Abstract
Current lifestyle and environmental factors contribute to obesity development, leading to low-grade chronic inflammation (LGCI). Apart from obesity, LGCI is also related to rheumatic diseases such as osteoporosis (OP) and osteoarthritis (OA). In these, an excessive accumulation of adipose tissue has been linked to an excessive production of proinflammatory factors, such as adipokines. This work’s aim is to stablish the effect of obesity-associated LGCI in major rheumatic diseases and to determine optimal strategies to reduce it. Obesity is a risk factor for developing OA, where a systemic LGCI state has been found. Concretely, obesity-associated LGCI has been described as an OA instauration and progression promoter. To avoid this, several therapeutical approaches (diet control, physical exercise, or nutraceuticals) have been tested. OP is another major rheumatic disease where a basal LGCI has been described, being worsened by obesity. As in OA, diet management and supplementation with vitamin D or probiotics have been proposed as approaches to treat obesity-associated LGCI in this pathology. Currently, the increase in the prevalence of rheumatic diseases is unstoppable. Nonetheless, obesity is a risk factor that can be controlled. Thus, the study of new interventions to control the impact of obesity-associated LGCI is a challenge for the management of patients with rheumatic diseases.
1. Introduction
Lifestyle and environmental factors play a crucial role in health. It is well known that the diet changes recently adopted in our society have had negative effects on our wellbeing [1]. Among these changes, an excess of caloric intake, the poor quality of micro- and macronutrients, such as an increase in saturated fatty acids, trans fats, and simple sugars, as well as a decrease in calcium, magnesium, and B vitamin intake should be highlighted [1]. These alterations contribute to chronic stress and low-grade chronic inflammation (LGCI) [1]. Obesity-related LGCI is also known as metainflammation [2]. Moreover, all these modifications of diet intake have also led to an increase in adiposity, which is related to inflammatory status and contributes to LGCI [1]. LGCI is defined as a persistent and unresolved inflammation, accompanied by a subclinical elevation (2- to 4-fold) of inflammatory cytokines or the presence of specific immune cells in peripheral blood [1]. LGCI is related to many chronic diseases including obesity, osteoporosis (OP), osteoarthritis (OA) [3], and sarcopenia [1]. An excessive local or systemic amount of proinflammatory cytokines, such as interleukin-1 (IL1), interleukin-6 (IL6), or tumor necrosis factor alpha (TNFα), alters bone remodeling by promoting osteoclastogenesis [4,5]. Indeed, the deleterious effects of excessive inflammation on bone metabolism have been previously described [6]. However, the inflammatory environment not only affects bone but also other skeletal tissues including cartilage. In cartilage, proinflammatory cytokines condition the expression of cartilage degradation molecules, such as matrix metalloproteinases (MMPs) or aggrecanases [7,8,9,10], among others.
1.1. Obesity and Obesity-Associated Low-Grade Chronic Inflammation
Obesity has been defined as an excessive accumulation of fat mass that implies a risk to health [11]. It is commonly measured through the Body Mass Index (BMI) that relates the height and the weight of patients, although currently, it has been displaced by waist size, which is a more accurate metric [12]. It is considered that a BMI value higher than 25 implies the patient is overweight, and a BMI value over 30 implies the patient is obese [11]. Regarding waist size, obesity is defined as a waist perimeter greater than 94 cm for men and 80 cm for women [12]. This pathology is considered an uncontrolled epidemic disease, since its numbers are continuously surpassing themselves both in adults and children [11]. Indeed, as the World Health Organization states, nowadays obesity and being overweight are deadlier than being underweight [11].
Obesity and its associated metabolic syndrome have been related to numerous rheumatic diseases [13]. Part of this link has been underpinned by the fact that the adipose tissue acts as an endocrine organ, secreting adipokines (lipocalin 2 (LCN2), adiponectin (ADIPOQ), leptin, and visfatin among others) and proinflammatory cytokines, such as TNFα, IL6, or IL1 [14,15]. Therefore, an excessive accumulation of adipose tissue has been linked to an excessive production of multiple factors that can boost the inflammatory responses typically found in multiple rheumatic diseases. Consistent with this overproduction of inflammatory factors in obesity, the presence of the LGCI environment has also been found in obese patients, which is characterized by increased TNFα levels [16]. Indeed, in this pathological context a relationship between altered bone metabolism and chronic inflammation has been observed [17]. Concretely, in obese mice, bone fractures had a worse evolution with a lower plasma concentration of growth factors and a greater plasma concentration of proinflammatory cytokines, such as TNFα [18].
Obesity has also been associated with significant changes in adipokine levels. Among the most well-known changes in adipokine levels associated with obesity are the increase in the circulating leptin concentration [16] and the decrease in ADIPOQ concentration levels [19]. Leptin is the most studied adipokine, and it controls appetite through the promotion of anorexia [20]. This molecule also exerts an outstanding modulation of the immune system. Its activities include the control of the expression and production of proinflammatory cytokines, such as IL6 and TNFα, by monocytes [21]. Accordingly, this activity has been suggested as a common link between this adipokine and inflammatory pathologies. In contrast to leptin, ADIPOQ levels have been found to be diminished in the blood of obese patients [19]. Nonetheless, they are increased in certain inflammatory pathologies [22] due to their implication in innate immune responses [23]. As with leptin, LCN2 expression and its production is increased under injury or inflammatory conditions [24], and simultaneously, LCN2 has been described to induce the expression of several proinflammatory cytokines [25]. Moreover, it has been pointed out that LCN2 influences the inflammation related to obesity and its comorbidities (type 2 diabetes mellitus, non-alcoholic fatty liver disease, and cardiovascular disorders) [26,27]. Another adipokine dysregulated in obese patients is visfatin, also known as NAMPT (Nicotinamide phosphoribosyltransferase). This adipokine is produced by fat and immune cells, such as β-lymphocyte precursors, among others. Thus, its augmented expression in obesity has not only been linked to the increased amount of adipose tissue in obesity but also to a greater production by immune cells [28,29,30,31]. Indeed, visfatin has been related to the inflammatory state in rheumatic diseases [32].
Aiming for the control of LGCI resulting from obesity, different therapeutical approaches have been studied, including nonpharmacological dietetic manipulation, caloric restriction as a weight loss measure, antioxidant foods, and physical exercise (PE). As a result, given the relevance of the inflammatory state in obese patients, this work’s objective is to establish the effect of obesity-associated LGCI in major rheumatic diseases, as well as to determine the optimal strategies to reduce LGCI.
