Abstract
Treponema pallidum subspecies pallidum causes syphilis, a sexually transmitted disease that infects more than 2.1 million pregnant women every year. Due to its maximum death rates and augmented risk of human immunodeficiency virus (HIV) infection, the disease is still a matter of debate in many low- and high-income countries. The infection has three stages that lead to several complications if left untreated and can lead to many tertiary complications in the brain, eyes, ears, heart, and pregnancy. Neurosyphilis is also known as the clinical result of infection of the central nervous system by Treponema pallidum subspecies pallidum. It can evolve at any time and from any stage of syphilis exposure. This review briefly explains the severe and multiple neurosyphilitic complications and recently identified cases related to neurosyphilis. We also explained computational neuroscience, neuroinformatics, and in silico models and techniques based on artificial intelligence and other computational and mathematical methods. These techniques have already been applied to several neurological and psychological brain complications and can be applied to neurosyphilis to better understand the persistence of the disease related to the brain that causes neurosyphilis.
1. Introduction
Syphilis is a sexually-transmitted disease caused by Treponema pallidum (Tp) subspecies pallidum infection that infects more than 2.1 million pregnant women every year. Due to its maximum death rates of neonates, augmented risk of human immunodeficiency virus (HIV) infection, and continued morbidity particularly in low-income countries [1,2] as well as in high-income countries [3,4], such as Japan, where the rate of cases is increasing at an alarming level in heterosexual men and women, syphilis is a disease of worldwide concern [5]. Principally, the infection is transmitted through sexual contact, exceptionally with blood transfusion and blood products, and transmits vertically from mother to child (Syphilis Transmission from Mother-to-Child (MTCT)) during pregnancy [4]. The Tp spirochete transmits vertically to the fetus, leading to congenital syphilis infections in poorly treated or utterly treated pregnant women, and causes multiple clinical manifestations, including stillbirth and neonatal death, skin and visceral manifestations, and other asymptomatic infections [6,7]. According to the World Health Organization (WHO), a recently published study estimating the burden of congenital syphilis showed more than half a million (almost 661,000) cases of congenital syphilis in 2016, consequently facing 200,000 stillbirths and neonatal deaths [8,9]. Congenital syphilis is the second leading cause of preventable stillbirth globally, preceded only by “Malaria” [8].
Following infection, syphilis can present as a chancre (primary syphilis/first site of infection) 10–90 days (average three weeks) after manifestation. If syphilis is not adequately treated, the chancre may disappear. Nevertheless, within a few weeks or months, the symptoms of secondary syphilis may appear and can be seen on the host body. The symptoms may vary and comprise rashes on the body, alopecia (loss of hair), condylomata lata, and vague symptoms with malaise, sore throat, weight loss, and low-grade fever. Secondary syphilis manifestation can also vanish within a few weeks, even without treatment; then, “latent syphilis” follows, where the patients do not display any symptoms and indications but remain infectious for up to a year following infection [4,9]. Latent infection can last for decades if untreated and leads to tertiary syphilis complications [10]. Tertiary syphilis can evolve with manifestations that depend on which organ is involved [11,12,13].
Neurosyphilis (NS), also known as the clinical result of infection of the central nervous system by the Tp spirochete [14,15], can evolve at any time of syphilis exposure. Due to the lack of susceptible and specific diagnostic tests, diagnosis and identification depend on clinical conclusions, cerebrospinal fluid (CSF) contrasts, and clinical decisions [16]. As there is no standardized and specific diagnostic, NS remains challenging [17]. NS involves all neurological disorders related to nervous system invasion by the Tp and can be seen during the primo-secondary (early NS) or tertiary stages [18]. Emphatically, NS has two forms: an early form often strikes the CSF, meninges, and vasculature; the late form hits the brain and spinal cord parenchyma, and in several cases, it goes unnoticed or unidentified, leading to multifarious neurological complications [12]. Early NS complications are asymptomatic neurosyphilis (ANS), CSF abnormalities with no neurological signs or symptoms, for example, gumma of the CNS (cervical spinal syphilitic gumma [18] and spinal intramedullary syphilitic gumma [14]). In early symptomatic neurosyphilis, complications such as acute syphilitic meningitis, neurorecurrence, neurosyphilitic meningitis, and meningovascular syphilis have been reported. Late symptomatic neurosyphilis (SNS) complications include parenchymatous syphilis, general paresis, and tabes dorsalis [13,19]. Ocular and otologic syphilitic manifestations can occur but commonly coexist with early NS acute meningitis [16]. ANS occurs in early NS infection, and ANS patients are highly susceptible and quickly develop forthcoming SNS complications; almost 35% of ANS patients develop SNS in a natural progression. However, the reason for SNS risk linked to SNS development is still unclear [20]. Over the last many decades, a firm rise in syphilitic meningitis and different forms of early NS have been observed in HIV-positive inhabitants, mainly in men who have sex with men (MSM) [16].
Briefly, rather than becoming an infection of historical ponderance, syphilis continues to be challenging for scientists, researchers, and clinicians due to its multiple and unclear neurological complications in the age of HIV [19]. This article focuses specifically on exceptional and continued controversial NS complications such as “neurosyphilitic meningitis” and “cerebral syphilitic gumma” of neurosyphilis for its clinical manifestations and diagnosis. We attempt to highlight several important questions that remain unanswered. Our focus is to describe the Tp invasion capability of crossing the brain’s vascular basement membrane (the blood–brain barrier; BBB) [21] and its neurocognitive and psychiatric transformations as an initial demonstration [22] in neurosyphilitic patients. We also describe the new field of neuroinformatics (NI) as “The field of science whose objective is to unite neuroscience data and introduce pioneer computational tools to enhance our understanding of the nervous system and its functions in health and disease” [23], and computational neuroscience (CN) as “A wide and interdisciplinary area for the development, simulation, and analysis of multiscale prototypes and principles of neural activities from the molecular level, via cells, and networks, up to cognition and behavior” [23,24] research in neurological disorders.
2. Treponema pallidum Pathogenesis in Neurological Complications
T. pallidum subspecies pallidum, a long lean (from 0.15 μm by 6 μm to 15 μm), is a gradually growing bacterium that cannot grow and be easily cultured for clinical aims [25]. The bacterium is instinctively very delicate and fragile due to its abnormal envelope arrangement. The peptidoglycan layer is enclosed with a cytoplasmic-membrane-adjoining location rather than the typical outer-membrane-adjoining location as in conventional Gram-negative bacteria. This abnormal ultrastructure and extremely fragile nature make laboratory manipulation enormously difficult [21,26,27]. Tp’s manifestation depends on the time instant, site, and immune condition of infected personnel [28,29]. The infection can clasp any body organ and its tertiary syphilis chronic lesion that causes various disease characteristics and conclusions. The tertiary syphilis lesions associated with internal organs can be separated into three different categories—(A) mononuclear cell reactions similar to secondary syphilis skin lesions, (B) gummas, and (C) deadly degenerative alterations to loss of vascular or nerve supply [28,29,30].
Neurological lesions are associated with gummas, meningovascular inflammation, cerebral vessels inflammation, and general paresis (dementia paralytica), which may lead to multiple disorders (Table 1; Figure 1). The meningeal response in late syphilis can be asymptomatic, but it has been associated with inflammatory cells in the CNS and a positive VDRL test [31,32]. Meningitis in syphilis is characterized by thickened meninges; lymphocytic perivascular pervades around small vessels; and in parenchymal syphilis, disseminated, inflammatory, and proliferative changes occur in the cerebral cortex. Long ago, large gummas were common and often connected with the periphery to the meninges [28,29]. The NS manifestations appear to be the outcome of Tp’s apparent efficacy in the tissues; organisms gather massive amounts due to either incapability of immune response to be at the site of infection or lack of the timely immune response to command the disease [28,33]. In the present-day scenario, infected patients do not demonstrate typical symptoms of tabes dorsalis, general paresis, or meningovascular syphilis. Alternatively, bows show multiple atypical complications, ophthalmic symptoms such as poor vision, strokes, confusion, or personality changes. The disease was discovered incidentally during different medical investigations for various causes in several findings. Moreover, viable Tp can be identified with early and untreated syphilis in the CSF of 30% of patients [21,34]. In every stage of syphilis, macrophages quickly produce several inflammatory cytokines that stimulate the mechanism that secretes inflammatory mediators that lead to tissue damage, which is the main reason for clinical manifestations of syphilis; yet, very little is known about the process of this particular mechanism [35]. The specific manifestations of Tp infection rely on the time, infection site, and immune condition of an infected person [28,35,36]. The capacity of the immune system of the infected person depends on the syphilis stages, particularly the impact of the strength of delayed-type hypersensitivity (DTH) mediated by CD4+ cells. CD8+ cytotoxic T cells are comparatively inefficient in controlling the advancement of lesions if the response to DTH is inadequate [28]. A recent study published by Wei Li in 2020 [37] showed that the recombinant Tp protein Tp0768, which is a stage-dependent antigen, plays an important role in Tp infection and encourages proinflammatory cytokine secretion of macrophages via endoplasmic reticulum stress and the ROS/NF-κB pathway. In the study, the authors showed that Tp0768 stimulation of macrophages is able to increase the expression levels of IL-1β, IL-6, and IL-8 mRNA in a dose- and time-dependent manner. Furthermore, an investigation pointed out that Tp0768 activated endoplasmic reticulum stress and the ROS/NF-κB pathway in macrophages [37]. Another study on neurosyphilis dispersal in West China published by Dongdong Li in 2020 [38] demonstrated that the role of CSF_CXCL13 and syphilis serology is able to provide an additional precise context for the clinical identification and diagnosis of neurosyphilis. The study showed that the sensitivity of serology in neurosyphilis accompanied by CSF_CXCL13 can be helpful in neurosyphilis monitoring and can also be a potential marker for neurosyphilis diagnosis [38].
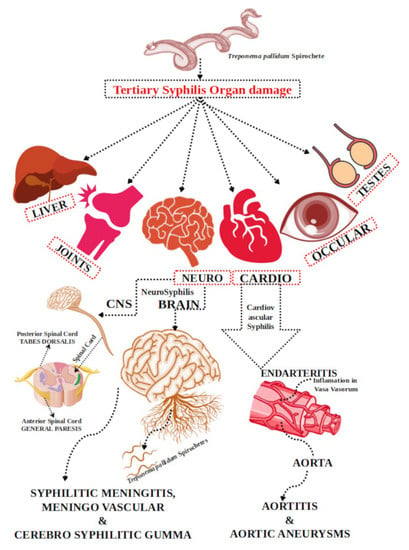
Figure 1.
Multiple tertiary syphilis is related to multiple organ damage and NS complications.
3. Treponema pallidum Neurological Invasion and Evasion Mechanism
Pathogens can employ multifold approaches for prolonged survival within the host, seeking to avoid and prevent their immune responses and going unnoticed [39]. Invasion of the CNS by pathogens is a grievous and often lethal phenomenon throughout several perfuse diseases. It can lead to hearing and vision loss, cerebral palsy, hydrocephalus, and cognitive impairment [40]. Spirochetes are strongly accommodated to encounter the host defense system on multiple leads to ensure long-term persistence inside the hosts. The symptoms and disease courses of spirochetes reflect their capability to expand themselves throughout the host without producing acute responses from the host immune recognition system, and these properties make them experts in immune evasion and persistence [41]. The extremely invasive character of Tp is well-established and has been described previously by various in vitro and in vivo laboratory studies [39,42,43,44,45,46]. The bacterium enters the body through the entire mucosal surface or skin abrasions during sexual intercourse before clinical manifestations. After entry, Tp unleashes to multiply locally and populate itself from the local infection site through blood and lymphatic vessels [47]. Coupling with host cells and the extracellular matrix is believed to be the initial and crucial step of Tp infection [26,48]. After reaching underneath the epithelium, Tp proliferates locally and disseminates via lymphatic vessels and the bloodstream. The spiral and flat-woven morphological characteristics of Tp make it robust and enable it to ubiquitously infiltrate tissue and vascular barriers in the host’s body. In contrast, its periplasmic mobility gears it farther through front-to-back stir and coordinates in response to poorly explained chemotactic signals. Although it is still unclear how Tp has advantages in invading deep visceral and musculoskeletal tissues, arriving and surviving in distant skin and mucosal sites increase the chances of subsequent transmission [26,45,49].
This extracellular pathogen avoids recognition from the host’s innate and adaptive immune responses. It does not contain lipopolysaccharide (LPS), a primarily proinflammatory glycolipid that is present in Gram-negative bacteria [50]. It is rich in lipoproteins that are adequate for activating macrophages and dendritic cells (DCs) through Toll-like receptor (TLR) 1, TLR2—dependent signaling pathway, and CD14 and mainly come to the lower surface of the outer membrane [51], which is why these pathogen-associated molecular patterns (PAMPs) are recognized as crucial proinflammatory agonists in spirochetal infection. However, Tp has a unique outer membrane (OM) structure. It comprises a scarcity of surface-exposed lipoproteins that empowers Tp to undergo dissemination and remain difficult to identify by the innate immune mechanism. It also explains the shortage of systemic inflammatory symptoms, which is the core characteristic of syphilis [49,51]. These multiple abnormal ultrastructural mechanisms may assist it in infection proliferation, evasion of the immune response, and bacterial persistence [21].
