeypox Virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972, 46, 593–597. [Google Scholar] [PubMed]
- Cho, C.T.; Wenner, H.A. Monkeypox Virus. Bacteriol. Rev. 1973, 37, 1–18. [Google Scholar] [CrossRef]
- Meyer, H.; Perrichot, M.; Stemmler, M.; Emmerich, P.; Schmitz, H.; Varaine, F.; Shungu, R.; Tshioko, F.; Formenty, P. Outbreaks of Disease Suspected of Being Due to Human Monkeypox Virus Infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002, 40, 2919–2921. [Google Scholar] [CrossRef]
- R Pebody Human Monkeypox in Kasai Oriental, Democratic Republic of Congo, February 1996–October 1997: Preliminary Report. Wkly. Releases 1997, 1, 1015. [CrossRef]
- Heymann, D.L.; Szczeniowski, M.; Esteves, K. Re-Emergence of Monkeypox in Africa: A Review of the Past Six Years. Br. Med. Bull. 1998, 54, 693–702. [Google Scholar] [CrossRef]
- Mukinda, V.; Mwema, G.; Kilundu, M.; Heymann, D.; Khan, A.; Esposito, J. Re-Emergence of Human Monkeypox in Zaire in 1996. Lancet 1997, 349, 1449–1450. [Google Scholar] [CrossRef]
- Hutin, Y.J.F.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of Human Monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef]
- Doty, J.; Malekani, J.; Kalemba, L.; Stanley, W.; Monroe, B.; Nakazawa, Y.; Mauldin, M.; Bakambana, T.; Liyandja Dja Liyandja, T.; Braden, Z.; et al. Assessing Monkeypox Virus Prevalence in Small Mammals at the Human–Animal Interface in the Democratic Republic of the Congo. Viruses 2017, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Yinka-Ogunleye, A.; Aruna, O.; Dalhat, M.; Ogoina, D.; McCollum, A.; Disu, Y.; Mamadu, I.; Akinpelu, A.; Ahmad, A.; Burga, J.; et al. Outbreak of Human Monkeypox in Nigeria in 2017–18: A Clinical and Epidemiological Report. Lancet Infect. Dis. 2019, 19, 872–879. [Google Scholar] [CrossRef]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The Detection of Monkeypox in Humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Past U.S. Cases and Outbreaks Monkeypox Poxvirus. Available online: https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html (accessed on 25 July 2022).
- Vaughan, A.; Aarons, E.; Astbury, J.; Brooks, T.; Chand, M.; Flegg, P.; Hardman, A.; Harper, N.; Jarvis, R.; Mawdsley, S.; et al. Human-to-Human Transmission of Monkeypox Virus, United Kingdom, October 2018. Emerg. Infect. Dis. 2020, 26, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Erez, N.; Achdout, H.; Milrot, E.; Schwartz, Y.; Wiener-Well, Y.; Paran, N.; Politi, B.; Tamir, H.; Israely, T.; Weiss, S.; et al. Diagnosis of Imported Monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019, 25, 980–983. [Google Scholar] [CrossRef]
- Yong, S.E.F.; Ng, O.T.; Ho, Z.J.M.; Mak, T.M.; Marimuthu, K.; Vasoo, S.; Yeo, T.W.; Ng, Y.K.; Cui, L.; Ferdous, Z.; et al. Imported Monkeypox, Singapore. Emerg. Infect. Dis. 2020, 26, 1826–1830. [Google Scholar] [CrossRef]
- World Health Organization. Monkeypox Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 25 July 2022).
- Center for Disease Control and Prevention. 2022 Monkeypox Outbreak Global Map. Poxvirus. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 21 July 2022).
- World Health Organization. Director-General Declares the Ongoing Monkeypox Outbreak a Public Health Emergency of International Concern. Available online: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern (accessed on 25 July 2022).