1.2. Obesity-Associated Low-Grade Chronic Inflammation in Osteoarthritis
Currently, osteoarthritis is the most prevalent rheumatic disease, being the main cause of pain and disability. The main characteristic of this pathology is the intra-articular space narrowing, as a consequence of cartilage degradation in this area [33]. In this pathology, systemic LGCI is observed. The inflammatory component of this pathology has been widely described, and cytokines, such as IL1β, are determinant in its ethology [3,34]. These molecules affect the functional unit formed by articular cartilage and subchondral bone [3,35] causing changes in the relationship between both tissues [3]. IL1β expression is downstream from the biochemical and mechanical loading alterations occurring in the osteoarthritic joint. These modifications not only affect the expression of proinflammatory mediators but also induce the expression of catabolic factors [36,37].
Osteoarthritis is a key factor for an increase in body weight, since the pain and disability caused by the disease cause a sedentary lifestyle, an obesity risk factor [38,39]. Interestingly, the relationship between both pathologies is circular since the increase in body weight and the associated metabolic syndrome contribute to an increase in the mechanical loading and osteoarthritic biochemical alterations [40]. In mice, it has been widely demonstrated how high fat diet (HFD)-induced obesity causes OA, increasing proinflammatory cytokine secretion, cartilage degradation, subchondral bone loss, etc., [41,42,43,44]. Obesity not only affects the OA establishment but also influences OA severity by increasing knee degeneration and proinflammatory cytokine (IL6) production by joint tissues [45]. Additionally, it has been demonstrated that the effects of obesity go beyond individuals, being also heritable. When breeding HFD-induced obese mice, the predisposition to weight gain was increased for up to two generations. Associated with this fact, the female mice in two generations of offspring were more prone to OA evidenced in several tissues [46].
Obesity’s prejudicial effects on OA are not only due to the excessive weight. In a study with human C-reactive protein (CRP) transgenic mice, body weight and OA severity showed no correlation; however, the latter was related to CRP induction [47]. Interestingly, the association between obesity and OA has been also described in nonbearing joints. Concretely, an increase in adipokines, such as adiponectin, resistin, and visfatin have been described with OA [34,48]. Therefore, the influence of obesity on OA could be potentially caused by the maintenance of the LGCI induced by the obesity-altered levels of adipokines and cytokines. Interestingly, the infrapatellar fat pad has arisen as a major adipokine and proinflammatory cytokine producer when compared to subcutaneous fat deposits [49]. This tissue has a more direct effect on the joint than the rest of the adipose tissue and visceral and subcutaneous fat deposits. In obese individuals and HFD-fed mice, the infrapatellar fat pad showed more proinflammatory molecules and adipokine secretion [49]. Moreover, the infrapatellar fat pad release of TNF was elevated in OA patients as compared to healthy patients [49]. However, it is not only fat tissue that is responsible for obesity’s influence on OA. It was determined in rats that high carbohydrate–fat diet-induced obesity caused synovitis prior to degradation of the cartilage, as well as an increase in the macrophage infiltration and proinflammatory profile, evidencing again, the proinflammatory effects of obesity on OA [41]. Moreover, in synovial fluid, obesity’s local effects (high fat and high sucrose diet-induced) caused an increase in the inflammatory molecules’ concentration, thus causing the induction of OA in the joint [50].
Certain studies have related the LGCI observed in obesity with dysregulation in the gut microbiota, and a role in OA development for this situation has been proposed through the increase in proinflammatory molecules that could activate innate immune responses [34].
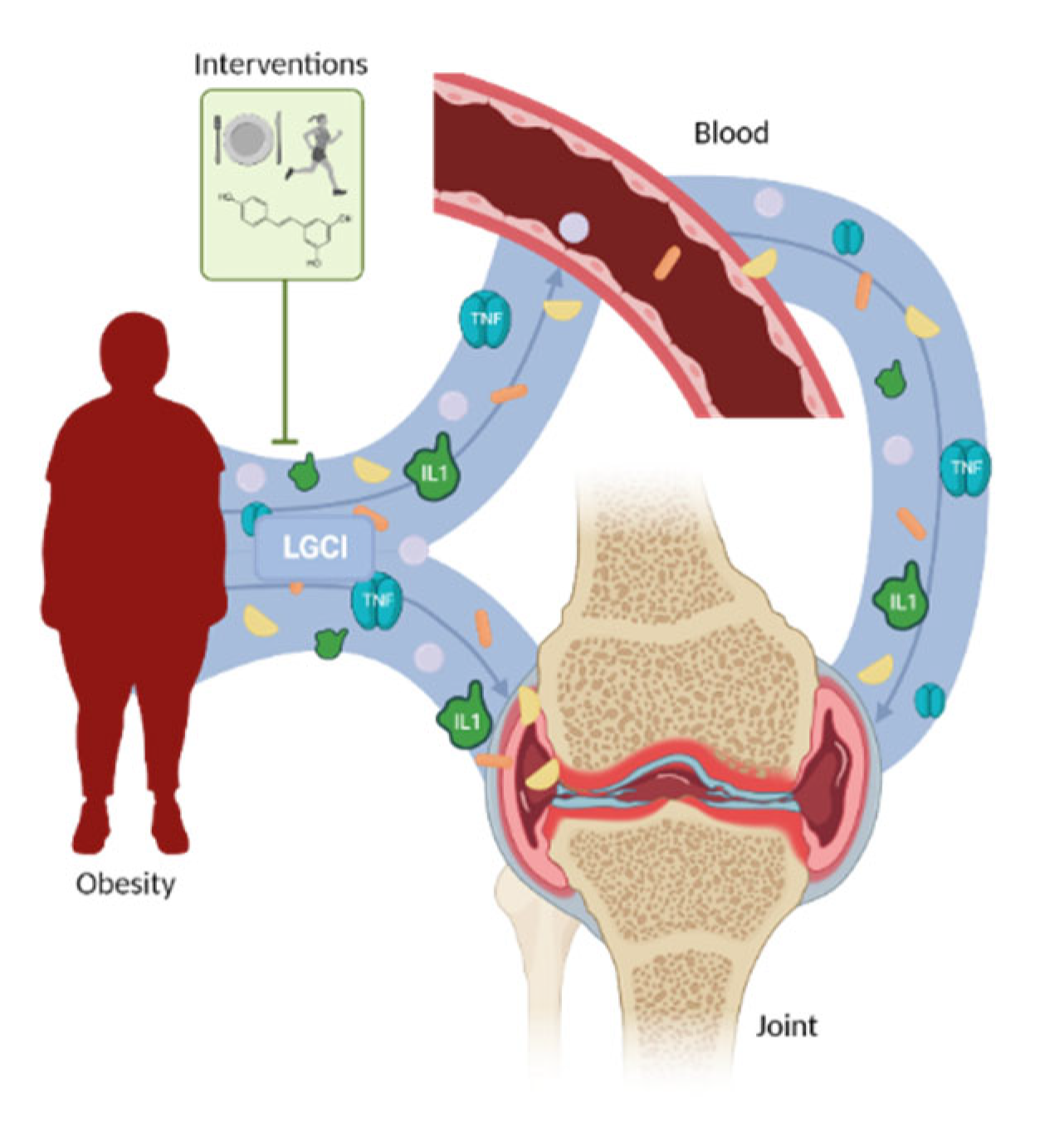
Regulation of obesity-associated LGCI in osteoarthritis is important. Obesity is a chronic disease, but it is also a preventable one. Considering how obesity promotes OA through both a contribution to the LGCI and the increase in joint loading, several approaches have been studied, with the most relevant being diet, PE, and the combination of both (Figure 1). Exercise as wheel running retarded HFD-induced OA progression in mice with obesity [44]. In addition, this exercise also impaired proinflammatory cytokine production, which has been associated with an improvement in glucose tolerance [44]. In a clinical study with obese and overweight African Americans, McLeod et al. observed how the combination of PE and diet ameliorated insulin sensitivity and fat accumulation [51]. Interestingly, PE itself was equally successful as diet+PE when referring to changes in inflammation parameters [51]. Diet itself has also been proposed as a non-pharmacological approach to treat obesity-associated OA. In another study with OA patients, the disease index (Western Ontario and McMaster Universities Osteoarthritis Index) correlated