4. Blood–Brain Barrier (BBB) and Central Nervous System (CNS) Crossing by Treponema pallidum towards Neurosyphilis
The neurological disorder’s expression by neuro-invading pathogens is usually connected via infiltration of the blood–brain barrier (BBB) and CNS invasion [52]. The BBB is a structural and functional barrier built by the brain’s microvascular endothelial cells (BMECs), astrocytes, and pericytes (Figure 2) and is one of the compact barriers inside the human body that defends the brain from various infirmities caused by pathogens. Importantly, it maintains the neural microenvironment by controlling molecular travel into and out of the CNS and permitting blood vessels to regulate ions and other molecules strongly. It protects the CNS against blood-borne microorganisms and toxins [53,54,55]. BMECs are essential for imparting nutrients to the brain with tight junctions and using the efflux pump mechanism for unrecognized particles to carry blood circulation [56]. Astrocytes and pericytes assist in properly handling BMECs [53,55]. However, their role in the microbial traversal of the barrier is still not adequately explained. In addition, astrocytes (interaction of endothelial cells via astrocyte end-feet, Figure 2) [57] and microglial cells [55,58] govern hematogenous cell recruitment and provide strength in the translocation of specific pathogens [53]. Diverse microorganisms have the potential to traverse the BBB, infect the CNS, and travel through the BBB Transcellularly (no evidence of Trojan horse disruption), Paracellularly (between the cells), and by infection of phagocytes via the Trojan horse mechanism (within infected phagocytes) [52,59]. Tp is an obligate human pathogen that can rapidly invade the circulatory system and traverse the blood–placenta, blood–retina, and blood–brain barriers [60].
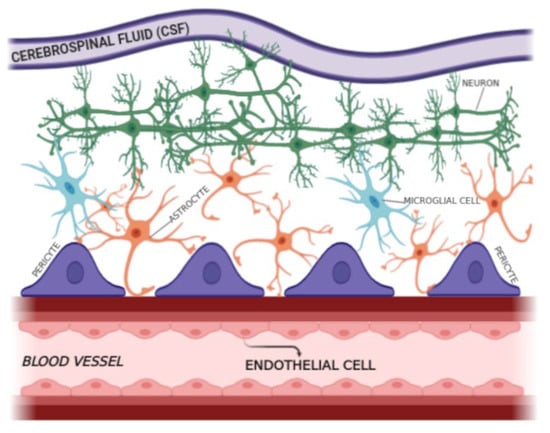
Figure 2.
The BBB is a structural and functional barrier in the human brain.
Syphilis pathogenesis shows Tp’s invasive properties, but the exact tissue invasion model is unknown. Tp could invade the host cells, according to Thomas et al., 1988, who characterized the destination of radio-labeled motile organisms collated to HeLa cell monolayer; 26 percent of treponemes were preceded by monolayer in trypsin-resistant protection, and presumably the monolayer and the surface to which they persisted but did not meet intracellularly. They found that Tp attachment by electrical resistance to cultured human and rabbit aortic and human umbilical vein endothelial cells was 2 times greater than that to HeLa cells. Tp aortic endothelial cells propagated on membrane filters with circumstances in which tight intercellular junctions had formed and Tp was able to walk via endothelial cell monolayers with no evidence of tight junction alteration. Heat-killed Tp and Treponema phagendis (nonpathogenic) biotype failed in the infiltration of the monolayer.
Moreover, transmission electron micrographs demonstrated Tp in intercellular junctions. In vitro examinations enlighten this highly motile spirochete that can leave circulation by penetrating the junctions amid endothelial cells (Figure 3) [45,59]. Additionally, an interaction between the Tp molecule Tp0751 and laminin may encourage tissue invasion [48,61].
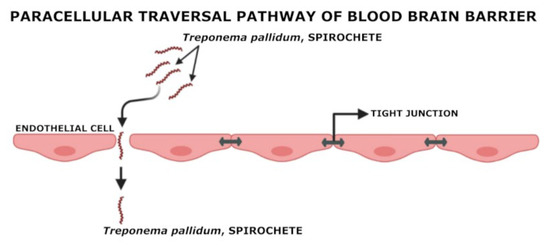
Figure 3.
Paracellular traversal pathway of Tp to cross BBB.
Some different studies have endorsed Tp’s distinct adhesion toward the vascular endothelium and separation of basement membranes. Tp0751 binds to laminin−1, −2, −4, −8, and −10 and amino acids positioned between 98 and 101, 127 and 128, and 182 and 185 in Tp0751 are sensitive to binding with laminin [62,63].
In their study, published in 2020, Lithgow and colleagues reported that recombinant Tp0751 adheres to human endothelial cells of macrovascular and microvascular origin, including a cerebral brain microvascular endothelial cell line of the molecular interactions of Tp0751-mediated adhesion to the vascular endothelium. Endothelial binding is confined to the lipocalin fold-containing domain of the protein, according to adhesion experiments employing recombinant Tp0751 N-terminal truncation. They also used live Tp to confirm this interaction and showed that Tp0751-specific antiserum disrupts spirochete adhesion to endothelium monolayers. Using affinity chromatography, coimmunoprecipitation, and plate-based binding techniques, they also identified the 67-kDa laminin receptor (LamR) as an endothelium receptor for Tp0751. LamR has also been a receptor for the adhesion of other neurotropic invasive bacterial pathogens to brain endothelial cells—including Haemophilus influenzae, Neisseria meningitidis, and Streptococcus pneumoniae—implying the existence of a similar mechanism for extravasation of insidious extracellular bacterial pathogens [64].
Tp is difficult to maintain in vitro for an extended period without the male rabbit testicle [65,66]. With the help of the hyaluronic acid enzyme, Tp removes the connective matrix of the capillary and the auxiliary polysaccharide matrix around the blood vessels. It eventually motivates tissue necrosis ulceration, which macrophages can remove through antibody-mediated phagocytosis [67,68,69]. A recent work performed by Bu-Fang Xu et al. in 2019 [69] demonstrated the critical role of exosomes in pathogenesis and inflammatory diseases, and tumor treatment. Bu-Fang Xu and his fellows analyzed the impact of Tp-induced macrophage-derived exosomes on vascular endothelial cells to identify their involvement in syphilis pathogenesis. Their study demonstrated that exosomes derived from Tp-infected macrophages increased cell adhesion and permeability of vascular endothelial cells and may play a significant role in syphilis pathogenesis.
5. Regulatory T Cell (Treg-Cell) during Neurosyphilis Complications
Regulatory T cells, also known as Treg-cells, are T cells that help prevent autoimmune illness by regulating other immune system cells and controlling the immune response to self and foreign particles (Antigens). Treg-cells are immunosuppressive T cells essential for immune homeostasis, inhibiting autoimmunity, promoting self-tolerance, and working as sensitive inflammatory regulators in different pathological conditions such as autoimmunity and injury, and nervous system degradation [70]. Understanding the potency of the T cell response is an elementary issue in immunology with entanglement for immunity to pathogens, autoimmunity, and immunotherapy. The early effects of the Treg-cell response are depicted by the summation of free antigen signals, costimulation, and cytokines. Treg-cells, CD4+, CD25+, and Foxp3+ cells perform a significant role in immune homeostasis, suppressing self-reactive T cell responses and binding pathogen-directed immune responses before damage occurs [71]. As explained above, Tp subsp. Pallidum—the etiological representative of syphilis—can propagate through any body organ, including the central nervous system (CNS). Neuroinvasion leads to asymptomatic neurosyphilis (ANS), such as gumma of the CNS and acute syphilitic meningitis/meningovascular syphilis as “early symptomatic neurosyphilis”, and it can also cause severe, even irreversible, late symptomatic neurosyphilis (SNS) as parenchymatous syphilis, general paresis, and Tabes dorsalis.
The neurological damage in neurosyphilis complication mechanisms is still not well-explained [13,18,19,72]. As an outcome of Tp infection, mammalian hosts organize substantial cellular and humoral immune responses to clear the Tp spirochete from infected regions. Patients with NS have contaminated CSF Treg-cell collection, and their immunosuppressive cytokines cannot suppress T-cell-mediated inflammatory tissue damage, leading to neurological complications [72,73]. According to Wang et al. in 2014, the Th17 response is probably involved in CNS injury and is associated with clinical symptoms in neurosyphilis patients. They performed flow cytometry analysis and IL-17 level quantification in cerebrospinal fluid with ELISA of neurosyphilis patients. Th17 was screened out of cytometry analysis in peripheral blood from 103 neurosyphilis patients, 69 syphilis patients without neurological symptoms, and 70 healthy donors. After a one-year follow-up, 44 neurosyphilis patients were examined further to determine whether Th17/IL-17 was involved in NS. As a result, a growing frequency of Th17 cells was identified in NS patients’ peripheral blood compared with normal donors. A total of 55.3% of NS patients (average of 2.29 (0 59.83) pg/mL) had IL-17 in CSF, particularly those with symptomatic neurosyphilis (61.9%). CSF IL-17 was obtained from Th17 cells in NS patients. The levels of IL-17 in the CSF of NS patients were certainly associated with the total CSF protein CSF VDRL (Venereal Disease Research Laboratory) titers. Regardless, the importance of the Th17 response in NS is still unclear [73]. In another study by Yu Q et al. in 2017 [72] on the potential role of humoral immunity in NS pathogenesis, the authors studied B-cell infiltration in the CSF of NS patients and the respective intrathecal immunoglobulin levels. As a result, they suggested that the upregulated expression of intrathecal CXCL13 in Treg-cells works as an essential regulator for the recruitment of peripheral B cells into the CNS and activates aberrant antibody responses. The tenacity and function of B cells/immuno-globulin-secreting cells can rely on activated B cells fostering ectopic germinal centers (EGCs) in the CNS of NS patients [72]. Tp can grow to affect the CNS, causing NS. Accumulating evidence suggests that Treg-cells may play a crucial role in syphilis pathogenesis. However, little is known about the Treg-cell response in neurosyphilis complications [73,74].
6. Immunological Changes/Adaptation of Syphilis–HIV Coinfections Associated with Treg-Cell
Syphilis has risen significantly in MSM, particularly in HIV-infected patients at supreme risk for syphilis [75]. Tp and HIV-1 are bidirectionally mutualistic, enhancing disease projections reciprocally in coinfected people, and syphilis patients are 3- to 5-fold more likely to acquire HIV if exposed to the virus via sexual intercourse [75,76,77]. Work published by Guo N et al. in 2019 showed the phenotypic and immunological variations in monocyte subsets and Tregs, and discovered the links between these cell types during Tp/HIV-1 coinfection. They applied cell-staining techniques to find alterations in monocyte subsets, Treg-cells, and any connections between these cells. In their identifications, the frequency of classical monocytes was higher in the rapid plasma regain (RPR+) group than in the healthy controls (HCs) and the chronic HIV-1 infection (CHI) plus RPR+ (CHI and RPR+) group. The frequencies of Foxp3+, CD25+, CD45RA+, and Foxp3+, Helios+, CD45RA+ Treg-cells were notably higher in the RPR+, CHI, and CHI and RPR+ groups than in HCs. However, the frequency of CD45RA+ Treg-cells was lower in the CHI and RPR+ group than in the CHI group. The frequencies of Foxp3+, CD25+, CD45RO+, and Foxp3+, Helios+, CD45RO+ Treg-cells were shorter in the RPR+, CHI, and CHI and RPR+ groups than in HCs. The frequency of intermediate monocytes was negatively correlated with CD45RA+ Tregs and positively correlated with the frequency of CD45RO+ Treg-cells. These results showed that intermediate monocytes control the differentiation of Treg-cell subsets in Tp/HIV-1 coinfection. Their findings provide new insight into an immunological mechanism that involves monocytes/Tregs in HIV-infected individuals with syphilis [76]. However, it is still unclear as to the immunological modifications in monocytes and Treg-cells, and the links between these cell types during syphilis infection among HIV-1-infected MSM [76,78].
7. Multiple Neurosyphilitic Maladies
7.1. Neurosyphilitic Meningitis or Syphilitic Meningitis/Meningovascular Syphilis (MVS)
Chronic and long-term disease and clinical outcomes of infection of the CNS by Tp can occur during any stage of neurosyphilitic meningitis. Approximately 30% of untreated syphilis cases have led to complications in neurology and psychiatry for the last two centuries [15,20]. Nevertheless, modern NS epidemiology is still not well-described because of the lack of data based on population. NS infections have been primarily identified in HIV patients [79], but there have been cases in non-HIV patients. For example, these patients have had both symptomatic and asymptomatic syphilis, and the form of meningitis, with space-occupying gummas, vasculitis, strokes, cranial neuropathy, myelopathy, dementia, and seizures is hard to diagnose [79,80]. Asymptomatic neurosyphilis (ASN) is a type of CNS infection in which patients have confirmed syphilis and have a CSF pleocytosis event (an increase in WBC count in the CSF) but are neurologically asymptomatic. ANS patients with constant infection or without medication are at high risk for disease progression toward symptomatic neurosyphilis (SNS; 35% of ANS patients evolve SNS with the natural progression [81]), notably with symptomatic syphilitic meningitis, MVS, intracranial gummas, general paresis, and tabes dorsalis [20]. Syphilitic meningitis comprises meningeal irritability and increases intracranial pressure, causing headaches, back pain, neck pain, vision problems, nausea, and vomiting before antibiotic discovery. According to Merritt, CSF was classified into three primary forms—hydrocephalic, vertical, and basilar—to classify syphilitic meningitis [16]. Untreated syphilis appears in the secondary and later stages of the infection and implicates all nervous system elements with brain disorders, spinal cord, and cranial and peripheral nerves. With the arrival of antibiotic treatments, NS has relocated its clinical demonstration from chronic and delayed forms, including the CNS parenchyma, to an older way to affect the meninges and CNS blood vessels. These are an unusual pathological representation of inboard NS, MVS, or meningovascular neurosyphilis [15,16,82]. MVS is a distinct form of NS described by a meningo-encephalopathic syndrome with superimposed cerebrovascular or myelovascular incidents and a fusion of chronic syphilitic meningitis and arthritis. MVS is an infection associated with inflammatory arteriopathy, causing outgoing damage to the blood vessels of the leptomeninges, brain, and spinal cord, leading to necrosis [83,84]. MVS can lead to dementia responsible for vascular dementia or hydrocephalus by clogging CSF permeation. The reason for syphilitic dementia is paretic neurosyphilis (or dementia paralytica, or general paralysis of the insane, or general paresis) [85]. Recent cases of MSV are mentioned in Table 2.
