Abstract
A number of antifungal drugs are based on polyene macrolides that cause severe side effects. Most of these compounds contain a single aminodeoxysugar, D-mycosamine. Toxicity can be reduced by increasing the extent of glycosylation. The aromatic heptaene 67-121C and two analogues of the degenerate heptaene nystatin have a second sugar attached to the C4′ hydroxyl of mycosamine. Another nystatin analogue has L-digitoxose as a second sugar attached to C35 on the macrolactone ring. The pentaene selvamicin has 4-O-methyl-L-digitoxose at C27, the equivalent position. To assist the production of new antifungals by synthetic biology, we explore further the utility of three classes of polyene glycosyltransferase: extending glycosyltransferases that form disaccharide-containing polyenes, glycosyltransferases that add the L-digitoxose sugars of nystatin A3 and selvamicin, and mycosaminyltransferases that add the primary aminodeoxysugar. In addition, we combine enzymatic hyperglycosylation with a known chemical method for adding sugars to the C3′ amino group of mycosamine. This was used to convert the disaccharide-containing 67-121C heptaene to forms containing branched trisaccharide or tetrasaccharide chains. These analogues are of interest for testing as anti-Leishmania drugs.
1. Introduction
Glycosylated polyene macrolides are synthesised by streptomycetes and related actinobacteria [1]. These compounds have antifungal activity that results from specific binding of ergosterol in target cell membranes. The group includes the antibiotics amphotericin B and nystatin A1, the preservative pimaricin, the pentaene eurocidin, and two classes of aromatic heptaene, exemplified by candicidin and partricin (Figure 1) [2]. These agents are also effective against Leishmania parasites that contain ergosterol-related sterols in their membranes [3]. Amphotericin B is highly active but shows severe toxicity that results from a moderate affinity for cholesterol in mammalian cell membranes [4]. Low water-solubility, a tendency to aggregate and instability contribute to adverse effects. Amphotericin B selectively complexes with ergosterol to form membrane-permeabilizing channels [5]. In addition, amphotericin B molecules self-assemble into structures that extract ergosterol from the membranes of sensitive cells [6,7]. This disrupts several membrane functions and accounts for the potency of amphotericin B and the low emergence of resistance in fungal pathogens.
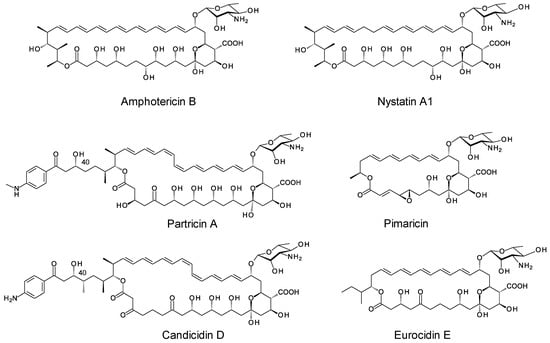
Figure 1.
Examples of glycosylated polyene macrolides.
Life-threatening fungal infections are increasing in incidence and very few classes of drugs are available to treat them [8]. The limited range of therapeutic options means that continued use of amphotericin B is essential, despite side effects that are almost unacceptable. Toxicity can be partially alleviated by liposomal formulation or by structural modification. Improved analogues can be made by chemical methods or by genetic engineering of producer organisms. Most polyenes have a single mycosamine sugar attached to the macrolactone ring. This sugar is essential for biological activity and is involved in sterol binding. Organic chemists have synthesised analogues with altered sugar residues [9,10,11]. The C2 hydroxyl of mycosamine is important as C2′-deoxy-amphotericin B and C2′-epi-amphotericin B show improved specificity for ergosterol over cholesterol. Chemical modification with longer carbohydrate chains increases water-solubility and can further reduce toxicity [12,13,14].
Next generation sequencing has revealed that biosynthetic gene clusters (BGCs) for nystatins, candicidins and pimaricins occur frequently in various actinomycete genomes. Genome mining and bioinformatic studies predict possible biosynthetic pathways for several types of hyperglycosylated polyenes [2]. However, only two classes of these compounds have been isolated and chemically characterized to date. The disaccharide-containing polyenes 67-121C, NPP and nystatin P1 have a second sugar attached to the C4′ hydroxyl of the mycosamine (Figure 2) [15,16,17]. The PegA, NppY and NypY proteins have been identified as the extending glycosyltransferases [2]. The second class of hyperglycosylated polyene contains two unlinked monosaccharides, the standard mycosamine and a neutral deoxysugar attached to the opposite end of the macrolactone ring. These include nystatin A3, nystatin analogues modified with L-mycarose at C35 (Figure 3), polyfungin, and candidinin [1,18,19,20,21,22,23]. The sugar residue on C35 does not block activity [18]. This is interesting because chemical studies have shown that the C35 hydroxyl group of amphotericin B is essential for membrane permeabilization but not for overall antifungal activity [24]. The pentaene selvamicin has D-rhamnose at C15 in place of mycosamine and 4″-O-methyl-L-digitoxose at C27, the position corresponding to C35 of nystatin [25]. The SelSV glycosyltransferase encoded within the selvamicin BGC is likely to catalyse addition of the L-digitoxosyl sugar.