positively with BMI and IL6 concentrations and also correlated between them [52]. As expected, adhesion to a healthy diet was inversely related to the BMI and the body fat percentage. Additionally, this high-quality diet was also negatively correlated with the IL6 concentration [52]. The serum concentration of the markers of collagen I, II, and III MMP-mediated degradation were measured following an intervention with diet, PE, and diet+PE during 18 months in obese osteoarthritic patients. The data showed how diet and the combination of diet+PE diminished weight and markers of cartilage degradation, thus affecting both obesity and OA [53]. Messier et al. also determined the beneficial effects of the combination of diet and PE on both obesity and OA [54]. The results obtained were similar to those from Loeser et al. [53], with diet and diet+PE as the most effective approaches to reduce inflammatory mediators, such as the IL6 concentration [54]. These results suggest that PE, ideally combined with diet, improves the LGCI associated with obesity and thus the OA markers and symptoms.
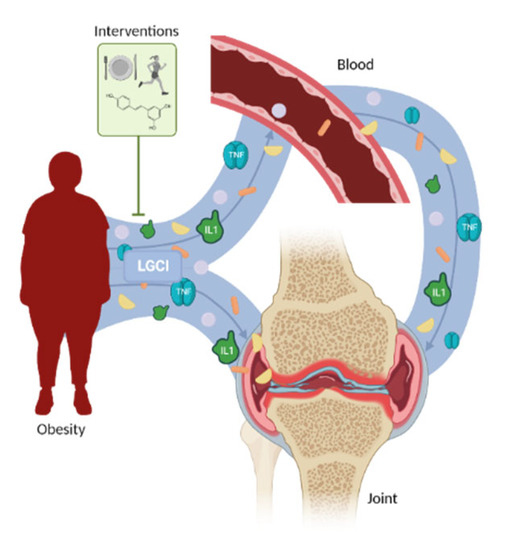
Figure 1.
Relation between obesity-associated low-grade chronic inflammation and osteoarthritis. The proinflammatory molecules secreted systemically and locally by adipose tissue promote osteoarthritis instauration and progression. This effect could be modulated by pharmacological and non-pharmacological approaches.
In addition to the non-pharmacological approaches as diet and PE, some pharmacological treatments have been tested to reduce obesity-induced OA LGCI. Asiatic acid (AA) impeded the NFκB route in human chondrocytes, impairing the induced inflammation and degradation of the extracellular matrix [55]. In vivo, AA administration reduced proinflammatory and adipokine levels in obese mice, and it also averted the instauration of obesity-associated OA, inhibiting the TLR4-mediated innate immune responses [55]. Other drugs were tested, such as follistatin (FST), which diminished the joint synovial fluid concentration of obesity-induced proinflammatory cytokines and adipokines in mice [56]. In addition, FST also exerted a protective effect against post-traumatic OA [56]. Resveratrol was also proposed as a treatment for obesity-induced OA. Despite not affecting weight, resveratrol controlled proinflammatory cytokine and adipokine levels classically associated with obesity-induced OA, such as IL1β, and leptin in obese mice treated with two different doses [57]. Similar to AA, resveratrol’s mechanism of action was described to involve the blockade of TLR4-mediated innate immune responses [57]. Among the natural extracts tested, grape seed proanthocyanidin extract (GSPE) showed interesting results. In diet-induced obesity (DIO) mice, this extract administration blocked obesity development, improving the associated fat and inflammatory profiles [58]. It also slowed arthritis progression in obese collagen-induced arthritis mice, through the regulation of immune cell responses and local inflammatory mediators’ concentrations [58].
Interestingly, some drugs associated with therapeutic weight loss have showed anti-inflammatory and anticatabolic effects on OA [59,60]. Metformin decreased proinflammatory cytokine expression, such as IL4 and IL1β, in primary chondrocytes [61,62]. This antidiabetic drug also showed chondroprotective effects through the increase in the main components of the extracellular matrix [62,63,64,65] and the decrease in the expression of disintegrin and metalloproteinases (ADAMs) [61,64]. Liraglutide, a glucagon-like peptide 1 (GLP-1) receptor agonist, also evidenced anti-inflammatory effects by reducing the IL6 and NFκB pathway expression in chondrocytes [66,67,68]. It has been suggested as a potential drug to target OA due to its effects in decreasing the catabolic activity of MMPs and aggrecanases [66,67,68].
1.3. Obesity-Associated Low-Grade Chronic Inflammation in Osteoporosis
OP is the most prevalent bone pathology. It is characterized by a loss of bone mineral density and the bony architecture, caused primarily by bone remodeling alterations. This mainly leads to bone fragility and fracture proneness. As with most rheumatic diseases, OP is highly related to age and sex, with a higher prevalence in older women [69,70]. In primary OP, caused either by menopause or aging, the regulation exerted by estrogens on IL1, TNFα, and IL6 [71,72] is lost [73]. Thus, the increase in the concentration of proinflammatory cytokines has been associated with osteoclastogenesis promotion [4]. These molecules stimulate osteoblast receptor activator of nuclear kappa-B ligand (RANKL) secretion [4,5]. Specifically, it was reported that TNFα and IL1β worked synergically to promote bone resorption through the promotion of osteoclastogenesis in a direct and indirect manner [4,5]. Indeed, in several OP animal models, deletion of key cytokines receptors, such as the IL1 receptor or the TNFα receptor, drastically diminished bone loss [6]. TNFα specifically affects bone growth directly affecting the growth plate, with this effect being reverted by its inhibitor [74]. Prolonged exposure to this cytokine in mice caused bone alterations similar to those presented in rheumatoid arthritis (RA) [75].