7.2. Syphilitic Myelitis (SM)
Syphilitic myelitis (SM) is a rare manifestation of NS caused by Tp. One-third (more than 30%) of early syphilitic patients have CNS manifestations. Recently, the reemergence of syphilis has been seen together with an increment in NS. Nevertheless, symptomatic syphilis, particularly SM and its clinical symptoms, has been occasionally mentioned, and fewer cases have been registered. Initial treatment and diagnosis are vital, as it demonstrates that myelopathy has a curable and perhaps irregular cause [1]. Unfortunately, most cases of SM are misdiagnosed, and there are not many research data available. Some articles describe its clinical manifestations and neuroimaging characteristics, but there are no consistent data on prognosis with long-term follow-up [86,87]. Cases of SM are mentioned in Table 2.
7.3. Cerebral Syphilitic Gumma (CSG)
A gumma is also known as granuloma and is formed during inflammation (immune response against foreign particles or microorganisms). Macrophages do not eliminate chronic disease [28,88]. If the pathogens can be removed, the gumma’s progression can be comprehensively cellular but can sustain growth if its antigens persist. The surrounding epithelial cells vary in lymphocytes in number, macrophages, plasma cells, fibroblasts, and connective tissue scarring, depending on the lesion’s progress stage. The gumma is a highly typical lesion of tertiary syphilis [28] that guides CSG as a demonstration of NS and is recognized to be infrequently included in the brain (first reported by Botalli in 1563 [89]). Due to its rare and miscellaneous features on imaging, CSG is mostly misdiagnosed, is comfortably confused with a brain tumor, and can be seen on any body part; misdiagnosis makes CSG identification difficult [90,91,92]. CSG is the consequence of the cellular immune response secondary to Tp invasion. Generally, it grows from the dura and pia mater (cerebro meningeal). Macroscopically, CSG is discovered as soft, well-defined lesions. It is considered a nonspecific, chronic inflammatory infiltrate, and the formation of an inflammatory “tumor-like” granulation possessing lymphocytes and plasma cells; abnormally, CSG intracerebral determines the problem of differential diagnosis with a malignant cerebral tumor [18,92]. Secret invasion, headache, nausea, and vomiting are the general clinical manifestations of CSG. The pathological signs of CSG are similar to tuberculosis, which comprises sizeable inflammatory intrusion of lymphocytes, plasma cells, and central caseous necrosis edged by epithelioid cells, multinucleated giant cells, and lymphocytes with intimal hyperplasia and peripheral arterial inflammation. CSG can be separated into multiple intracranial lesions growing from the meninges, with asymmetrical growth and surrounding edema that nearly resembles a brain tumor. CSG is infrequently misdiagnosed as a brain neoplasm, which requires surgery [89]. Cases of CSG are mentioned in Table 2.
7.4. Atypical Behavior and Neuropsychiatric Symptoms in NS
Neurology and psychiatry both fight to employ disorders that evade different classifications. NS is one of the most extensive and deadly forms of degenerative mental complications. Neuropsychiatric symptoms of syphilis habitually occur in the late stage, representing tertiary syphilis, presumed as general paresis, also known as general paralysis of the insane paralytic dementia or meningoencephalitis—an outcome of direct Tp invasion into the brain [93,94,95,96,97]. Initial symptoms comprise memory loss, irritability, insomnia, and personality change. A developing dementia malady can grow over many years and lasts as confusion and disorientation, loss of judgment, seizures, and psychiatric symptoms, such as depression, mania, and psychosis. A physical check-up can be expected, but patients generally display various complications. Among them are dysarthria, hypomimia, limb hypotonia, facial and limb intention tremor, hyperreflexia, tabes dorsalis, sensory ataxia, and Argyll Robertson pupils, a small number of these mimic early-onset Alzheimer’s disease [98,99,100]. Cases are mentioned in Table 1.

Table 1.
Reported cases of multiple disorders in neurosyphilitic patients.
Table 1.
Reported cases of multiple disorders in neurosyphilitic patients.
| Complication | Disorders | Case Report/References |
|---|---|---|
| Neurosyphilitic Patients | Attention Deficit Disorder | [101] |
| Anger/Violent Behavior | [102,103,104] | |
| Anxiety | [102] | |
| Bipolar Disorder | [105] | |
| Behavioral/Neuropsychiatric Changes | ||
| Complex Condition | [106] | |
| Drug/Alcohol | [107,108,109] | |
| Dissociative Disorder | [110] | |
| Hearing Disorder | [111,112,113] | |
| Hormonal Disabilities | [114,115] | |
| Memory Loss and Dementia | [116,117,118] | |
| Psychotic Mania and Hypomania | [105,119] | |
| Panic Disorder | ||
| Personality Disorder | [104] | |
| Post-Traumatic Disorder | ||
| Sleep Disorder/Insomnia | [19,120] | |
| Suicidal Thoughts | [102] | |
| Traumatic Brain Injury | ||
| Trigeminal Nerve Dysfunction | [121] | |
| Weight Loss | [122] |

Table 2.
Recent reported specific NS complications cases (MRI—Magnetic resonance imaging, CT—Computed Tomography).
Table 2.
Recent reported specific NS complications cases (MRI—Magnetic resonance imaging, CT—Computed Tomography).
| Types of Neurosyphilis | Age/Sex | Symptoms | Treatment/Recovery | References |
|---|---|---|---|---|
| NS Meningitis, MVS, SM | 31/M | Paresis of upper extremities, Predominantly in the right arm Intense holocranial headache | Crystalline Sodium Penicillin | [79] |
| 28/M | Low CRP (10 mg/L, reference value: <8 mg/L) with HIV-positive | Benzyl-penicillin | [123] | |
| 43/M | Frontal headache, fever, nausea, vomiting, HIV-positive with tuberculous meningitis | Antiretroviral therapy | [82] | |
| 49/M | Recurrent strokes in the left middle cerebral artery territory; dysphasia, higher cognitive deficits, motor deficits, and subsequent infarcts in the right middle cerebral and anterior cerebral artery territories manifest with seizures and behavioral and social problems | Injection procaine penicillin, 1.8–2.4 million units intramuscularly; Probenecid, 500 mg orally | [124] | |
| 24/F | Severe and persistent headache, migraine headache, significant dizziness, vertigo | Benzathine Penicillin G intramuscularly, 1.6 million units | [80] | |
| 43/F | Rash of legs, numbness, and weakness in the bilateral feet; lesions in the cervical and thoracic cord | Penicillin G intravenous, 24 million units | [86] | |
| 19 patients included M and F | Sensory disturbance, paraparesis, urinary retention | Penicillin | [1] | |
| 29/F | Progressive bilateral lower extremities’ numbness and weakness | Penicillin G, 4 million units; dexamethasone, 5 mg | [87] | |
| 63/M | Progressive lumbago, weakness of both lower extremities, bilateral lower-limb weakness with motor power of 4–5, lower-limb hyporeflexia | Ceftriaxone, Methylprednisolone | [125] | |
| CSG | 62/M | Speech disturbance, medical history of hypertension | The clinical diagnosis was a glioma; patients admitted for the surgery | [126] |
| 52/F | Headache with intensity from very mild to severe attacks and dizziness; presence of a metastatic tumor | Water-soluble penicillin-G administered intravenously | [91] | |
| 44/M Bisexual | General fatigue and rash, HIV-positive; later showed headache, nausea, and vomiting; brain mass lesion detected in the right temporal lobe through MRI | Oral amoxicillin; later ceftriaxone intravenous, 2 g | [127] | |
| 45/M | Severe headache, left-sided weakness MRI identified a small lesion near to sagittal sinus in the right frontal lobe; surgery was performed | Intravenous penicillin, 2.5 million units; intramuscular injections of benzathine penicillin, 2.4 million units | [90] | |
| 59/F | Dysarthria showed a mass in the brain; after surgery, fever and rash were reported with infiltration on the chest | Ceftriaxone | [128] | |
| 50/F | The MRI and CT scan identified headaches and speech disturbances (mixed aphasia), left parietal injury, and later left temporal recurrence; the last relapse of the tumor lesion in the left temporal region was identified with MRI | Intravenous benzathine penicillin | [92] | |
| 6 Patients between 32–61/4M-2F | All 6 patients exhibited 10 lesions, nine of which were located in the cerebral hemisphere, primarily in the grey matter identified by MRI neuroimaging; surgery was performed | High dose of penicillin after surgery | [129] | |
| 52/F | MRI identified intermittent headache lasting for 5 months, vomiting, history of hypertension and hyperlipidemia, multiple nodules with evident perilesional edema in the right temporal lobe; severe edema in the brain tissue of the right temporal lobe was also observed; surgery was performed | Penicillin treatment, 18 million units | [89] | |
| 58/M | Extradural cervical spinal syphilitic gumma; the epidural lesion was removed via a posterior approach; brain MRI revealed a cerebro-meningeal syphilitic gumma | An antibiotic regime based on aqueous penicillin G | [18] | |
| 66/M | MRI identified affective disorder, hypomnesia, convulsion, cerebral swelling, hyperintensity in the cortex/subcortex, and multiple lacunar cerebral infarctions. The presence of a pial arteriovenous fistula was also detected by CT angiography | Diazepam was used for convulsion and antibiotic therapy | [130] | |
| 46/M | Numbness of bilateral lower limbs, lower back pain, irregular defecation, homogeneous peripheral enhancement, and the intramedullary nodule was identified at the T7 level with extensive thoracic cord edema; MRI syphilitic gumma was considered | Penicillin G | [131] | |
| 47/M | History of diabetes mellitus, the patient had generalized seizures, multiple brain tumors were identified through MRI, and multiple cerebral syphilitic gummas were diagnosed | High dose of penicillin | [132] |
8. Advantages of Bioinformatics (BI), Computational Neuroscience (CN), and Neuroinformatics (NI) in Neuroscience
Bioinformatics (BI) is a research area where biologists, computer scientists, physicians, mathematicians, and chemists blend their skills and fetch various fields, such as molecular biology, genetics, microbiology, mathematics, chemistry, biochemistry, physics, and informatics. The communication of these fields helps resolve distinct tasks to discover new evidence in complex biological systems to justify health organizations to better comprehend life and disease mechanisms [133,134,135]. BI was initially defined as comparing and databasing the genome (DNA), individual molecules such as RNA and proteins, and modeling the structure and function of existing and newly made proteins. It is a biological concept in terms of biological molecules (DNA, protein). It applies “Information Technology (IT)” obtained from fields on a large scale—e.g., applied mathematics, computer science, and statistics—to better perceive and assemble the information allied with these molecules. In short, BI manages information systems for molecular biology and possesses diverse uses, which lead to several research areas [128,129,130]. Further outbreaks in BI—for example, the vast amount of data generated after the advent of next-generation sequencing technologies and large-scale investigation of the genomes of several microorganisms to humans—formed comprehensive transformations in biology to deem multiple dissimilar complications in humans caused by diverse microorganisms [136,137,138].
The human brain is a complex and biological organ ranging from 3 pounds to 1.4 kg in weight (2% of total body weight) that expends almost 20% of oxygen with a high energy requirement, and its temporal behavior corresponds to development, structure, and function. Coding and automation are crucial to model, analyze, and understand the 86.1 ± 8.1 billion neurons. The non-neuronal glial cells and the human brain’s neuronal networks contain approximately 100 trillion (excitatory and inhibitory synapses) connections. Almost 13% of global disease sets are established by neurological disorders of the human brain (more than the cardiovascular problem representing only 5% of global disease sets). Physiologists, theoretical and experimental physicists, mathematicians, computer scientists, engineers, molecular biologists, physicians, clinicians, BI, psychologists, and philosophers, among others, are all involved in neuroscience study today [24,139].
The computational neuroscience (CN) area has evolved quickly in the last two decades. CN comprises computational (theoretical and mathematical) techniques to comprehend neural events at divergent hierarchical levels of neural organization, as well as how does the human brain make calculations [140]? CN is primarily reliant on biology, physics, mathematics, and computation- and direction-type issues. At the same time, the archival, mounting, and combining of the large amount of data generated by clinical records, scientific articles, and unique databases are drifted by “Neuroinformatics (NI)” [24]. NI is an exceptionally interdisciplinary area of study that engages several techniques and perspectives from the area of computer science, information systems, and integrative biology to identify, analyze, digest, simulate, and compute large-scale neuroscientific data [141] and thus works as an interface between computer science and experimental neuroscience. Although, in a broader scenario, NI includes neuromorphic engineering and computational neuroscience and is connected with software tools and ontologies; database integration, sharing, transformation, visualization; and quantification of neuroscience research. Despite the pioneering rising start from the 1990–2000 “Decade of the Human Brain” [142], the informatics organization’s infrastructure for neuroscience constantly needs adequate advancement. The exceptional diversity of neuroscience, representing multiscale and multimodal study direction by brain complexity and interspecies diversity, has reduced information management culture and data sharing policies. However, these scientific complexities and data heterogeneity challenges make the integration of informatics more imperative for neuroscience [24,143].
9. Application of Computational Neuroscience (CN) and Neuroinformatics (NI) and Their Benefits in Brain Complications
Computational neuroscience (CN) reflects the perspective of making the hypothesis of brain functions about the information processing quality of structures that make up nervous systems. The CN is the analytical study of the brain to discover the theory and procedure that escorts the growth, organization, information processing, and mental functions of the nervous system [24,144]. The CN techniques advance our awareness of brain function and assist in converting the obtained knowledge into technological applications from a scientific viewpoint to identify how the brain thinks. Currently, CN can also specify the brain circuits or network study to determine how the human brain processes multiple actions, as maintained by precise information and properties of structural and functional activities with the assistance of computational strength [24].