- Shchelkunov, S.N.; Totmenin, A.V.; Babkin, I.V.; Safronov, P.F.; Ryazankina, O.I.; Petrov, N.A.; Gutorov, V.V.; Uvarova, E.A.; Mikheev, M.V.; Sisler, J.R.; et al. Human Monkeypox and Smallpox Viruses: Genomic Comparison. FEBS Lett. 2001, 509, 66–70. [Google Scholar] [CrossRef]
- Alakunle, E.; Moens, U.; Nchinda, G.; Okeke, M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses 2020, 12, 1257. [Google Scholar] [CrossRef]
- Orthopoxvirus. ViralZone. Available online: https://viralzone.expasy.org/149?outline=all_by_species (accessed on 26 July 2022).
- Henderson, D.A.; Borio, L.L. Chapter 58—Smallpox and Monkeypox. In Tropical Infectious Diseases, 2nd ed.; Guerrant, R.L., Walker, D.H., Weller, P.F., Eds.; Churchill Livingstone: Philadelphia, PA, USA, 2006; pp. 621–636. ISBN 978-0-443-06668-9. [Google Scholar]
- Srinivasan, K.; Rao, M. Poxvirus Driven Human Diseases and Emerging Therapeutics. Med. Phamacol. 2022, 2022070300. [Google Scholar] [CrossRef]
- Law, M.; Carter, G.C.; Roberts, K.L.; Hollinshead, M.; Smith, G.L. Ligand-Induced and Nonfusogenic Dissolution of a Viral Membrane. Proc. Natl. Acad. Sci. USA 2006, 103, 5989–5994. [Google Scholar] [CrossRef] [Green Version]
- ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323482554001326 (accessed on 27 July 2022).
- Moyer, R.W.; Graves, R.L. The Mechanism of Cytoplasmic Orthopoxvirus DNA Replication. Cell 1981, 27, 391–401. [Google Scholar] [CrossRef]
- Vanderplasschen, A.; Hollinshead, M.; Smith, G.L. Intracellular and Extracellular Vaccinia Virions Enter Cells by Different Mechanisms. J. Gen. Virol. 1998, 79, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shang, J.; Weng, S.; Aliyari, S.R.; Ji, C.; Cheng, G.; Wu, A. Genomic Annotation and Molecular Evolution of Monkeypox Virus Outbreak in 2022. J. Med. Virol. 2022, jmv.28036. [Google Scholar] [CrossRef] [PubMed]
- Isidro, J.; Borges, V.; Pinto, M.; Sobral, D.; Santos, J.D.; Nunes, A.; Mixão, V.; Ferreira, R.; Santos, D.; Duarte, S.; et al. Phylogenomic Characterization and Signs of Microevolution in the 2022 Multi-Country Outbreak of Monkeypox Virus. Nat. Med. 2022, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Sohail, M.S.; Quadeer, A.A.; McKay, M.R. Vaccinia Virus Vaccination Is Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. BioRxiv 2022. [Google Scholar] [CrossRef]
- Douglass, N.J.; Dumbell, K.R. DNA Sequence Variation as a Clue to the Phylogenesis of Orthopoxviruses. J. Gen. Virol. 1996, 77, 947–951. [Google Scholar] [CrossRef]
- Likos, A.M.; Sammons, S.A.; Olson, V.A.; Frace, A.M.; Li, Y.; Olsen-Rasmussen, M.; Davidson, W.; Galloway, R.; Khristova, M.L.; Reynolds, M.G.; et al. A Tale of Two Clades: Monkeypox Viruses. J. Gen. Virol. 2005, 86, 2661–2672. [Google Scholar] [CrossRef]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence Differences between Monkeypox Virus Isolates from West Africa and the Congo Basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef]
- Parker, S.; Buller, R.M. A Review of Experimental and Natural Infections of Animals with Monkeypox Virus between 1958 and 2012. Future Virol. 2013, 8, 129–157. [Google Scholar] [CrossRef]
- Zdenek, J.; Fenner, F. Human MonkeyPox; Karger: Basel, Switzerland, 1988. [Google Scholar]
- Gigante, C.M.; Korber, B.; Seabolt, M.H.; Wilkins, K.; Davidson, W.; Rao, A.K.; Zhao, H.; Hughes, C.M.; Minhaj, F.; Waltenburg, M.A.; et al. Multiple Lineages of Monkeypox Virus Detected in the United States, 2021–2022. BioRxiv 2022. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Tegally, H.; Pigott, D.M.; Dasgupta, A.; Sheldon, J.; Wilkinson, E.; Schultheiss, M.; Han, A.; Oglia, M.; Marks, S.; et al. Tracking the 2022 Monkeypox Outbreak with Epidemiological Data in Real-Time. Lancet Infect. Dis. 2022, 22, 941–942. [Google Scholar] [CrossRef]
- World Health Organization. 2022 Monkeypox Outbreak: Global Trends—Detailed Case Data. Available online: https://worldhealthorg.shinyapps.io/mpx_global/#3_Detailed_case_data (accessed on 30 August 2022).