It has been suggested that obesity is a protective factor towards OP [76,77,78]. This mistaken interpretation is led by the fact that the mechanical loading increase, caused by the increase in weight in these patients, promotes bone mineral density through the activation of the Wnt-wingless (WNT) pathway, among other mechanisms [79,80,81]. However, it has been described that the presence of obesity in childhood diminishes the bone mass peak critical in this stage [82]. Moreover, in obese mice and humans the increased amount of fat mass is related to a lower bone quality [83,84,85,86]. Thus, the LGCI present in obesity has been suggested as a contributing factor to the reduction in bone mineral density and thus OP development [4,87].
Due to the impact of diet and inflammation on bone health, studies have pointed to diet management as a key factor in the prevention of bone-related diseases such as OP [88,89]. Thus, n-3 polyunsaturated fatty acids’ (PUFAs) consumption has been described as a bone health promoter due to their capacity to decrease LGCI [88,89]. In contrast, n-6 PUFAs intake exhibited deleterious effects on bones, since its consumption contributed to LGCI and an increase in reactive oxygen species. Moreover, these factors impacted mesenchymal stem cells’ (MSC) differentiation towards adipogenesis [88,89]. Hence, the n-6/n-3 PUFAs ratio has been suggested as an interesting tool to manage LGCI in the context of bone health [88,89].
Other studies have proposed vitamin D supplementation during pregnancy as a promoter of bone mineral content [90]. However, its impact on bone structure has not been elucidated yet [90]. Finally, probiotics could be a potential tool to prevent OP due to their capacity to increase calcium absorption and thus improve bone density [91]. Nonetheless, the influence of these treatments in LGCI has not been described so far. Notwithstanding, it is important to notice that a decrease in vitamin D levels has been described in obese patients [92] and recently related to chronic inflammation [93]. Thus, it could be a potential obesity-LGCI treatment in rheumatic diseases.
OP pharmacological tools are scarce. Among them, selective estrogen receptor modulators (SERMs) have demonstrated a positive effect on decreasing body weight and thus obesity [94]. Concretely, Raloxifene and its association with shock waves showed beneficial effects controlling OP in ovariectomized rats by decreasing the obesity-associated LGCI [95]. Surprisingly, the bisphosphonate clodronate liposome also had an antiobesity effect by limiting energy intake. Nonetheless, this process was associated with deleterious side effects that impair its use in this disease [96,97].
1.4. Obesity-Associated Low-Grade Chronic Inflammation in Other Rheumatic Diseases
A good example of inflammatory rheumatic disease is RA. Nonetheless, the inflammatory profile in this pathology significantly differs from the LGCI present in diseases including obesity, OA, and OP. The levels of serum proinflammatory cytokine concentrations are lower in OA patients than in RA patients, as well as in synovial fluid [98,99]. Moreover, the inflammatory environment and the mechanisms involved in it also vary [100]. Nonetheless, RA has also been related to obesity, with the activity of this pathology being higher in obese patients [101]. CRP, a major rheumatic proinflammatory indicator, positively correlated with BMI, waist circumference, and pathology activity [101]. Differing from OA, hygienic approaches, such as diet and PE, to control RA inflammatory levels were not successful [102], probably due to the differences between LGCI and acute inflammation in both pathologies.
Gout is another rheumatic pathology with an outstanding inflammatory component. Similarly, it has also been related to obesity, since obesity is one of the major risk factors for this pathology [103]. Surprisingly, in vivo administration of monosodium urate (MSU) crystals to induce gout reduced IL6 and MCP1 macrophage basal and elevated production in obese mice [104]. Nonetheless, in the same subjects, MSU treatment increased the IL1β production similar to that in the control mice [104].
2. Methods
To analyze the impact of diet and its relationship with the inflammatory state, we performed a search in Pubmed using the words “diet”, “inflammation”, “obesity”, and “arthritis” or “osteoporosis” according to Mesh terminology (Table 1 and Table 2).

Table 1.
The inclusion and exclusion criteria used to limit the results to the study object.

Table 2.
The searches performed, with the total results, the accepted results, and the dismissed articles.
3. Conclusions
LGCI is a common denominator in numerous rheumatic diseases [1,2]. The sustained and slightly increased level of proinflammatory cytokines in these conditions provoke alterations along the musculoskeletal system, affecting cartilage integrity [6,7,8,9], bone remodeling and architecture [3,4], and synovia integrity [40]. However, LGCI is not only present in rheumatic diseases. It is a characteristic of metabolic pathologies such as type 2 diabetes mellitus and obesity [25,26]. Obesity and being overweight are major risk factors for rheumatic diseases [12]. Traditionally, their deleterious effects were attributed to the increase in the mechanical loading due to the excess weight present in this pathology [39]. Nonetheless, the higher prevalence of pathologies as OA in nonbearing joints in obese individuals versus healthy ones suggested another mechanism for the predisposition to rheumatic diseases in obese patients [45]. LGCI has been understood as the alteration through which obesity affects the musculoskeletal system, with the mechanism of this damage being the excessive production of proinflammatory cytokines and adipokines by adipose tissue [13,14,15,18,19]. In order to control obesity and the LGCI associated with the most frequent rheumatic diseases, both hygienic and pharmacologic approaches were tested. When comparing the reduction in weight obtained through diet control, PE, or the combination of both, diet and diet+PE emerged as the most effective treatments for weight and fat loss, as well as for the control of the excessive production of proinflammatory cytokines and adipokines in OA patients [43,50,51,52,53] It has been also shown that in aiming to reduce obesity-associated LGCI, weight loss is not crucial; yet, it is beneficial for the patients’ health. Regarding pharmacological approaches, several extracts and fatty acids showed effective results in reducing the LGCI secondary to obesity either in osteoarthritic or in osteoporotic patients [54,55,56,57]. Further studies need to be conducted to evaluate these drugs effect in vivo, even though the obtained results were promising.
As society is aging, all the age-related diseases are increasing in prevalence. Therefore, the increase in rheumatic diseases is, for the moment, unstoppable. Nonetheless, obesity is a risk factor that can be controlled. Thus, the study of new interventions and approaches to control the impact of this disease and its associated LGCI is a challenge for the management of patients with rheumatic diseases.