Artificial intelligence (AI) underpins cellular and synaptic activities and the biophysical underpinning of neuronal computing and algorithms. AI refers to a computer system’s ability to perform tasks that would generally need human intelligence. In contrast, artificial neural networks (ANN) are computational models built on many small neural units that are utilized in computer science and research (artificial neurons). Roughly analogous to the observed behavior of a biological brain’s axons, the field has provided systemic and computational abilities to simulate artificial human or animal brain models. In doing so, it aims to comprehend how the brain functions and understand cognition and behavior through the inspection of neuronal data by Artificial Brains [145,146]. Neuroinformatics (NI) plays an essential part in neuroscience and clinical research for managing scientific questions and practicing medicine. NI facilitates the secure storage of neuroscience databases and is linked to the development of scientific tools and computer models for data exchange, knowledge integration, and big data analysis in neuroscience [24,136]. NI is also thought to hold more conventional BI and recently renowned computational systems’ biology research for neuroscience. The NI area has grown expeditiously to expand present-time information and communication technology (ICT)-based tools. Briefly, an expansion in NI incorporates (a) the accumulation and organization of neuroscience data (for both wet and dry laboratories) and the development of a knowledge foundation; (b) workflow enlargement to manage data, metadata, and other research-related documents; (c) generation of multipurpose tools, automated data acquisition, analysis, and visualization; (d) the invention of tools and mounting depositories for data distribution; and (e) the advancement of tools for the hypothesis, computation, and simulation of a neural event [23]. In silico modeling in NI may create a new, reciprocal tool for chemical design and potential neurotoxicity predictions (damage to the brain or peripheral nervous system by exposure to natural or artificial toxic substances) and in neurotoxicity, screening to assist in clarifying the early speculations attained in vitro and in vivo. Certified in silico models can be applied in pharmacological target recognition to help connect with in vitro and in vivo studies and, finally, to establish safe chemicals and powerful therapeutic strategies [23] in neurological disorders such as NS complications.
10. Computational Neuroscience and Neuroinformatics in Neurosyphilis Complications
One of the most complex organs in humans is the brain, and the unusual brain behavior in psychiatric or mental illness is still comprehensively unknown. Despite having intended advancements in diagnostics and therapy in several medical areas in the last few decades, our insights into mental deformation are limited and outdated [141]. The bacterium Tp subsp. pallidum, responsible for syphilis, can cause several complications in the CNS and can be recognized in CSF in the early phase of the complication. Neurosyphilis can arise at any time from the first infection, as explained above, with several life-threatening severe psychiatric consequences in diverse brain parts later, for example, in early NS complications—meninges, cerebrospinal fluid, cranial nerves, and vasculature—and late NS complications—the brain with spinal cord parenchyma has been reported [17,147]. One of the milestone studies by Lukehart et al. [37] illustrated that approximately 12 out of 40 patients had neuroinvasion proof in the early phase of the disease compared with the late phase [17,37]. However, CSF examination is still the central component [148]. CN and NI are cutting-edge neuroscience research fields to integrate computer science, physics, and applied mathematics. In this review article, we explain multiple new CN techniques, as explained above. NI techniques (which are already in use for several mental illnesses) can be applied to various NS complications to better understand them. These techniques may also help researchers generate new theories, create computational and simulation environments for modeling, and better understand NS complications. They may also help develop tools for data acquisition, exploration, visualization, and distribution of NS.
10.1. Artificial Intelligence (AI)-, Machine Learning (ML)-, and Deep Learning (DL)-Based Techniques for Neurological Maladies
For the past several decades, medical institutions and healthcare centers have generated significant data, for example, medical imaging (MI) data, genomic information, and free text through screening applications. Exploring these types of data has transformed the previous techniques applied by medical researchers, doctors, and brain scientists to better understand and distinguish brain disease diagnoses from the importance of therapy behaviors. However, this remarkable outcome generated in recent years has increased the volume and complexity of data. Several old computer-based programs and algorithms are not sufficient to handle these datasets [149,150]. In the present scenario, AI (Figure 4) became a significant computer science area and has been massively applied in complex data analysis and in identifying significant and influential connections in given datasets. In neurological complications and the brain supervision domain, AI has been used to innovate diverse applications, accomplished outstanding outcomes, and unlocked various perspectives for disease diagnosis, analysis, and outcome, especially in brain research and related complications [151,152]. Thus, AI approaches have fascinated brain research and CN in recent decades. Amidst these techniques, ML (Figure 4), a subapproach of AI, has gained experience, identified and categorized unknown conditions, learned from earlier experiences using data, and became a famous and comprehensively applied technique to understand brain complications. Currently, DL (Figure 4)—another subarea of AI—has also reformed several neurological assignments. Exclusively, DL algorithms have been enhanced and have become an essential technique in computational vision from other approaches to diverse brain-related image analysis [151,153].
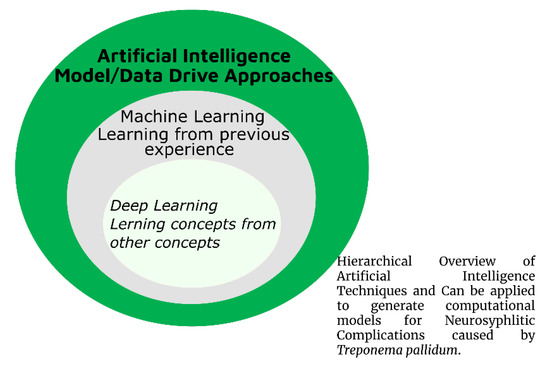
Figure 4.
Hierarchical overview of artificial intelligence (AI).
10.1.1. Computational Models and BBB Permeability Detection in NS
Outstanding computational technique growth has increased the biological and chemical data by a vast amount and played an essential role in chemical and biological research. ML and DL are branches of AI that use mathematical algorithms and statistical strategies to deal with many complications and problems, and the continuous development of ML benefited drug discovery by focusing on the identification, analysis, and prediction of the physicochemical character and biological behavior of the compounds [154]. The BBB is a part of the neurovascular unit (NVU) composed of pericytes, glial cells, neuronal cells, and endothelial cells (ECs) and works as an interface between the brain and CNS, transports essentials (explained above) to the brain cells, and protects the brain against the neuroinvasion of pathogens [155]. Tp’s extremely invasive character is demonstrated by the spirochetes’ extensive vascular dissemination early in the disease’s course, with spirochetes crossing tissue barriers and the highly protected blood–brain, placental, and retinal barriers. A recent study published in 2019 by Wang F. et al. stated that high levels of HbA1c (5.7–64%) may modify the ordinary course of syphilis by increasing the permeability of the BBB and speeding up the progression of NS in syphilis patients with high HbA1c levels. However, because syphilis can mimic any ailment and hence go undetected, it might cause a delay in receiving adequate treatment. Rather than being a historically significant illness, syphilis continues to pose fresh diagnostic and treatment obstacles [156].
A recent study showed that 98% of micro and macro drug molecules could not pass over the BBB. This characteristic points to the direct drug-likeness of a molecule. Thus, novel in silico computational tools are needed in the CNS drug search process that could be less expensive and clinically compatible and can significantly benefit traumatic brain disorders (TBI) and neurological complications [154,157,158]. In recent years, several research works on the BBB have been published. For example, a work published by Chai et al. in 2014 [159] demonstrated that rabies virus infection with the laboratory-attenuated rabies virus (RABV) helps in brain drug penetration, and their research has guided scientists for new drugs in BBB permeability [154,158]. An enormous quantity of research related to the BBB and in silico models has been excellent. The in silico models (Figure 5) identify molecular properties and activities in enhancing the drug discovery process. The primary purpose of in silico models for BBB is to use ML techniques such as support vector machines (SVM) and artificial neural networks (ANN). A recent study published by Singh et al., 2020, developed BBB classification models based on the machine learning algorithm, random forest, multilayer perception, and minimal sequential optimization to enhance the permeability capability of the BBB by using 605 molecules in a large, curated data set with two categorization thresholds. A training set of 479 compounds for threshold-1, 432 compounds for threshold-2, and a test set of 126 compounds for threshold-1 and 110 compounds for threshold-2 were created from the data set. The consensus QSAR methodology was applied to generate and predict BBB models. The consensus model produced better results than individual models and outperformed other available techniques. The BBB compound substructure study found a list of fragments that were more prevalent among BBB compounds than among BBB–compounds. The concord BBB classification model generated in their study was good, and it could be beneficial for the early screening of drugs in discovery projects to determine their likely permeability across the BBB [160].
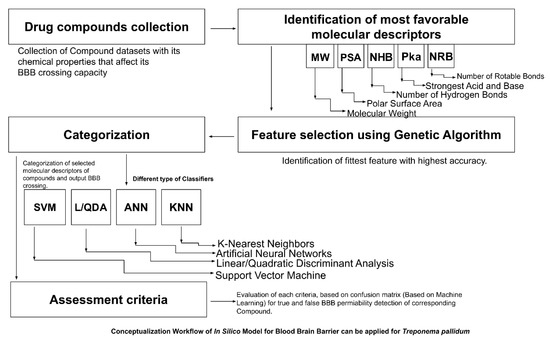
Figure 5.
Workflow of in silico model for blood–brain barrier can be applied for Treponema pallidum.
Several studies have been reviewed in BBB permeability detection through ML and DL algorithms, mainly in image diagnosis when predicting brain drug penetration [154,159] and could also apply for Tp brain invasion.
10.1.2. Computational/In Silico Approaches Based on AI for NS Meningitis or SM
Meningitis is a severe inflammation of the meninges caused by pathogens (mostly- bacteria and viruses). Intense symptoms include fever, headache, neck stiffness, altered consciousness, and vomiting if untreated. Due to its sensitivity, it depends on abnormalities that can affect its activity. Meningitis infection is an example of an abnormal meningeal layer [161]. As explained above, NS meningitis or SM—a rare clinical outcome of infection of the CNS by Tp—can occur during any stage of syphilis and leads to several complications (Table 3); a minimal number of SM cases have been documented. In this section, we explain methods based on AI, and the existing model was used to simulate meningitis caused by various pathogens and can be beneficial to better understanding NS meningitis or SM. Mostafa Langarizadeh et al., 2015, developed the system based on fuzzy logic and a technique for order performance by similarity to an ideal solution (TOPSIS—89% identification precision, 92% singularity, and 97% individual sensitivity) for meningitis in adults. The system was developed to elucidate meningitis with high-level discovery [162,163]. The Bacterial Meningitis Diagnosis System (BMDS) is a mathematical model system established by N. D. Oye and Thomas., in 2019, for diagnosing bacterial meningitis using L. L. MATLAB R2015a, using fuzzy logic to assess bacterial meningitis. Their research aims to use fuzzy logic methods to simulate and construct an expert system for diagnosing bacterial meningitis. The researcher engaged in a series of tasks to achieve the stated goal. Initially, all input variables (symptoms) needed to establish the presence of bacterial meningitis in a patient were determined through interviews with the domain expert, who also defined the system input variables’ membership degree. The system employed the triangle membership function. The triangle fuzzifier was used to fuzzify the input variables, and the fuzzy output was used as the inference input. Mamdani is the inference type. The logical AND operator combined the preceding IF and the subsequent THEN (IF–THEN statements) to build 177 rules that are triggered to produce an output, which became the defuzzifier’s input. The system employs the centroid defuzzification approach, with the system’s output being a graph displayed on the surface viewer. Depending on the input data, different graphs are displayed. A mathematical model was created to determine whether a patient had bacterial meningitis or not. MSSQL Server Management Studio 2012 was used to create the knowledgebase component containing all required data. Microsoft Visual Studio 2015 and the C# object-oriented language were used to develop the expert system for the diagnosis. The system’s features include a home page with three menus, a patient registration page, a patient diagnostic page, a data page with a tabular representation of the patient’s information, and reports with graphical representations depending on gender, age group, and LGA of residence. This study developed a fuzzy model for bacterial meningitis detection and implemented it as a system named the Bacterial Meningitis Diagnosis System (BMDS). BMDS will go a long way toward assisting medical staff in saving the lives of bacterial meningitis sufferers. BMDS can assist medical workers in maintaining a computerized patient record, analyzing the record, and generating reports in a graphical format for easy study. Any approved medical personnel or paramedical workers can utilize the system [164].
Abubakar A. M. et al., 2019, developed an artificial intelligence (AI)-based method to diagnose meningitis. This study uses image processing techniques and an artificial neural network (ANN) to demonstrate a recently created system based on the AI method for the automatic diagnosis of meningitis from Gram-stained sputum smear microscopy pictures. Blood samples were obtained from the patient and placed in a special dish for microorganism growth observation, mainly bacteria, to develop an intelligent method of meningitis diagnosis using ANN employing image processing techniques. Meningitis was also examined using imaging data extracted from patient cerebrospinal fluid (CSF) samples. Due to Gram-staining, meningitis bacilli were segmented by pixel intensity value using a cascade adaptive-threshold-based technique. A multilayer (ML) ANN with scaled conjugate gradient descent backpropagation training approach was utilized to classify the presence or absence of TB bacilli in the preprocessed input image. The system was simulated using MATLAB image processing and Neural Network Toolboxes. The ANN classifier had a mean squared error (MSE) of 0.025 and 94.7 percent accuracy. These findings show that image processing can aid in detecting meningitis bacilli in Gram-stained CSF smear samples [165].