- Center for Disease Control and Prevention. Technical Report: Multi-National Monkeypox Outbreak, United States, 2022; CDC: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/technical-report.html (accessed on 6 August 2022).
- Center for Disease Control and Prevention. Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/index.html (accessed on 20 July 2022).
- Center for Disease Control and Prevention. Monkeypox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics.html (accessed on 30 August 2022).
- Center for Disease Control and Prevention. Monkeypox in the U.S.—Case Definitions for Use in the 2022 Monkeypox Response. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/case-definition.html (accessed on 9 August 2022).
- Khodakevich, L.; Ježek, Z.; Messinger, D. Monkeypox Virus: Ecology and Public Health Significance. Bull. World Health Organ. 1988, 66, 747–752. [Google Scholar] [PubMed]
- Formenty, P.; Muntasir, M.O.; Damon, I.; Chowdhary, V.; Opoka, M.L.; Monimart, C.; Mutasim, E.M.; Manuguerra, J.-C.; Davidson, W.B.; Karem, K.L.; et al. Human Monkeypox Outbreak Caused by Novel Virus Belonging to Congo Basin Clade, Sudan, 2005. Emerg. Infect. Dis. 2010, 16, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Aarons, E.; Astbury, J.; Balasegaram, S.; Beadsworth, M.; Beck, C.R.; Chand, M.; O’Connor, C.; Dunning, J.; Ghebrehewet, S.; et al. Two Cases of Monkeypox Imported to the United Kingdom, September 2018. Eurosurveillance 2018, 23, 1800509. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Lapa, D.; Carletti, F.; Mazzotta, V.; Matusali, G.; Pinnetti, C.; Meschi, S.; Gagliardini, R.; Colavita, F.; Mondi, A.; Minosse, C.; et al. Monkeypox Virus Isolation from a Semen Sample Collected in the Early Phase of Infection in a Patient with Prolonged Seminal Viral Shedding. Lancet Infect. Dis. 2022, 22, P1267–P1269. [Google Scholar] [CrossRef]
- Alakunle, E.F.; Okeke, M.I. Monkeypox Virus: A Neglected Zoonotic Pathogen Spreads Globally. Nat. Rev. Microbiol. 2022, 20, 507–508. [Google Scholar] [CrossRef]
- Mbala, P.K.; Huggins, J.W.; Riu-Rovira, T.; Ahuka, S.M.; Mulembakani, P.; Rimoin, A.W.; Martin, J.W.; Muyembe, J.-J.T. Maternal and Fetal Outcomes Among Pregnant Women with Human Monkeypox Infection in the Democratic Republic of Congo. J. Infect. Dis. 2017, 216, 824–828. [Google Scholar] [CrossRef]
- Dashraath, P.; Nielsen-Saines, K.; Mattar, C.; Musso, D.; Tambyah, P.; Baud, D. Guidelines for Pregnant Individuals with Monkeypox Virus Exposure. Lancet 2022, 400, 21–22. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Monkeypox in the U.S—Clinical Recognition. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html (accessed on 6 August 2022).