Author Contributions
Conceptualization, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G.; methodology, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G.; validation, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G.; investigation, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G.; data curation, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G.; writing—original draft preparation, A.A.-P., M.G.-F., M.L.-F., A.P.-P. and A.C.-G.; writing—review and editing: A.A.-P., M.G.-F., M.P.-R., V.L., A.J.-M. and R.G.; visualization, A.A.-P., M.G.-F.; supervision, M.P.-R., V.L., A.J.-M. and R.G.; project administration, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G.; funding acquisition, A.A.-P., M.G.-F., M.L.-F., A.P.-P., A.C.-G., M.P.-R., V.L., A.J.-M. and R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Instituto de Salud Carlos III (ISCIII) (grant numbers PI19/01446 and FI20/00210) and co-funded by the European Union via ”Fondo de Investigación Sanitaria” from Fondo Europeo de Desarrollo Regional (FEDER); Fundación IDIS; Mutua Madrileña Foundation (grant numbers MMA 2018 and MMA 2020) and the Ministry of Science, Innovation, and Education (grant numbers FPU17/01706 and UDC Margarita Salas RS1.UDC.MS06). The support was associated with the following personnel: A.A.-P. (IDIS 2019), M.G.-F. (FPU17/01706), M.L.-F. (FI20/00210). A.P.-P. (MMA 2020), A.C.-G. (Rio Hortega CM20/00186), A.J.-M. (SERGAS no grant number), V.L. (PT20/00009), M.P.-R. (UDC Margarita Salas RSU.UDC.MS06), and R.G. (ISCIII and SERGAS via Miguel Servet II program CPII20/00026).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ilich, J.Z.; Gilman, J.C.; Cvijetic, S.; Boschiero, D. Chronic Stress Contributes to Osteosarcopenic Adiposity via Inflammation and Immune Modulation: The Case for More Precise Nutritional Investigation. Nutrients 2020, 12, 989. [Google Scholar] [CrossRef] [PubMed]
- Chuan, L.; Xu, M.J.; Kepeng, W.; Adler, A.J.; Vella, A.T.; Zhou, B. Macrophage polarization and Metainflammation. Traslational Res. 2018, 191, 29–44. [Google Scholar] [CrossRef]
- Gómez, R.; Villalvilla, A.; Largo, R.; Gualillo, O.; Herrero-Beaumont, G. TLR4 signalling in osteoarthritis-finding targets for candidate DMOADs. Nat. Rev. Rheumatol. 2014, 11, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.; Cooper, M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009, 201, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Clowes, J.A.; Riggs, B.L.; Khosla, S. The role of the immune system in the pathophysiology of osteoporosis. Immunol. Rev. 2005, 208, 207–227. [Google Scholar] [CrossRef]
- Vargas, S.J.; Naprta, A.; Glaccum, M.; Lee, S.K.; Kalinowski, J.; Lorenzo, J.A. Interleukin-6 expression and histomorphometry of bones from mice deficient in receptors for interleukin-1 or tumor necrosis factor. J. Bone Miner. Res. 1996, 11, 1736–1744. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol. 2013, 9, 400–410. [Google Scholar] [CrossRef]
- Husa, M.; Liu-Bryan, R.; Terkeltaub, R. Shifting HIFs in osteoarthritis. Nat. Med. 2010, 16, 641–644. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell. Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Obesity. Available online: https://www.who.int/health-topics/obesity#tab=tab_1 (accessed on 5 September 2022).
- Obesity—NHS. Available online: https://www.nhs.uk/conditions/obesity/ (accessed on 5 September 2022).
- Anandacoomarasamy, A.; Caterson, I.; Sambrook, P.; Fransen, M.; March, L. The impact of obesity on the musculoskeletal system. Int. J. Obesity 2022, 32, 211–222. [Google Scholar] [CrossRef]
- Mraz, M.; Lacinova, Z.; Drapalova, J.; Haluzikova, D.; Horinek, A.; Matoulek, M.; Trachta, P.; Kavalkova, P.; Svacina, S. The effect of very-low-calorie diet on mRNA expression of inflammation-related genes in subcutaneous adipose tissue and peripheral monocytes of obese patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2011, 201, 96. [Google Scholar] [CrossRef]
- Buchowski, M.S.; Hongu, N.; Acra, S.; Wang, L.; Warolin, J.; Roberts, L.J. Effect of modest caloric restriction on oxidative stress in women, a randomized trial. PLoS ONE 2012, 7, e47079. [Google Scholar] [CrossRef]
- Canavan, B.; Salem, R.O.; Schurgin, S.; Koutkia, P.; Lipinska, I.; Laposata, M.; Grinspoon, S. Effects of physiological leptin administration on markers of inflammation, platelet activation, and platelet aggregation during caloric deprivation. J. Clin. Endocrinol. Metab. 2005, 90, 5779–5785. [Google Scholar] [CrossRef]
- Cao, J.J. Effects of obesity on bone metabolism. J. Orthop. Surg. Res. 2011, 6, 30. [Google Scholar] [CrossRef]
- Gao, F.; Lv, T.R.; Zhou, J.C.; Qin, X.D. Effects of obesity on the healing of bone fracture in mice. J. Orthop. Surg. Res. 2018, 13, 145. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Taeda, K.; Miyagawa, J.I.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Ahima, R.S. Revisiting leptin’s role in obesity and weight loss. J. Clin. Investig. 2008, 118, 2380–2383. [Google Scholar] [CrossRef]
- Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 1999, 194, 6–11. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Therapy Insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Rodrigues, A.; Sousa, J.; Coppola, G.; Geschwind, D.; Sousa, N.; Correia-Neves, M.; Palla, J.A. Lipocalin 2 is a Choroid Plexus Acute-Phase Protein. J. Cereb. Blood Flow Metab. 2008, 28, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaun, C.; Rauscher, S.; Gröger, M.; Klinger, M.; Wojta, J.; Neumayer, C.; Huk, I.; et al. Neutrophil Gelatinase-Associated Lipocalin (NGAL) is Associated with Symptomatic Carotid Atherosclerosis and Drives Pro-inflammatory State in Vitro. Eur. J. Vasc. Endovasc. Surg. 2016, 51, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Li, H.; Zhou, M.; Fang, Q.; Bao, Y.; Xu, A.; Jia, W. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab. Res. Rev. 2014, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Abella, V.; Scotece, M.; Conde, J.; Gómez, R.; Lois, A.; Pino, J.; Gómez-Reino, J.J.; Lago, F.; Mobasheri, A.; Gualillo, O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 2015, 20, 565–571. [Google Scholar] [CrossRef]