In 2019, Zaccari K. et al. developed a machine learning (ML) approach using multiple algorithms such as adaptative boosting (AdaBoost), decision tree, gradient boosting, K-nearest neighbors (KNN), logistic regression, random forest, and support vector machines (SVM) to identify meningitis in patients in Sao Paulo, Brazil. Their decision tree algorithm was the best, with 94.56% and 96.18% accuracy for the training and testing data, respectively. The outcomes showed valuable help to doctors in early meningitis diagnosis [166]. In 2020, an open and coordinated clinical choice emotionally supportive network was developed by Viviane M. L. et al. to examine meningitis based on tree-based machine learning, also known as decision trees, with 88% detection accuracy in patients [167]. Work published by Solomon O. A. et al. in 2020 developed an AI-based method known as Bayesian belief network (which gains experience based on experience). They simulated a model to identify patients’ meningococcal meningitis and serogroup types. Their model had 99.99% prediction accuracy with 97.12% susceptibility to meningococcal meningitis. The model also showed 95.42% sensitivity with Neisseria meningitidis serogroup types A, B, and C [168].
10.1.3. Computational/In Silico Modeling for CSG
A gumma (CSG) is also known as granuloma and is formed during inflammation. It is a soft, granulomatous, tumorlike lump that occasionally becomes evident during late syphilis. It primarily exists under the skin and mucous membranes. It can also be seen in bones, nervous systems, multiple tissues, and organs, mimicking a metastatic tumor, also known as primary cancer or primary tumor. CSG is misdiagnosed and often mistaken for a brain neoplasm, leading to surgery [91,127]. Several specialists, researchers, clinicians, and doctors have been trying to predict cancer for many decades based on their personal experiences. After the advent of the digital information period, most scientists and clinicians acknowledge the power and benefits of AI-based techniques (ML and DL) as decision-making supporting tools and techniques, which have been applied to the diagnosis and classification [169]. Recently published work by Huang et al., 2019, used AI to distinguish the optimal prognosis index for brain metastases by ML. In 700 cancer patients with brain metastases, they developed mutual algorithm information and a rough set with particle swarm optimization (MIRSPSO) approaches to forecast a patient’s prognosis with the most remarkable accuracy. A total of 700 cancer patients with brain metastases were enrolled in this trial, and divided into 446 training and 254 testing cohorts. To assess the performance of cancer prognosis for each patient, 7 features and 7 prediction methods were used. The area under the curve (AUC) = 0.978 ± 0.06 forecasts the patient’s prognosis with the highest accuracy using MIRSPSO techniques. MIRSPSO outperformed the classic statistical methods of sequential feature selection (SFS), mutual information with particle swarm optimization (MIPSO), and mutual information with sequential feature selection (MISFS) in terms of AUC by 1.72 percent, 1.29 percent, and 1.83 percent, respectively.
Moreover, in terms of accuracy, sensitivity, and specificity, the clinical performance of the best prognosis outperformed the conventional statistical technique. Clinical applications must find the best machine-learning approaches for predicting overall survival in brain metastases. Machine learning has a far greater accuracy rate than traditional statistical methods and could be a realistic and convenient way to find optimum index markers for clinical usage [169,170].
ML-based techniques for predicting brain metastases have become crucial in clinical applications [170]. AI develops technological systems that can solve complex tasks in systems that would traditionally need human intelligence.
Simeone Marino and colleagues devised a computational biology method in 2015 to determine how macrophages influence bacterial control, polarization, and function in granulomas during mycobacterium TB infection. They created a computational tool in granulomas to explore the mechanisms that drive macrophage polarization, function, and bacterial control. A “macrophage polarization ratio” is a metric to understand how cytokine signaling translates into the polarization of single macrophages in a granuloma, which regulates cellular functions such as antimicrobial activity and cytokine production. The created approach determined the macrophage polarization ratio to understand how cytokine signaling defines single macrophage polarization in a granuloma, which governs cell functions and produces antimicrobial cytokines. After expanding the macrophage polarization ratio to the tissue level, they dubbed the granuloma polarization ratio and explained the mean polarization value for complete granulomas. Their findings show that NF-kB signaling is a more plausible therapeutic target for macrophage polarization during the early stages of infection. They used experimental data from TB granulomas in nonhuman primates to create a computational model that predicted two new and testable hypotheses about macrophage profiles in TB aftermath. First, the temporal dynamics of granuloma polarization ratios are indicative of granuloma outcome. Second, short NF-kB signal activation intervals describe stable necrotic granulomas with low CFU numbers and minor inflammation. These findings imply that NF-kB signaling dynamics could be used as a therapeutic target to enhance M1 polarization early in infection and improve outcomes [171].
AI techniques are useful in specific gene mutation discovery through pathological tumor images. For example, New York University School of Medicine researchers developed DL techniques to analyze pathological images of lung tumors. The group’s DL technique can determine the differences between two different lung cancers (adenocarcinoma and squamous cell carcinoma) with high accuracy and can also discover gene mutations via images [172]. The future model mentioned above and techniques based on AI and in silico computation will help innovate new CSG techniques and systems. The existing AI models are mentioned in Table 3.

Table 3.
In silico models for multiple brain disorders based on AI, AL, and DL.
Table 3.
In silico models for multiple brain disorders based on AI, AL, and DL.
| Different Neurological Complications | In Silico Model, Systems/Techniques | Motive/Brain Complications | Reference |
|---|---|---|---|
| Meningitis | Fuzzy expert system | Bacterial and aseptic meningitis | [163] |
| Fuzzy cognitive map with TOPSIS | Assessment of meningitis ratio in adults | ||
| Based on decision trees | Meningitis diagnosis | [167] | |
| Based on machine learning algorithms | Prediction of meningitis outbreaks in the Nigerian population | [161] | |
| Based on the genetic algorithm and decision tree | Distinguishing between bacterial and viral meningitis | [173] | |
| Mathematical model | Meningococcal meningitis | [174] | |
| Mathematical modeling | Bacterial meningitis transmission dynamics with control measures | [175] | |
| Cancer/Gumma/Granuloma | Mining prognosis index based on AI and ML | To identify the optimum prognosis index for brain metastases | [170] |
| Deep learning | Lung cancer histopathology images | [172] | |
| Atypical Behavior | Bayesian model | To diagnose psychiatric disorders | |
| Artificial neural networks using cerebral perfusion SPECT data | To identify Alzheimer’s | [176] | |
| Dynamical bifurcation model based on learned expectation and asymmetry | Bipolar disorders | [177] | |
| Deep neural networks | Anxiety | [178] | |
| Linear discriminant analysis based on ML | Depression | [179] | |
| Random forest | Healthy aging | [180] | |
| Through ML text analysis | Cognitive distortions | [181] | |
| In silico modeling based on support vector machine | Stress | [182] | |
| Multicenter ML | Schizophrenia | [183] |
10.1.4. In Silico Model and Techniques for Atypical Behavior and Neuropsychiatric Symptoms in NS
Many brain research projects have been supported in the study to better understand psychiatric disorders. Even though psychiatric disorders belong to brain science and research, psychotherapists yet diagnose and treat patients with experiences instead of the diseases’ pathophysiology [184]. As explained above, NS is one of the most extensive and deadly forms of degenerative mental complications. Neuropsychiatric symptoms of syphilis habitually occur in the late stage and demonstrate memory loss, irritability, insomnia, personality change, and mimic Alzheimer’s disease. Recent work published by Dariusz and his colleague in 2019 illustrated the ANN method to diagnose Alzheimer’s disease by utilizing brain single-photon emission computed tomography (SPECT) data. The sensitivity and differentiation studies were 93.8% and 86.1%, respectively, in Alzheimer’s disease diagnosis [176]. Eduardo Nigri and Nivio Ziviani—professors at the Federal University of Minas Gerais Department of Computer Science—and their colleagues released a Deep Convolutional Neural Network, MRI-based computational model for Alzheimer’s Disease Diagnosis in 2020. They proposed a different manner of explanation tailored to the brain scan task. The Swap Test approach was employed in their research. It creates heatmaps that illustrate the parts of the brain that are most symptomatic of Alzheimer’s disease and provide interpretability for the model’s decisions in a format that physicians can comprehend. The method was created especially for registered brain MRI scans, and experimental findings utilizing an axiomatic evaluation suggest that the proposed method is better for explaining Alzheimer’s disease diagnosis using MRI. According to their findings, 2D+C models have higher selectivity numbers, whereas 3D models have somewhat higher continuity numbers. Furthermore, they discovered that the proposed Swap Test explanation methodology identifies regions traditionally associated with Alzheimer’s disease diagnosis by visually evaluating the results [185].
Several techniques have already been developed for psychiatric disorders, demonstrating how AI techniques could help discover biomarkers for psychiatric disorders and atypical behavior in patients. These techniques could be beneficial to treat and diagnose psychiatric disorders related to NS. Detailed techniques are explained in Table 3 for psychiatric disorder identification and treatment.
11. Conclusions
The central nervous system (CNS) plays an essential role in decision making and communication. Formed by nerves, the brain, and the spinal cord, the peripheral nervous system (PNS) manages to regulate and control every step of the daily routine. The CNS has significant anatomic and cellular heterogeneity, and participates deeply in responding to numerous brain functions and behaviors in disease and its pathogenesis during complications. However, acute variation in the brain condition due to inflammation symbolizes CNS complications with neurological pathogens. Treponema pallidum subspecies pallidum was identified almost 500 years ago, causing sexually-transmitted syphilis in people, and if it goes untreated, can cause significant complications in patients. NS is known as the clinical result of Tp infection of the CNS. Due to the lack of susceptible and specific diagnostic tests, diagnosis and identification depend on the clinical conclusion, cerebrospinal fluid (CSF) contrasts, and clinical decision. As there is no standardized and specific diagnostic, NS remains challenging. This review article broadly explained NS, its various complications, and recent identified cases. We have also briefly described BI, NI, CN, in silico models (based on AI, ML, and DL), and their importance and applications. The existing or developed in silico models and tools for various neurological complications with outstanding outcomes help researchers, doctors, and scientists better understand the diseases. These models and techniques may also apply to NS complications. They can lead to a better understanding of NS and treat this life-threatening complication.
Funding
The present work was supported by the State of Minas Gerais Research Foundation (FAPEMIG) to support Post-Doctoral Scholarship (Junior) under grant number RED-00132-16.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the collaboration and assistance of the team members of all involved institutions, the support of the Rede de Ciências Ômicas—RECOM; Minas Gerais State Agency for Research and Development—FAPEMIG; and Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, J.-L.; Wang, W.-X.; Hu, W.-L. Clinical features of syphilitic myelitis with longitudinally extensive myelopathy on spinal magnetic resonance imaging. World J. Clin. Cases 2019, 7, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Klausner, J.D. An Update on the Global Epidemiology of Syphilis. Curr. Epidemiol. Rep. 2018, 5, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Keuning, M.W.; Kamp, G.A.; Schonenberg-Meinema, D.; Dorigo-Zetsma, J.W.; van Zuiden, J.M.; Pajkrt, D. Congenital syphilis, the great imitator—case report and review. Lancet Infect. Dis. 2020, 20, e173–e179. [Google Scholar] [CrossRef]
- Spiteri, G.; Unemo, M.; Mårdh, O.; Amato-Gauci, A.J. The resurgence of syphilis in high-income countries in the 2000s: A focus on Europe. Epidemiol. Infect. 2019, 147, e143. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Arima, Y.; Yamagishi, T.; Nishiki, S.; Kanai, M.; Ishikane, M.; Matsui, T.; Sunagawa, T.; Ohnishi, M.; Oishi, K. Rapid Increase in Reports of Syphilis Associated With Men Who Have Sex With Women and Women Who Have Sex With Men, Japan, 2012 to 2016. Sex. Transm. Dis. 2018, 45, 139–143. [Google Scholar] [CrossRef]
- The Lancet. Congenital syphilis in the USA. Lancet 2018, 392, 1168. [Google Scholar] [CrossRef]
- Hussain, S.A.; Vaidya, R. Congenital Syphilis. Clin. Dermatol. 2021, 2, 143–161. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537087/ (accessed on 13 September 2021).
- WHO. WHO Publishes New Estimates on Congenital Syphilis, (n.d.). Available online: https://www.who.int/news/item/26-02-2019-who-publishes-new-estimates-on-congenital-syphilis (accessed on 13 March 2021).
- Jaiswal, A.K.; Tiwari, S.; Jamal, S.B.; de Castro Oliveira, L.; Alves, L.G.; Azevedo, V.; Ghosh, P.; Oliveira, C.J.F.; Soares, S.C. The pan-genome of Treponema pallidum reveals differences in genome plasticity between subspecies related to venereal and non-venereal syphilis. BMC Genom. 2020, 21, 33. [Google Scholar] [CrossRef]
- Landry, T.; Smyczek, P.; Cooper, R.; Gratrix, J.; Bertholet, L.; Read, R.; Romanowski, B.; Singh, A.E. Retrospective review of tertiary and neurosyphilis cases in Alberta, 1973–2017. BMJ Open 2019, 9, e025995. [Google Scholar] [CrossRef]
- Aral, S.O.; Over, M.; Manhart, L.; Holmes, K.K. Sexually Transmitted Infections, Disease Control Priorities in Developing Countries. 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK11734/ (accessed on 13 September 2021).