- Baetselier, I.D.; Dijck, C.V.; Kenyon, C.; Coppens, J.; den Bossche, D.V.; Smet, H.; Liesenborghs, L.; Vanroye, F.; de Block, T.; Rezende, A.; et al. Asymptomatic Monkeypox Virus Infections among Male Sexual Health Clinic Attendees in Belgium. MedRxiv 2022. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Fontoura, D.D.S.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.M.; Damon, I.K. Human Monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Adalja, A.; Inglesby, T. A Novel International Monkeypox Outbreak. Ann. Intern. Med. 2022, 175, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Girometti, N.; Byrne, R.; Bracchi, M.; Heskin, J.; McOwan, A.; Tittle, V.; Gedela, K.; Scott, C.; Patel, S.; Gohil, J.; et al. Demographic and Clinical Characteristics of Confirmed Human Monkeypox Virus Cases in Individuals Attending a Sexual Health Centre in London, UK: An Observational Analysis. Lancet Infect. Dis. 2022, 22, P1321–P1328. [Google Scholar] [CrossRef]
- Li, Y.; Olson, V.A.; Laue, T.; Laker, M.T.; Damon, I.K. Detection of Monkeypox Virus with Real-Time PCR Assays. J. Clin. Virol. 2006, 36, 194–203. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. HHS Expanding Monkeypox Testing Capacity to Five Commercial Laboratory Companies. Available online: https://www.hhs.gov/about/news/2022/06/22/hhs-expanding-monkeypox-testing-capacity-five-commercial-laboratory-companies.html (accessed on 28 July 2022).
- Center for Disease Control and Prevention. Laboratory Procedures and Biosafety Guidelines—Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/lab-personnel/lab-procedures.html (accessed on 28 July 2022).
- Center for Disease Control and Prevention. Monkeypox in the U.S.—Preparation and Collection of Specimens. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html (accessed on 9 August 2022).
- Center for Disease Control and Prevention. Monkeypox in the U.S—Guidance for Tecovirimat Use Under Expanded Access Investigational New Drug Protocol during 2022 U.S. Monkeypox Cases. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html (accessed on 9 August 2022).
- SIGA Technologies. A Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety, Tolerability, and Pharmacokinetics of TPOXX When Administered Orally for 28 Days in Adult Subjects; SIGA Technologies: New York, NY, USA, 2022. [Google Scholar]
- Adler, H.; Gould, S.; Hine, P.; Snell, L.B.; Wong, W.; Houlihan, C.F.; Osborne, J.C.; Rampling, T.; Beadsworth, M.B.; Duncan, C.J.; et al. Clinical Features and Management of Human Monkeypox: A Retrospective Observational Study in the UK. Lancet Infect. Dis. 2022, 22, 1153–1162. [Google Scholar] [CrossRef]
- Rao, A.K.; Schulte, J.; Chen, T.-H.; Hughes, C.M.; Davidson, W.; Neff, J.M.; Markarian, M.; Delea, K.C.; Wada, S.; Liddell, A.; et al. Monkeypox in a Traveler Returning from Nigeria—Dallas, Texas, July 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 509–516. [Google Scholar] [CrossRef]
- Hutson, C.L.; Kondas, A.V.; Mauldin, M.R.; Doty, J.B.; Grossi, I.M.; Morgan, C.N.; Ostergaard, S.D.; Hughes, C.M.; Nakazawa, Y.; Kling, C.; et al. Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model. mSphere 2021, 6, e00927-20. [Google Scholar] [CrossRef]
- Smee, D.F. Progress in the Discovery of Compounds Inhibiting Orthopoxviruses in Animal Models. Antivir. Chem. Chemother. 2008, 19, 115–124. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Monkeypox in the U.S.—Treatment Information for Healthcare Professionals. Available online: https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html (accessed on 9 August 2022).
- Center for Disease Control and Prevention. Prevention—Monkeypox in the U.S. Available online: https://www.cdc.gov/poxvirus/monkeypox/prevention.html (accessed on 28 July 2022).
- Hammarlund, E.; Lewis, M.W.; Carter, S.V.; Amanna, I.; Hansen, S.G.; Strelow, L.I.; Wong, S.W.; Yoshihara, P.; Hanifin, J.M.; Slifka, M.K. Multiple Diagnostic Techniques Identify Previously Vaccinated Individuals with Protective Immunity against Monkeypox. Nat. Med. 2005, 11, 1005–1011. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Considerations for Monkeypox Vaccination. Available online: https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html (accessed on 28 July 2022).
- Rao, A.K. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Research for B.E. and ACAM2000; FDA: Silver Spring, MD, USA, 2019.
- U.S. Food and Drug Administration. Research for B.E. and JYNNEOS; FDA: Silver Spring, MD, USA, 2021.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).