- Friebe, D.; Neef, M.; Kratzsch, J.; Erbs, S.; Dittrich, K.; Garten, A.; Petzold-Quinque, S.; Blüher, S.; Reinehr, T.; Stumvoll, M.; et al. Leucocytes are a major source of circulating nicotinamide phosphoribosyltransferase (NAMPT)/pre-B cell colony (PBEF)/visfatin linking obesity and inflammation in humans. Diabetologia 2011, 54, 1200–1211. [Google Scholar] [CrossRef]
- Curat, C.A.; Wegner, V.; Sengenès, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumié, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rodríguez, A.; Ramírez, B.; Silva, C.; Rotellar, F.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. Association of increased visfatin/PBEF/NAMPT circulating concentrations and gene expression levels in peripheral blood cells with lipid metabolism and fatty liver in human morbid obesity. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 245–253. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Dorweiler, B.; Cui, D.; Wang, T.; Woo, C.W.; Brunkan, C.S.; Wolberger, C.; Ima, S.; Tabas, I. Extracellular nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J. Biol. Chem. 2008, 283, 34833–34843. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Guillán-Fresco, M.; Alonso-Pérez, A.; Jorge-Mora, A.; Francisco, V.; Gualillo, O.; Gómez, R. Visfatin Connection: Present and Future in Osteoarthritis and Osteoporosis. J. Clin. Med. 2019, 8, 1178. [Google Scholar] [CrossRef]
- Findlay, D.M.; Atkins, G.J. Osteoblast-chondrocyte interactions in osteoarthritis. Curr. Osteoporos. Rep. 2014, 12, 127–134. [Google Scholar] [CrossRef]
- Silvestre, M.P.; Rodrigues, A.M.; Canhão, H.; Teixeira, D.; Calha, C.; Branco, J. Cross-Talk between Diet-Associated Dysbiosis and Hand Osteoarthritis. Nutrients 2020, 12, 3469. [Google Scholar] [CrossRef]
- Sharma, A.R.; Jagga, S.; Lee, S.-S.; Nam, J.-S. Interplay between Cartilage and Subchondral Bone Contributing to Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. Int. J. Mol. Sci. 2013, 14, 19805. [Google Scholar] [CrossRef]
- Sanchez, C.; Pesesse, L.; Gabay, O.; Delcour, J.; Msika, P.; Baudoin, C.; Henrotin, Y. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Care Res. 2012, 64, 1193–1203. [Google Scholar] [CrossRef]
- Zhen, G.; Cao, X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol. Sci. 2014, 35, 227–236. [Google Scholar] [CrossRef]
- Lee, J.; Chang, R.W.; Ehrlich-Jones, L.; Kwoh, C.K.; Nevitt, M.; Semanik, P.A.; Sohn, L.S.M.; Song, J.; Dunlop, D.D. Sedentary Behavior and Physical Function: Objective Evidence From the Osteoarthritis Initiative. Arthritis Care Res. 2015, 67, 366–373. [Google Scholar] [CrossRef]
- Shields, M.; Tremblay, M.S. Sedentary Behaviour and Obesity. 2008. Available online: www.statcan.ca (accessed on 17 April 2022).
- Creamer, P.; Hochberg, M.C. Osteoarthritis. Lancet 1997, 350, 503–509. [Google Scholar] [CrossRef]
- Sun, A.R.J.; Panchal, S.K.; Friis, T.; Sekar, S.; Crawford, R.; Brown, L.; Xiao, Y.; Prasadam, I. Obesity-associated metabolic syndrome spontaneously induces infiltration of pro-inflammatory macrophage in synovium and promotes osteoarthritis. PLoS ONE 2017, 12, e0183693. [Google Scholar] [CrossRef]
- Warmink, K.; Rios, J.L.; van Valkengoed, D.R.; Korthagen, N.M.; Weinans, H. Sprague Dawley Rats Show More Severe Bone Loss, Osteophytosis and Inflammation Compared toWistar Han Rats in a High-Fat, High-Sucrose Diet Model of Joint Damage. Int. J. Mol. Sci. 2022, 23, 3725. [Google Scholar] [CrossRef]
- Jang, W.Y.; Jeong, J.; Kim, S.; Kang, M.; Sung, Y.; Choi, M.; Park, S.; Kim, M.; Kim, S.; Ryoo, Z. Serum amyloid A1 levels and amyloid deposition following a high-fat diet challenge in transgenic mice overexpressing hepatic serum amyloid A1. Appl. Physiol. Nutr. Metab. 2016, 41, 640–648. [Google Scholar] [CrossRef]
- Griffin, T.M.; Huebner, J.L.; Kraus, V.B.; Yan, Z.; Guilak, F. Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: Effects of short-term exercise. Arthritis Rheumatol. 2012, 64, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Louer, C.R.; Furman, B.D.; Huebner, J.L.; Kraus, V.B.; Olson, S.A.; Guilak, F. Diet-induced obesity significantly increases the severity of posttraumatic arthritis in mice. Arthritis Rheumatol. 2012, 64, 3220–3230. [Google Scholar] [CrossRef] [PubMed]
- Harasymowicz, N.S.; Choi, Y.R.; Wu, C.L.; Iannucci, L.; Tang, R.; Guilak, F. Intergenerational Transmission of Diet-Induced Obesity, Metabolic Imbalance, and Osteoarthritis in Mice. Arthritis Rheumatol. 2020, 72, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Gierman, L.M.; van der Ham, F.; Koudijs, A.; Wielinga, P.Y.; Kleemann, R.; Kooistra, T.; Stoop, R.; Kloppenburg, M.; van Osch, G.J.V.M.; Stojanovic-Susulic, V.; et al. Metabolic stress-induced inflammation plays a major role in the development of osteoarthritis in mice. Arthritis Rheumatol. 2012, 64, 1172–1181. [Google Scholar] [CrossRef]
- Yusuf, E.; Nelissen, R.; Ioan-Facsinay, A.; Stojanovic-Susulic, V.; DeGroot, J.; van Osch, G.; Middeldorp, S.; Huizinga, T.; Kloppenburg, M. Association between weight or body mass index and hand osteoarthritis: A systematic review. Ann. Rheum. Dis. 2010, 69, 761–765. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. Osteoarthritis: Inflammation and fibrosis in adipose tissue of osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 325–326. [Google Scholar] [CrossRef]
- Collins, K.H.; Hart, D.A.; Reimer, R.A.; Seerattan, R.A.; Herzog, W. Response to diet-induced obesity produces time-dependent induction and progression of metabolic osteoarthritis in rat knees. J. Orthop. Res. 2016, 34, 1010–1018. [Google Scholar] [CrossRef]
- McLeod, A.; Schiffer, L.; Castellanos, K.; DeMott, A.; Olender, S.; Fitzgibbon, M.; Hughes, S.; Fantuzzi, G.; Tussing-Humphreys, L. Impact of Physical Activity and Weight Loss on Fat Mass, Glucose Metabolism, and Inflammation in Older African Americans with Osteoarthritis. Nutrients 2020, 12, 3299. [Google Scholar] [CrossRef]