- Buitrago-Garcia, D.; Martí-Carvajal, A.J.; Jimenez, A.; Conterno, L.O.; Pardo, R. Antibiotic therapy for adults with neurosyphilis. Cochrane Database Syst. Rev. 2019, 5, CD011399. [Google Scholar] [CrossRef]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.-S.; Radolf, J.D.; Benzaken, A.S. Syphilis. Nat. Rev. Dis. Primers 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, G.; Fang, J.; Liu, H.; Yang, B.; Xu, Y. Spinal Intramedullary Syphilitic Gumma: An Unusual Presentation of Neurosyphilis. World Neurosurg. 2016, 95, 622.e17–622.e23. [Google Scholar] [CrossRef]
- Ropper, A.H. Neurosyphilis. N. Engl. J. Med. 2019, 381, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Koralnik, I.J.; Marra, C.M. Neurosyphilis. Semin. Neurol. 2019, 39, 448–455. [Google Scholar] [CrossRef]
- Tuddenham, S.; Ghanem, K.G. Neurosyphilis: Knowledge Gaps and Controversies. Sex. Transm. Dis. 2018, 45, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mejdoubi, A.; Khoulali, M.; Raouzi, N.; Nasri, S.; Mebrouk, Y.; Oulali, N.; Moufid, F. Neurosyphilis revealed by compressive cervical spine syphilitic gumma: A case report. Spinal Cord Ser. Cases 2020, 6, 56. [Google Scholar] [CrossRef]
- Ghanem, K.G. Review: Neurosyphilis: A Historical Perspective and Review. CNS Neurosci. Ther. 2010, 16, e157–e168. [Google Scholar] [CrossRef]
- Li, W.; Jiang, M.; Xu, D.; Kou, C.; Zhang, L.; Gao, J.; Qin, K.; Wu, W.; Zhang, X. Clinical and Laboratory Characteristics of Symptomatic and Asymptomatic Neurosyphilis in HIV-Negative Patients: A Retrospective Study of 264 Cases. BioMed Res. Int. 2019, 2019, 2426313. [Google Scholar] [CrossRef]
- Houston, S.; Cameron, C.E. Treponema pallidum Dissemination; Facilitating Immune Evasion and Bacterial Persistence. In The Pathogenic Spirochetes: Strategies for Evasion of Host Immunity and Persistence; Springer: Boston, MA, USA, 2012; pp. 3–18. ISBN 9781461454045. [Google Scholar] [CrossRef]
- Costiniuk, C.T.; MacPherson, P.A. Neurocognitive and psychiatric changes as the initial presentation of neurosyphilis. CMAJ 2013, 185, 499–503. [Google Scholar] [CrossRef][Green Version]
- Linne, M.-L. Neuroinformatics and Computational Modelling as Complementary Tools for Neurotoxicology Studies. Basic Clin. Pharm. Toxicol. 2018, 123 (Suppl. S5), 56–61. [Google Scholar] [CrossRef]
- Nayak, L.; Dasgupta, A.; Das, R.; Ghosh, K.; De, R.K. Computational neuroscience and neuroinformatics: Recent progress and resources. J. Biosci. 2018, 43, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- Hook, E.W. Syphilis. Lancet 2017, 389, 1550–1557. [Google Scholar] [CrossRef]
- Izard, J.; Renken, C.; Hsieh, C.E.; Desrosiers, D.C.; Dunham-Ems, S.; la Vake, C.; Gebhardt, L.L.; Limberger, R.J.; Cox, D.L.; Marko, M.; et al. Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete. J. Bacteriol. 2009, 191, 7566–7580. [Google Scholar] [CrossRef]
- Liu, J.; Howell, J.K.; Bradley, S.D.; Zheng, Y.; Zhou, Z.H.; Norris, S.J. Cellular Architecture of Treponema pallidum: Novel Flagellum, Periplasmic Cone, and Cell Envelope as Revealed by Cryo Electron Tomography. J. Mol. Biol. 2010, 403, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.A.; Dabiri, G.; Cribier, B.; Sell, S. The immunopathobiology of syphilis: The manifestations and course of syphilis are determined by the level of delayed-type hypersensitivity. Am. J. Dermatopathol. 2011, 33, 433–460. [Google Scholar] [CrossRef]
- MacCallum, W.G. A Text-Book of Pathology; WB Saunders Company: Philadelphia, PA, USA, 1924. [Google Scholar]
- Tavora, F.; Burke, A. Review of isolated ascending aortitis: Differential diagnosis, including syphilitic, Takayasu’s and giant cell aortitis. Pathology 2006, 38, 302–308. [Google Scholar] [CrossRef]
- Kofman, O. The changing pattern of neurosyphilis. Can. Med. Assoc. J. 1956, 74, 807–812. Available online: https://pubmed.ncbi.nlm.nih.gov/13316674/ (accessed on 13 September 2021).
- Timmermans, M.; Carr, J. Neurosyphilis in the modern era. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1727–1730. [Google Scholar] [CrossRef]
- Hooshmand, H.; Escobar, M.R.; Kopf, S.W. Neurosyphilis: A study of 241 patients. JAMA 1972, 219, 726–729. [Google Scholar] [CrossRef]
- Lukehart, S.A.; Hook, E.W.; Baker-Zander, S.A.; Collier, A.C.; Critchlow, C.W.; Handsfield, H.H. Invasion of the central nervous system by Treponema pallidum: Implications for diagnosis and treatment. Ann. Intern. Med. 1988, 109, 855–862. [Google Scholar] [CrossRef]
- Domantay-Apostol, G.P.; Handog, E.B.; Gabriel, M.T.G. Syphilis: The international challenge of the great imitator. Dermatol. Clin. 2008, 26, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Hook, E.W. The pathogenesis of syphilis: The Great Mimicker, revisited. J. Pathol. 2006, 208, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, X.; Cai, J.; Zhao, F.; Cao, T.; Ning, L.; Luo, C.; Xiao, X.; Liu, S. Recombinant Treponema pallidum protein Tp0768 promotes proinflammatory cytokine secretion of macrophages through ER stress and ROS/NF-κB pathway. Appl. Microbiol. Biotechnol. 2021, 105, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, X.; Shi, M.; Luo, L.; Tao, C. Diagnostic role of CXCL13 and CSF serology in patients with neurosyphilis. Sex. Transm. Infect. 2021, 97, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Embers, M.E. The pathogenic spirochetes: Strategies for evasion of host immunity and persistence. In The Pathogenic Spirochetes: Strategies for Evasion of Host Immunity and Persistence; Springer Science & Business Media: Berlin, Germany, 2013; pp. 1–265. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Milioli, G.; Elias, S.G.; Teixeira, A.L. Pathophysiology of bacterial infection of the central nervous system and its putative role in the pathogenesis of behavioral changes. Rev. Bras. Psiquiatr. 2013, 35, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, A.; Boyadjian, A.; Kelesidis, T. Spirochetal Lipoproteins and Immune Evasion. Front. Immunol. 2017, 8, 364. [Google Scholar] [CrossRef]
- Cumberland, M.C.; Turner, T.B. The rate of multiplication of Treponema pallidum in normal and immune rabbits. Am. J. Syph. Gonorrhea Vener. Dis. 1949, 33, 201–212. [Google Scholar]
- Raiziss, G.W.; Severac, M. Rapidity with which spirochaeta pallida invades the blood stream. Arch. Dermatol. Syphilol. 1937, 35, 1101–1109. [Google Scholar] [CrossRef]
- Riviere, G.R.; Thomas, D.D.; Cobb, C.M. In vitro model of Treponema pallidum invasiveness. Infect. Immun. 1989, 57, 2267–2271. [Google Scholar] [CrossRef]
- Thomas, D.D.; Navab, M.; Haake, D.A.; Fogelman, A.M.; Miller, J.N.; Lovett, M.A. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl. Acad. Sci. USA 1988, 85, 3608–3612. [Google Scholar] [CrossRef]
- Thomas, D.D.; Fogelman, A.M.; Miller, J.N.; Lovett, M.A. Interactions of Treponema pallidum with endothelial cell monolayers. Eur. J. Epidemiol. 1989, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.C.; Hazlett, K.R.; Radolf, J.D. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes Infect. 2002, 4, 1133–1140. [Google Scholar] [CrossRef]
- LaFond, R.E.; Lukehart, S.A. Biological basis for syphilis. Clin. Microbiol. Rev. 2006, 19, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef]
- Fraser, C.M.; Norris, S.J.; Weinstock, G.M.; White, O.; Sutton, G.G.; Dodson, R.; Gwinn, M.; Hickey, E.K.; Clayton, R.; Ketchum, K.A.; et al. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 1998, 281, 375–388. [Google Scholar] [CrossRef]
- Cruz, A.R.; Ramirez, L.G.; Zuluaga, A.V.; Pillay, A.; Abreu, C.; Valencia, C.A.; la Vake, C.; Cervantes, J.L.; Dunham-Ems, S.; Cartun, R.; et al. Immune Evasion and Recognition of the Syphilis Spirochete in Blood and Skin of Secondary Syphilis Patients: Two Immunologically Distinct Compartments. PLoS Negl. Trop. Dis. 2012, 6, e1717. [Google Scholar] [CrossRef] [PubMed]
- Pulzova, L.; Bhide, M.R.; Andrej, K. Pathogen translocation across the blood-brain barrier. FEMS Immunol. Med. Microbiol. 2009, 57, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S. Mechanisms of microbial traversal of the blood–brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- Coureuil, M.; Lecuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood-brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J.; et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Mucke, L.; Eddleston, M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 1993, 7, 1226–1232. [Google Scholar] [CrossRef]
- Tkáčová, Z.; Káňová, E.; Jiménez-Munguía, I.; Čomor, Ľ.; Širochmanová, I.; Bhide, K.; Bhide, M. Crossing the Blood-Brain Barrier by Neuroinvasive Pathogens. Folia Vet. 2018, 62, 44–51. [Google Scholar] [CrossRef][Green Version]
- Church, B.; Wall, E.; Webb, J.R.; Cameron, C.E. Interaction of Treponema pallidum, the syphilis spirochete, with human platelets. PLoS ONE 2019, 14, e0210902. [Google Scholar] [CrossRef]
- Forrester, J.V.; McMenamin, P.G.; Dando, S.J. CNS infection and immune privilege. Nat. Rev. Neurosci. 2018, 19, 655–671. [Google Scholar] [CrossRef]
- Cameron, C.E. Identification of a Treponema pallidum laminin-binding protein. Infect. Immun. 2003, 71, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Houston, S.; Hof, R.; Francescutti, T.; Hawkes, A.; Boulanger, M.J.; Cameron, C.E. Bifunctional role of the Treponema pallidum extracellular matrix binding adhesin Tp0751. Infect. Immun. 2011, 79, 1386–1398. [Google Scholar] [CrossRef]
- Lithgow, K.V.; Church, B.; Gomez, A.; Tsao, E.; Houston, S.; Swayne, L.A.; Cameron, C.E. Identification of the Neuroinvasive Pathogen Host Target, LamR, as an Endothelial Receptor for the Treponema pallidum Adhesin Tp0751. MSphere 2020, 5, e00195-20. [Google Scholar] [CrossRef]
- Sell, S.; Salman, J.; Norris, S.J. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am. J. Pathol. 1985, 118, 248–255. [Google Scholar]
- Edmondson, D.G.; Hu, B.; Norris, S.J. Long-Term In Vitro Culture of the Syphilis Spirochete Treponema pallidum subsp. Pallidum. MBio 2018, 9, e01153-18. [Google Scholar] [CrossRef] [PubMed]
- Pulzová, L.; Mlynárčik, P.; Bencúrová, E.; Bhide, M. It Takes Two to Tango: Protein-Protein Interactions in the Translocation of Pathogens Across. In The Blood-Brain Barrier: New Research, 1st ed.; Montenegro, P.A., Juárez, S.M., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 79–116. [Google Scholar]
- Hawley, K.L.; Cruz, A.R.; Benjamin, S.J.; la Vake, C.J.; Cervantes, J.L.; LeDoyt, M.; Ramirez, L.G.; Mandich, D.; Fiel-Gan, M.; Caimano, M.J.; et al. IFNγ Enhances CD64-Potentiated Phagocytosis of Treponema pallidum Opsonized with Human Syphilitic Serum by Human Macrophages. Front. Immunol. 2017, 8, 1227. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.-F.; Wang, Q.-Q.; Zhang, J.-P.; Hu, W.-L.; Zhang, R.-L. Treponema pallidum induces the activation of endothelial cells via macrophage-derived exosomes. Arch. Dermatol. Res. 2019, 311, 121–130. [Google Scholar] [CrossRef]
- Duffy, S.S.; Keating, B.A.; Perera, C.J.; Moalem-Taylor, G. The role of regulatory T cells in nervous system pathologies. J. Neurosci. Res. 2018, 96, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.R.; Kan, A.; Heinzel, S.; Marchingo, J.M.; Hodgkin, P.D.; Hawkins, E.D. Regulatory T Cells Suppress Effector T Cell Proliferation by Limiting Division Destiny. Front. Immunol. 2018, 96, 2461. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cheng, Y.; Wang, Y.; Wang, C.; Lu, H.; Guan, Z.; Huang, J.; Gong, W.; Shi, M.; Ni, L.; et al. Aberrant Humoral Immune Responses in Neurosyphilis: CXCL13/CXCR5 Play a Pivotal Role for B-Cell Recruitment to the Cerebrospinal Fluid. J. Infect. Dis. 2017, 216, 534–544. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Gao, Z.; Guan, Z.; Lu, H.; Shi, M.; Gao, Y.; Xu, H.; Yang, X.F.; Zhou, P. Increased Interleukin-17 in Peripheral Blood and Cerebrospinal Fluid of Neurosyphilis Patients. PLoS Negl. Trop. Dis. 2014, 8, e3004. [Google Scholar] [CrossRef]
- Li, K.; Wang, C.; Lu, H.; Gu, X.; Guan, Z.; Zhou, P. Regulatory T Cells in Peripheral Blood and Cerebrospinal Fluid of Syphilis Patients with and without Neurological Involvement. PLoS Negl. Trop. Dis. 2013, 7, e2528. [Google Scholar] [CrossRef]
- Santos, A.M.G.; Júnior, V.R.D.S.; Melo, F.L.D.; Aquino, A.E.C.D.A.; Ramos, M.O.A.; Araújo, L.M.; de Lira, C.R.; Sobral, P.M.; Figueiroa, F.; de Melo, H.R.L.; et al. Prevalence and risk factors of syphilis and human immunodeficiency virus co-infection at a university hospital in Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 813–818. [Google Scholar] [CrossRef]
- Guo, N.; Liu, L.; Yang, X.; Song, T.; Li, G.; Li, L.; Jiang, T.; Gao, Y.; Zhang, T.; Su, B.; et al. Immunological Changes in Monocyte Subsets and Their Association With Foxp3+ Regulatory T Cells in HIV-1-Infected Individuals with Syphilis: A Brief Research Report. Front. Immunol. 2019, 10, 714. [Google Scholar] [CrossRef]
- Solomon, H.; Moraes, A.N.; Williams, D.B.; Fotso, A.S.; Duong, Y.T.; Ndongmo, C.B.; Voetsch, A.C.; Patel, H.; Lupoli, K.; McAuley, J.B.; et al. Prevalence and correlates of active syphilis and HIV co-Infection among sexually active persons aged 15–59 years in Zambia: Results from the Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016. PLoS ONE 2020, 15, e0236501. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Osbak, K.K.; Crucitti, T.; Kestens, L. The immunological response to syphilis differs by HIV status; a prospective observational cohort study. BMC Infect. Dis. 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Barragán, E.P.; Hernández, E.U.; Orozco, B.P.; González, M.S. Meningovascular neurosyphilis with basilar artery thrombosis in HIV patient. J. Infect. Public Health 2018, 11, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, S.; Burrascano, J. Neurosyphilis: An Unresolved Case of Meningitis. Case Rep. Infect. Dis. 2015, 2015, 634259. [Google Scholar] [CrossRef]
- Pastuszczak, M.; Zeman, J.; Jaworek, A.K.; Wojas-Pelc, A. Cerebrospinal Fluid Abnormalities in HIV-Negative Patients with Secondary and Early Latent Syphilis and Serum VDRL ≥ 1:32. Indian J. Dermatol. 2013, 58, 325. [Google Scholar] [CrossRef]
- Zamora, J.A.G.; Espinoza, L.A.; Nwanyanwu, R.N. Neurosyphilis with Concomitant Cryptococcal and Tuberculous Meningitis in a Patient with AIDS: Report of a Unique Case. Case Rep. Infect. Dis. 2017, 2017, 4103858. [Google Scholar] [CrossRef]
- Ha, T.; Tadi, P.; Dubensky, L. Neurosyphilis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Thibodeau, R.; Goel, A.; Jafroodifar, A.; Klumpp, M.; Mirchia, K.; Swarnkar, A. Cerebral syphilitic gumma presenting with intracranial gumma and pathologic vertebrae fractures. Radiol. Case Rep. 2021, 16, 916–922. [Google Scholar] [CrossRef]
- Nitrini, R.; de Paiva, A.R.B.; Takada, L.T.; Brucki, S.M.D. Did you rule out neurosyphilis? Dement. Neuropsychol. 2010, 4, 338–345. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W. Neurosyphilis presenting with myelitis-case series and literature review. J. Infect. Chemother. 2020, 26, 296–299. [Google Scholar] [CrossRef]
- Dong, H.; Liu, Z.; Duan, Y.; Li, D.; Qiu, Z.; Liu, Y.; Huang, J.; Wang, C. Syphilitic meningomyelitis misdiagnosed as spinal cord tumor: Case and review. J. Spinal Cord Med. 2019, 44, 789–793. [Google Scholar] [CrossRef]
- Daumas, A.; Coiffard, B.; Chartier, C.; Amara, A.B.; Alingrin, J.; Villani, P.; Mege, J.-L. Defective Granuloma Formation in Elderly Infected Patients. Front. Cell. Infect. Microbiol. 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, J.; Zhang, W.; Xu, Z.; Hou, H. The Application of MR Spectroscopy and MR Perfusion in Cerebral Syphilitic Gumma: A Case Report. Front. Neurosci. 2020, 14, 544802. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.; Huang, K.; Jiang, T.; Zhou, G.; Wu, T. Cerebral syphilitic gumma masquerading as cerebral metastatic tumors: Case report. Neurosurg. Focus 2019, 47, E15. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Li-Min, L. Cerebral syphilis mimicking metastatic tumors: Report and review of the literature. Neurol. India 2018, 66, 1170. [Google Scholar] [CrossRef] [PubMed]
- Barahona, E.A.; Olvera, J.L.N.; Liquidano, M.A.E.; Cobos, A.M.; Arroyo, Á.D.R.; Apo, E.G. Left temporal cerebral syphilitic gumma: Case report and literature review. Rev. Médica Del Hosp. Gen. México 2017, 80, 119–124. [Google Scholar] [CrossRef]
- Beauchemin, P.; Laforce, R. Neurocognitive Changes in Tertiary Neurosyphilis: A Retrospective Chart Review. Can. J. Neurol. Sci. 2014, 41, 452–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crozatti, L.L.; de Brito, M.H.; Lopes, B.N.A.; de Campos, F.P.F. Atypical behavioral and psychiatric symptoms: Neurosyphilis should always be considered. Autops. Case Rep. 2015, 5, 34–47. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Hou, L.; Zhong, X.; Chen, X.; Li, L.; Wu, Z.; Zheng, D.; Zhang, Y.; Tan, Y.; et al. Clinical and neuropsychological characteristics of general paresis misdiagnosed as primary psychiatric disease. BMC Psychiatry 2016, 16, 230. [Google Scholar] [CrossRef]
- Swain, K. ‘Extraordinarily arduous and fraught with danger’: Syphilis, Salvarsan, and general paresis of the insane. Lancet Psychiatry 2018, 5, 702–703. [Google Scholar] [CrossRef]
- Sharma, S.R.; Hussain, M.; Roy, D. General Paresis of Insane: A Forgotten Entity. Neurol. India 2020, 68, 487. [Google Scholar] [CrossRef]
- Read, P.J.; Donovan, B. Clinical aspects of adult syphilis. Intern. Med. J. 2012, 42, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Antunes, A.P.; Dias, M.C.; Verdelho, A. Neuropsychiatric Symptoms in Reversible Dementias; Springer International Publishing: Basel, Switzerland, 2017; pp. 93–139. [Google Scholar] [CrossRef]
- Mehrabian, S.; Raycheva, M.; Traykova, M.; Stankova, T.; Penev, L.; Grigorova, O.; Traykov, L. Neurosyphilis with dementia and bilateral hippocampal atrophy on brain magnetic resonance imaging. BMC Neurol. 2012, 12, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Hsu, J.W.; Huang, K.L.; Bai, Y.M.; Ko, N.Y.; Su, T.P.; Li, C.T.; Lin, W.C.; Tsai, S.J.; Pan, T.L.; et al. Sexually Transmitted Infection Among Adolescents and Young Adults With Attention-Deficit/Hyperactivity Disorder: A Nationwide Longitudinal Study. J. Am. Acad. Child Adolesc. Psychiatry 2018, 57, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Saini, H.S.; Sayre, M.; Saini, I.; Elsharkawy, N. Neurosyphilis Presenting as Intermittent Explosive Disorder and Acute Psychosis. Cureus 2019, 11, e6337. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, S.; Kenyon, C.; Léonard, N.; Vlieghe, E. The big imitator strikes again: A case report of neurosyphilis in a patient with newly diagnosed HIV. Acta Clin. 2017, 72, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Prynn, J.; Hussain, A.; Winnett, A. Diagnosing neurosyphilis: A case of confusion. BMJ Case Rep. 2016, 2016, bcr2016216582. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.H.; Yang, H.J.; Kim, S.H.; Park, J.H.; Yoon, H.-J. Psychotic mania as the solitary manifestation of neurosyphilis. Ann. Gen. Psychiatry 2018, 17, 24. [Google Scholar] [CrossRef]
- Neetu, R.; Sukhani, P.K.; Dubey, R. Neurosyphilis—A Forgotten Disease: Case Reports with Ten Years Follow-Up and Review of Literature. Neurol. India 2020, 68, 889–893. [Google Scholar] [CrossRef]
- Nguyen, A.; Berngard, S.C.; Lopez, J.P.; Jenkins, T.C. A Case of Ocular Syphilis in a 36-Year-Old HIV-Positive Male. Case Rep. Infect. Dis. 2014, 2014, 352047. [Google Scholar] [CrossRef]
- Muylaert, B.; Almeidinha, Y.; Borelli, N.; Esteves, E.; Oliveira, A.R.; Cestari, M.; Garbelini, L.; Eid, R.; Michalany, A.; De Oliveira Filho, J. Malignant syphilis and neurosyphilis in an immunocompetent patient. J. Am. Acad. Dermatol. 2016, 74, AB152. [Google Scholar] [CrossRef]
- Su, S.; Mao, L.; Zhao, J.; Chen, L.; Jing, J.; Cheng, F.; Zhang, L. Epidemics of HIV, HCV and syphilis infection among synthetic drugs only users, heroin-only users and poly-drug users in Southwest China. Sci. Rep. 2018, 8, 6615. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.; Zafar, R.; Riley, V.; Lindesay, J. Neurosyphilis presenting with dissociative symptoms. J. Neurol. Neurosurg. Psychiatry. 1995, 59, 452–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borges, C.R.; de Almeida, S.M.; Sue, K.; Koslyk, J.L.A.; Sato, M.T.; Shiokawa, N.; Teive, H.A.G. Neurosyphilis and ocular syphilis clinical and cerebrospinal fluid characteristics: A case series. Arq. De Neuro-Psiquiatria. 2018, 76, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Arain, Z.; Abbas, Y.; Adams, A. Pediatric otosyphilis—An unusual cause of conductive hearing loss. Radiol. Case Rep. 2020, 15, 65–70. [Google Scholar] [CrossRef]
- De Goffau, M.J.; Doelman, J.C.; van Rijswijk, J.B. Unilateral sudden hearing loss due to otosyphilis. Clin. Pract. 2011, 1, 296–298. [Google Scholar] [CrossRef]
- Eren, F.; Aygül, R.; Ekmekci, H.; Öztürk, Ş. Neurosyphilis Presenting with Ptosis and Diplopia as the First Complaints: Case Report. Turk. J. Neurol. 2018, 24, 330–333. [Google Scholar] [CrossRef]
- Pozzobon, T.; Facchinello, N.; Bossi, F.; Capitani, N.; Benagiano, M.; di Benedetto, G.; Zennaro, C.; West, N.; Codolo, G.; Bernardini, M.; et al. Treponema pallidum (syphilis) antigen TpF1 induces angiogenesis through the activation of the IL-8 pathway. Sci. Rep. 2016, 6, 18785. [Google Scholar] [CrossRef]
- Akinci, E.; Oncu, F.; Topcular, B. Neurosyphilis in Psychiatric Settings: Three Case Reports. Turk. J. Psychiatry 2016, 28, 61–66. [Google Scholar] [CrossRef]
- Tatar, Z.B.; Cansiz, A.; Köksal, A.; Kurt, E. A Case of Neurosyphilis Presenting with Dementia and Psychiatric Symptoms. J. Neuropsychiatry Clin. Neurosci. 2014, 26, E39–E40. [Google Scholar] [CrossRef]
- Blažeković, A.; Ozretić, D.; Habek, M.; Bilić, E.; Borovečki, F. Neurosyphilis: The shape of a rising threat. Int. J. Infect. Dis. 2018, 76, 1–3. [Google Scholar] [CrossRef]
- Chen, S.F.; Wang, L.Y.; Chiang, J.H.; Shen, Y.C. Bipolar Disorder Is Associated With an Increased Risk of Sexually Transmitted Infections: A Nationwide Population-based Cohort Study. Sex. Transm. Dis. 2018, 45, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-R.; Zhang, H.-L.; Huang, S.-J.; Zeng, Y.-L.; Xi, Y.; Guo, X.-J.; Liu, G.-L.; Tong, M.-L.; Zheng, W.-H.; Liu, L.-L.; et al. Psychiatric Manifestations as Primary Symptom of Neurosyphilis Among HIV-Negative Patients. J. Neuropsychiatry Clin. Neurosci. 2014, 26, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, L.J.D.; Marchiori, E.; Bahia, P.R.V. Neurosyphilis manifesting as trigeminal nerve dysfunction. Rev. Soc. Bras. Med. Trop. 2018, 51, 404. [Google Scholar] [CrossRef] [PubMed]
- Bezalely, S.; Jacob, G.; Flusser, G.; Ablin, J. Syphilis: An unusual manifestation? Case Rep. 2014, 2014, bcr2014204871. [Google Scholar] [CrossRef]
- Wagemakers, A.; Hepp, D.; Killestein, J.; Peferoen, L.; Ang, W. Acute syphilitic meningitis in an HIV-infected patient. IDCases 2018, 13, e00423. [Google Scholar] [CrossRef]
- Munshi, S.; Raghunathan, S.K.; Lindeman, I.; Shetty, A.K. Meningovascular syphilis causing recurrent stroke and diagnostic difficulties: A scourge from the past. BMJ Case Rep. 2018, 2018, bcr2018225255. [Google Scholar] [CrossRef]
- He, D.; Jiang, B. Syphilitic myelitis: Magnetic resonance imaging features. Neurol. India 2014, 62, 89–91. [Google Scholar] [CrossRef]
- Xi, Y.; Liang, Z.; Zhang, S.; Chu, M.; Lu, Y. MRI of neurosyphilis presenting as brain tumor: A case report. Radiol. Infect. Dis. 2015, 2, 197–200. [Google Scholar] [CrossRef]