- Mears, M.; Tussing-Humphreys, L.; Cerwinske, L.; Tangney, C.; Hughes, S.L.; Fitzgibbons, M.; Gomez-Perez, S. Associations between Alternate Healthy Eating Index-2010, Body Composition, Osteoarthritis Severity, and Interleukin-6 in Older Overweight and Obese African American Females with Self-Reported Osteoarthritis. Nutrients 2018, 11, 26. [Google Scholar] [CrossRef]
- Loeser, R.F.; Beavers, D.P.; Bay-Jensen, A.C.; Karsdal, M.A.; Nicklas, B.J.; Guermazi, A.; Hunter, D.J.; Messiery, S.P. Effects of dietary weight loss with and without exercise on interstitial matrix turnover and tissue inflammation biomarkers in adults with knee osteoarthritis: The Intensive Diet and Exercise for Arthritis trial (IDEA). Osteoarthr. Cartil. 2017, 25, 1822–1828. [Google Scholar] [CrossRef]
- Messier, S.P.; Mhaliko, S.L.; Legault, C.; Miller, G.D.; Nicklas, B.J.; DeVita, P.; Beavers, D.P.; Hunter, D.J.; Lyles, M.F. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: The IDEA randomized clinical trial. JAMA 2013, 310, 1263–1273. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, G.; Hu, Z.; Tang, S.; Xu, J.; Shang, P.; Tang, Q.; Liu, H. Asiatic acid ameliorates obesity-related osteoarthritis by inhibiting myeloid differentiation protein-2. Food Funct. 2020, 11, 5513–5524. [Google Scholar] [CrossRef]
- Tang, R.; Harasymowicz, N.S.; Wu, C.L.; Collins, K.H.; Choi, Y.R.; Oswald, S.J.; Guilak, F. Gene therapy for follistatin mitigates systemic metabolic inflammation and post-traumatic arthritis in high-fat diet-induced obesity. Sci. Adv. 2020, 6, eaaz7492. [Google Scholar] [CrossRef]
- Jiang, M.; Li, X.; Yu, X.; Liu, X.; Xu, X.; He, J.; Gu, H.; Liu, L. Oral Administration of Resveratrol Alleviates Osteoarthritis Pathology in C57BL/6J Mice Model Induced by a High-Fat Diet. Mediat. Inflamm. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Jhun, J.Y.; Moon, S.J.; Yoon, B.Y.; Byun, J.K.; Kim, E.K.; Yang, E.J.; Ju, J.H.; Hong, Y.S.; Min, J.K.; Park, S.H.; et al. Grape seed proanthocyanidin extract-mediated regulation of STAT3 proteins contributes to Treg differentiation and attenuates inflammation in a murine model of obesity-associated arthritis. PLoS ONE 2013, 8, e78843. [Google Scholar] [CrossRef]
- Jing, Y.; Wu, F.; Li, D.; Yang, L.; Li, Q.; Li, R. Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol. Cell. Endocrinol. 2018, 461, 256–264. [Google Scholar] [CrossRef]
- Gudbergsen, H. Liraglutide after diet-induced weight loss for pain and weight control in knee osteoarthritis: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 314–323. [Google Scholar] [CrossRef]
- Schadler, P. The Effect of Body Mass Index and Metformin on Matrix Gene Expression in Arthritic Primary Human Chondrocytes. Cartilage 2021, 13, 1004S–1018S. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Zhang, Y.; Liu, J.; Yao, Z.; Zhang, C. Protective effects of metformin against osteoarthritis through upregulation of SIRT3-mediated PINK1/Parkin-dependent mitophagy in primary chondrocytes. Biosci. Trends 2019, 12, 605–612. [Google Scholar] [CrossRef]
- Xing, H.; Liang, C.; Wang, C.; Xu, X.; Hu, Y.; Qiu, B. Metformin mitigates cholesterol accumulation via the AMPK/SIRT1 pathway to protect osteoarthritis chondrocytes. Biochem. Biophys. Res. Commun. 2022, 632, 113–121. [Google Scholar] [CrossRef]
- Li, H.; Gou, Y.; Tian, F.; Zhang, Y.; Lian, Q.; Hu, Y.; Zhang, L. Combination of metformin and exercise alleviates osteoarthritis in ovariectomized mice fed a high-fat diet. Bone 2022, 157, 116323. [Google Scholar] [CrossRef]
- Li, H.; Xiang, D.; Terkeltaub, R.; Lin, H.; Zhang, Y.; Zhou, B.; He, K.; Li, K.; Liu, Z.; Wei, J.; et al. Exploration of metformin as novel therapy for osteoarthritis: Preventing cartilage degeneration and reducing pain behavior. Arthritis Res. Ther. 2020, 22, 2020. [Google Scholar] [CrossRef]
- Mei, J.; Sun, J.; Wu, J.; Zheng, X. Liraglutide suppresses TNF-α-induced degradation of extracellular matrix in human chondrocytes: A therapeutic implication in osteoarthritis. Am. J. Transl. Res. 2019, 11, 4800. [Google Scholar]
- Chen, J.; Xie, J.; Shi, K.; Gu, Y.; Wu, C.; Xuan, J.; Ren, Y.; Chen, L.; Wu, Y.; Zhang, X.; et al. Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis. 2018, 9, 212. [Google Scholar] [CrossRef]
- Meurot, C.; Martin, C.; Sudre, L.; Breton, J.; Bougault, C.; Rattenbach, R.; Bismuth, K.; Jacques, C.; Berenbaum, F. Liraglutide, a glucagon-like peptide 1 receptor agonist, exerts analgesic, anti-inflammatory and anti-degradative actions in osteoarthritis. Sci. Rep. 2022, 12, 1567. [Google Scholar] [CrossRef]
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61. [Google Scholar] [CrossRef]
- Jilka, R.L. Cytokines, bone remodeling, and estrogen deficiency: A 1998 update. Bone 1998, 23, 75–81. [Google Scholar] [CrossRef]
- Pacifici, R. Cytokines, estrogen, and postmenopausal osteoporosis--the second decade. Endocrinology 1998, 139, 2659–2661. [Google Scholar] [CrossRef]
- Pacifici, R.; Rifas, L.; Teitelbaum, S.; Slatopolsky, E.; McCracken, R.; Bergfeld, M.; Lee, W.; Avioli, L.V.; Peckt, W.A. Spontaneous release of interleukin 1 from human blood monocytes reflects bone formation in idiopathic osteoporosis. Proc. Natl. Acad. Sci. USA 1987, 84, 4616–4620. [Google Scholar] [CrossRef]
- Fernandez-Vojvodich, P.; Palmblad, K.; Karimian, E.; Andersson, U.; Sävendahl, L. Pro-Inflammatory Cytokines Produced by Growth Plate Chondrocytes May Act Locally to Modulate Longitudinal Bone Growth. Horm. Res. Paediatr. 2012, 77, 180–187. [Google Scholar] [CrossRef]
- Guo, R.; Yamashita, M.; Zhang, Q.; Zhou, Q.; Chen, D.; Reynolds, D.G.; Awad, H.A.; Yanoso, L.; Zhao, L.; Schwarz, E.M.; et al. Ubiquitin Ligase Smurf1 Mediates Tumor Necrosis Factor-induced Systemic Bone Loss by Promoting Proteasomal Degradation of Bone Morphogenetic Signaling Proteins. J. Biol. Chem. 2008, 283, 23084. [Google Scholar] [CrossRef]
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef]