- Koizumi, Y.; Watabe, T.; Ota, Y.; Nakayama, S.I.; Asai, N.; Hagihara, M.; Yuka, Y.; Hiroyuki, S.; Toyonori, T.; Masakazu, T. Cerebral Syphilitic Gumma Can Arise Within Months of Reinfection: A Case of Histologically Proven Treponema pallidum Strain Type 14b/f Infection With Human Immunodeficiency Virus Positivity. Sex. Transm. Dis. 2018, 45, e1–e4. [Google Scholar] [CrossRef]
- Yoon, Y.K.; Kim, M.J.; Chae, Y.S.; Kang, S.-H. Cerebral Syphilitic Gumma Mimicking a Brain Tumor in the Relapse of Secondary Syphilis in a Human Immunodeficiency Virus-Negative Patient. J Korean Neurosurg. Soc. 2013, 53, 197–200. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Tang, G.; Liu, L.; Chen, G. Neuroimaging findings of cerebral syphilitic gumma. Exp. Ther. Med. 2019, 18, 4185–4192. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Han, F. Neurosyphilis complicated with pial arteriovenous fistula: A rare case report. Medicine 2019, 98, e17770. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, Z.; Hou, H. Diagnosis and Treatment of Spinal Syphilitic Gumma: A Case Report. Front. Neurol. 2020, 10, 1352. [Google Scholar] [CrossRef]
- Sasaki, R.; Tanaka, N.; Okazaki, T.; Yonezawa, T. Multiple cerebral syphilitic gummas mimicking brain tumor in a non-HIV-infected patient: A case report. J. Infect. Chemother. 2019, 25, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Luscombe, N.M.; Greenbaum, D.; Gerstein, M. What is bioinformatics? An introduction and overview. Yearb. Med. Inform. 2018, 10, 83–100. [Google Scholar] [CrossRef]
- Kasabov, N. Springer Handbook of Bio-/Neuroinformatics; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Bilotta, M.; Tradigo, G.; Veltri, P. Bioinformatics data models, representation and storage. Encycl. Bioinform. Comput. Biol. ABC Bioinform. 2018, 1, 110–116. [Google Scholar] [CrossRef]
- Morse, T.M. Neuroinformatics: From bioinformatics to databasing the brain. Bioinform. Biol. Insights 2008, 2, BBI.S540. [Google Scholar] [CrossRef]
- Nazipova, N.N.; Isaev, E.A.E.; Kornilov, V.V.; Pervukhin, D.V.E.; Morozova, A.A.E.; Gorbunov, A.A.; Ustinin, M.N. Big Data in Bioinformatics. Mat. Biolog. Bioinform. 2017, 12, 102–119. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Tiwari, S.; Tavares, G.C.; da Silva, W.M.; Oliveira, L.D.; Ibraim, I.C.; Guimarães, L.C.; Gomide, A.C.P.; Jamal, S.B.; Pantoja, Y.; et al. Pan-omics focused to Crick’s central dogma. In Pan-Genomics: Applications, Challenges, and Future Prospects; Elsevier: Amsterdam, Netherlands, 2020. [Google Scholar] [CrossRef]
- Kiernan, M.C. A fine neuroscience vintage. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1–2. [Google Scholar] [CrossRef][Green Version]
- Érdi, P. Teaching computational neuroscience. Cogn. Neurodyn. 2015, 9, 479–485. [Google Scholar] [CrossRef]
- Wood, H. A rapid e-volution. Nat. Rev. Neurol. 2011, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, G.M.; Mirsky, J.S.; Healy, M.D.; Singer, M.S.; Skoufos, E.; Hines, M.S.; Nadkarni, P.M.; Miller, P.L. The Human Brain Project: Neuroinformatics tools for integrating, searching and modeling multidisciplinary neuroscience data. Trends Neurosci. 1998, 21, 460–468. [Google Scholar] [CrossRef]
- Polavaram, S.; Ascoli, G. Neuroinformatics. Scholarpedia 2015, 10. [Google Scholar] [CrossRef]
- Trappenberg, T. Foundations of Computational Neuroscience, 2nd ed; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Thomas, J.I. Current Status of Consciousness Research from the Neuroscience Perspective. Acta Sci. Neurol. 2019, 2, 38–44. [Google Scholar]
- Jangid, A.; Chaudhary, L.; Sharma, K. Computational Neuroscience and Its Applications: A Review. Intell. Energy Manag. Technol. 2021, 1, 159–169. [Google Scholar] [CrossRef]
- Marra, C.M. Other central nervous system infections: Cytomegalovirus, Mycobacterium tuberculosis, and Treponema pallidum. Handb. Clin. Neurol. 2018, 152, 151–166. [Google Scholar] [CrossRef]
- Singh, A.E. Ocular and neurosyphilis: Epidemiology and approach to management. Curr. Opin. Infect. Dis. 2020, 33, 66–72. [Google Scholar] [CrossRef]
- Yasaka, K.; Abe, O. Deep learning and artificial intelligence in radiology: Current applications and future directions. PLoS Med. 2018, 15, e1002707. [Google Scholar] [CrossRef]
- Senders, J.T.; Arnaout, O.; Karhade, A.V.; Dasenbrock, H.H.; Gormley, W.B.; Broekman, M.L.; Smith, T.R. Natural and Artificial Intelligence in Neurosurgery: A Systematic Review. Neurosurgery 2018, 83, 181–192. [Google Scholar] [CrossRef]
- Segato, A.; Marzullo, A.; Calimeri, F.; de Momi, E. Artificial intelligence for brain diseases: A systematic review. APL Bioeng. 2020, 4, 041503. [Google Scholar] [CrossRef]
- Agrebi, S.; Larbi, A. Use of artificial intelligence in infectious diseases. Artif. Intell. Precis. Health 2020, 415–438. [Google Scholar] [CrossRef]
- Valliani, A.A.-A.; Ranti, D.; Oermann, E.K. Deep Learning and Neurology: A Systematic Review. Neurol. Ther. 2019, 8, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Alsenan, S.; Al-Turaiki, I.; Hafez, A. A Recurrent Neural Network model to predict blood–brain barrier permeability. Comput. Biol. Chem. 2020, 89, 107377. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. In-vitro blood–brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb. Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ge, H.; Su, X.; Wang, R.; Zeng, J.; Miao, J. High HbA1c level is correlated with blood-brain barrier disruption in syphilis patients. Neurol. Sci. 2019, 41, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.-O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood–brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Saber, R.; Rihana, S.; Mhanna, R. In silico and in vitro Blood-Brain Barrier models for early stage drug discovery. In Proceedings of the International Conference on Advances in Biomedical Engineering, ICABME, Tripoli, Lebanon, 17–19 October 2019. [Google Scholar] [CrossRef]
- Chai, Q.; He, W.Q.; Zhou, M.; Lu, H.; Fu, Z.F. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J. Virol. 2014, 88, 4698–4710. [Google Scholar] [CrossRef]
- Singh, M.; Divakaran, R.; Konda, L.S.K.; Kristam, R. A classification model for blood brain barrier penetration. J. Mol. Graph. Model. 2020, 96, 107516. [Google Scholar] [CrossRef]
- Tian, X.; Xu, Q.; Wang, Y. Prediction of Meningitis Outbreaks in Nigeria Using Machine Learning Algorithms. In Proceedings of the 2019 2nd Artificial Intelligence and Cloud Computing Conference, Kobe, Japan, 21–23 December 2019. [Google Scholar] [CrossRef]
- Merline, W. Risk Factors of Meningitis in Adults-An Analysis Using Fuzzy Cognitive Map with TOPSIS. Int. J. Sci. Innov. Math. Res. 2014, 2, 418–425. Available online: www.arcjournals.org (accessed on 15 September 2021).
- Langarizadeh, M.; Khajehpour, E.; Khajehpour, H.; Farokhnia, M.; Eftekhari, M. A Fuzzy Expert System for Distinguishing between Bacterial and Aseptic Meningitis. Iran. J. Med. Phys. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Oye, N.D.; Thomas, L.L. Fuzzy Model for Diagnosis of Bacterial Meningitis. Int. J. Comput. Appl. Technol. Res. 2019, 8, 33–51. [Google Scholar] [CrossRef]
- Abubakar, A.M.; Abdulsalam, K.A.; Adebisi, J.A. Application of Artificial Neural Network for Diagnosis of Cerebrospinal Meningitis. J. Eng. Res. 2019, 24, 12–25. Available online: http://jer.unilag.edu.ng/article/view/575 (accessed on 15 September 2021).
- Zaccari, K.; Marujo, E.C. Machine Learning for Aiding Meningitis Diagnosis in Pediatric Patients. Int. J. Med. Health Sci. 2019, 13, 411–419. [Google Scholar] [CrossRef]
- Lelis, V.M.; Guzman, E.; Belmonte, M.V. Non-invasive meningitis diagnosis using decision trees. IEEE Access 2020, 8, 18394–18407. [Google Scholar] [CrossRef]
- Alile, S.; Bello, M. A Machine Learning Approach for Diagnosing Meningococcal Meningitis. Int. J. Sci. Res. Comput. Sci. Eng. 2020, 8, 13–25. [Google Scholar]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. Cancer Lett. 2020, 471, 61–71. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Mining Prognosis Index of Brain Metastases Using Artificial Intelligence. Cancers 2019, 11, 1140. [Google Scholar] [CrossRef]
- Marino, S.; Cilfone, N.A.; Mattila, J.T.; Linderman, J.J.; Flynn, J.L.; Kirschner, D.E. Macrophage polarization drives granuloma outcome during Mycobacterium tuberculosis infection. Infect. Immun. 2015, 83, 324–338. [Google Scholar] [CrossRef]
- Coudray, N.; Ocampo, P.S.; Sakellaropoulos, T.; Narula, N.; Snuderl, M.; Fenyö, D.; Moreira, A.L.; Razavian, N.; Tsirigos, A. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat. Med. 2018, 24, 1559–1567. [Google Scholar] [CrossRef]
- D’Angelo, G.; Pilla, R.; Tascini, C.; Rampone, S. A proposal for distinguishing between bacterial and viral meningitis using genetic programming and decision trees. Soft Comput. 2019, 23, 11775–11791. [Google Scholar] [CrossRef]
- Martínez, M.J.F.; Merino, E.G.; Sánchez, E.G.; Sánchez, J.E.G.; del Rey, A.M.; Sánchez, G.R. A Mathematical Model to Study the Meningococcal Meningitis. Procedia Comput. Sci. 2013, 18, 2492–2495. [Google Scholar] [CrossRef]
- Asamoah, J.K.K.; Nyabadza, F.; Seidu, B.; Chand, M.; Dutta, H. Mathematical Modelling of Bacterial Meningitis Transmission Dynamics with Control Measures. Comput. Math. Methods Med. 2018, 2018, 2657461. [Google Scholar] [CrossRef]
- Świetlik, D.; Białowąs, J. Application of Artificial Neural Networks to Identify Alzheimer’s Disease Using Cerebral Perfusion SPECT Data. Int. J. Environ. Res. Public Health 2019, 16, 1303. [Google Scholar] [CrossRef]
- Chang, S.-S.; Chou, T. A Dynamical Bifurcation Model of Bipolar Disorder Based on Learned Expectation and Asymmetry in Mood Sensitivity. Comput. Psychiatry 2018, 2, 205. [Google Scholar] [CrossRef]
- Tran, T.; Kavuluru, R. Predicting mental conditions based on “history of present illness” in psychiatric notes with deep neural networks. J. Biomed. Inform. 2017, 75S, S138–S148. [Google Scholar] [CrossRef]
- Khondoker, M.; Dobson, R.; Skirrow, C.; Simmons, A.; Stahl, D. A comparison of machine learning methods for classification using simulation with multiple real data examples from mental health studies. Stat. Methods Med. Res. 2016, 25, 1804–1823. [Google Scholar] [CrossRef]
- Caballero, F.F.; Soulis, G.; Engchuan, W.; Sánchez-Niubó, A.; Arndt, H.; Ayuso-Mateos, J.L.; Haro, J.M.; Chatterji, S.; Panagiotakos, D.B. Advanced analytical methodologies for measuring healthy ageing and its determinants, using factor analysis and machine learning techniques: The ATHLOS project. Sci. Rep. 2017, 7, 43955. [Google Scholar] [CrossRef]
- Simms, T.; Ramstedt, C.; Rich, M.; Richards, M.; Martinez, T.; Giraud-Carrier, C. Detecting Cognitive Distortions Through Machine Learning Text Analytics. In Proceedings of the 2017 IEEE International Conference on Healthcare Informatics, ICHI 2017, Park City, UT, USA, 23–26 August 2017; pp. 508–512. [Google Scholar] [CrossRef]
- SahaKoustuv, D. ChoudhuryMunmun, Modeling Stress with Social Media Around Incidents of Gun Violence on College Campuses. Proc. ACM Hum. Comput. Interact. 2017, 1, 1–27. [Google Scholar] [CrossRef]
- Dluhoš, P.; Schwarz, D.; Cahn, W.; van Haren, N.; Kahn, R.; Španiel, F.; Horáček, J.; Kašpárek, T.; Schnack, H. Multi-center machine learning in imaging psychiatry: A meta-model approach. Neuroimage 2017, 155, 10–24. [Google Scholar] [CrossRef]
- di Liu, G.; Li, Y.C.; Zhang, W.; Zhang, L. A Brief Review of Artificial Intelligence Applications and Algorithms for Psychiatric Disorders. Engineering 2020, 6, 462–467. [Google Scholar] [CrossRef]
- Nigri, E.; Ziviani, N.; Cappabianco, F.; Antunes, A.; Veloso, A. Explainable Deep CNNs for MRI-Based Diagnosis of Alzheimer’s Disease. In Proceedings of the International Joint Conference on Neural Networks, Glasgow, UK, 19–24 July 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).