- Reid, I.R.; Ames, R.; Evans, M.; Sharpe, S.; Gamble, G.; France, J.; Lim, T.; Cundy, T. Determinants of total body and regional bone mineral density in normal postmenopausal women--a key role for fat mass. J. Clin. Endocrinol. Metab. 1992, 75, 45–51. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in older adults: Technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr. 2005, 82, 923–934. [Google Scholar] [CrossRef]
- Felson, D.T.; Zhang, Y.; Hannan, M.T.; Anderson, J.J. Effects of weight and body mass index on bone mineral density in men and women: The Framingham study. J. Bone Miner. Res. 1993, 8, 567–573. [Google Scholar] [CrossRef]
- Reid, I.R.; Plank, L.D.; Evans, M.C. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J. Clin. Endocrinol. Metab. 1992, 75, 779–782. [Google Scholar] [CrossRef]
- Ravn, P.; Cizza, G.; Bjarnason, N.H.; Thompson, D.; Daley, M.; Wasnich, R.R.; McClung, M.; Hosking, D.; Yates, A.J.; Christiansen, C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J. Bone Miner. Res. 1999, 14, 1622–1627. [Google Scholar] [CrossRef]
- Weiler, H.A.; Janzen, L.; Green, K.; Grabowski, J.; Seshia, M.M.; Yuen, K.C. Percent body fat and bone mass in healthy Canadian females 10 to 19 years of age. Bone 2000, 27, 203–207. [Google Scholar] [CrossRef]
- Hamrick, M.W.; Pennington, C.; Newton, D.; Xie, D.; Isales, C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 2004, 34, 376–383. [Google Scholar] [CrossRef]
- Goulding, A.; Taylor, R.W.; Jones, I.E.; McAuley, K.A.; Manning, P.J.; Williams, S.M. Overweight and obese children have low bone mass and area for their weight. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.K.; Laing, E.M.; Baile, C.A.; Hamrick, M.W.; Hall, D.B.; Lewis, R.D. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am. J. Clin. Nutr. 2007, 86, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Harris, S.; Must, A.; Naumova, E.; Phillips, S.; Rand, W.; Dawson-Hughes, B. Leptin, body composition and bone mineral density in premenopausal women. Calcif. Tissue Int. 2003, 73, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Haas, S.; Krins, S.; Knauerhase, A.; Löhr, M. Altered bone metabolism and bone density in patients with chronic pancreatitis and pancreatic exocrine insufficiency. JOP 2015, 16, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Ilich, J.Z.; Kelly, O.J.; Kim, Y.; Spicer, M.T. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arh. Hig. Rada Toksikol. 2014, 65, 139–148. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr. Res. 2013, 33, 521–533. [Google Scholar] [CrossRef]
- Villa, C.R.; Chen, J.; Wen, B.; Sacco, S.; Taibi, A.; Ward, W.; Comelli, E. Maternal Dietary Vitamin D Does Not Program Systemic Inflammation and Bone Health in Adult Female Mice Fed an Obesogenic Diet. Nutrients 2016, 8, 675. [Google Scholar] [CrossRef]
- Florowska, A.; Krygier, K.; Florowski, T.; Dłużewska, E. Prebiotics as functional food ingredients preventing diet-related diseases. Food Funct. 2016, 7, 2147–2155. [Google Scholar] [CrossRef]
- Walsh, J.S.; Bowles, S.; Evans, A.L. Vitamin D in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 389–394. [Google Scholar] [CrossRef]
- Zhou, A.; Nen, E.H. Vitamin D deficiency and C-reactive protein: A bidirectional Mendelian randomization study. Int. J. Epidemiol. 2022, dyac087. [Google Scholar] [CrossRef]
- Xu, B.; Lovre, D.; Mauvais-Jarvis, F. The effect of selective estrogen receptor modulators on type 2 diabetes onset in women: Basic and clinical insights. J. Diabetes Its Complicat. 2017, 31, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Lama, A.; Santoro, A.; Corrado, B.; Pirozzi, C.; Paciello, O.; Pagano, T.B.; Russo, S.; Calignano, A.; Raso, G.M.; Meli, R. Extracorporeal shock waves alone or combined with raloxifene promote bone formation and suppress resorption in ovariectomized rats. PLoS ONE 2017, 12, e0171276. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, Y.; Luo, P.; Yu, H.; Guo, S.; Liu, F.; Gao, J.; Xu, J.; Wang, S.; Zhang, C. Glucocorticoid-induced expansion of classical monocytes contributes to bone loss. Exp. Mol. Med. 2022, 54, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Bader, J.E.; Emos, R.T.; Velázquez, K.T.; Carson, M.S.; Sougiannis, A.T.; McGuiness, O.P.; Robinson, C.M.; Murphy, E.A. Repeated clodronate-liposome treatment results in neutrophilia and is not effective in limiting obesity-linked metabolic impairments. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E358–E372. [Google Scholar] [CrossRef] [PubMed]
- Nettelbladt Erik, G.; Sundblad Lars, K.M. Protein Patterns in Synovial Fluid and Serum in Rheumatoid Arthritis and Osteoarthritis. Arthritis Rheumatol. 1959, 2, 144–151. [Google Scholar] [CrossRef]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Ehart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriachi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef]
- Naghashian, F.; Hosseinzadeh-Attar, M.J.; Akhlaghi, M.; Yekaninejad, M.S.; Aryaeian, N.; Derakhshanian, H. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Naghashian, F.; Hosseinzadeh-Attar, M.J.; Akhlaghi, M.; Yekaninejad, M.S.; Aryaeian, N.; Derakhshanian, H. The relationship between anthropometric status and rheumatoid arthritis. Exploring the role of nesfatin and asymmetric dimethylarginine. Acta Reumatol. Port. 2019, 44, 126–131. [Google Scholar]
- Stavropoulos-Kalinoglou, A.; Metsios, G.; Smith, J.; Panoulas, V.; Douglas, K.; Jamurtas, A.; Koutedakis, Y.; Kitas, G. What predicts obesity in patients with rheumatoid arthritis? An investigation of the interactions between lifestyle and inflammation. Int. J. Obes. 2010, 34, 295–301. [Google Scholar] [CrossRef]
- Choi, H.K.; Mount, D.B.; Reginato, A.M. Pathogenesis of gout. Ann. Intern. Med. 2005, 143, 499–516. [Google Scholar] [CrossRef]
- Shaw, O.M.; Pool, B.; Dalbeth, N.; Harper, J.L. The effect of diet-induced obesity on the inflammatory phenotype of non-adipose-resident macrophages in an in vivo model of gout. Rheumatology 2014, 53, 1901–1905. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).