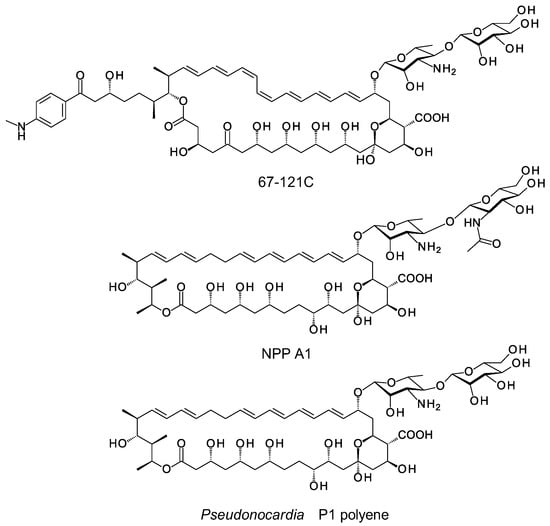
Figure 2.
Naturally ocurring disaccharide-containing polyenes. 67-121C is produced by Couchioplanes caeruleus DSM43634, NPP A1 is obtained from Pseudonocardia autotrophica KCTC9441, nystatin P1 is obtained from Pseudonocardia species P1.
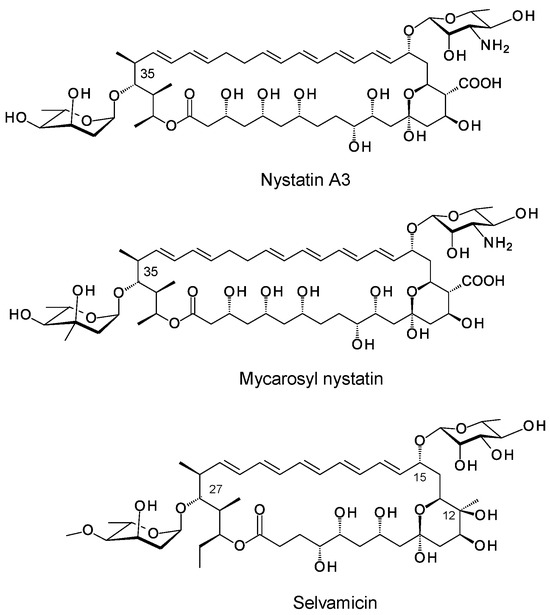
Figure 3.
Examples of polyenes containing two unlinked monosaccharides.
Recent studies have characterized the three extending glycosyltransferases that function in biosynthesis of disaccharide-containing polyenes. The PegA enzyme adds mannose to the C4′ OH of the mycosamine residue of 67-121A to form 67-121C. (67-121A is identical to partricin A, shown in Figure 1). NppY adds N-acetylglucosamine (GlcNAc) to 10-deoxynystatin during the biosynthesis of NPP and NypY adds the second sugar residue of nystatin P1 [2].
The Korean group, led by Eung-Soo Kim, has carried out extensive genetic engineering of NPP A1 biosynthesis [26,27,28,29,30]. Analogues were generated by inactivating the C10 hydroxylase P450, by engineering the PKS to make a heptaene rather than a tetraene, and by substituting NypY for NppY to replace GlcNAc with mannose. Out of five available disaccharide-containing nystatins, the best was NPP B2, a 10-deoxy heptaene analogue containing GlcNAc as the second sugar. The yields of NPP B2 have been improved by chemical mutagenesis and genetic engineering.
NypY can be used to generate 4′-O-mannosyl analogues of some of the available amphotericins–these are amphotericins A and B, 16-ketoamphotericins A and B and 8-deoxyamphotericins A and B [31]. These mannosyl-amphotericins are produced in low yields. Mannosyl-8-deoxyamphotericin B has been characterized by NMR spectroscopy [32].
In the 1970s, Polish medicinal chemists led by the late Edward Borowski found that amphotericin B reacts with glucose to form a disaccharide analogue [33]. The amino group of the mycosamine sugar forms an imine with the aldehyde of glucose. The imine rearranges to give N-fructosyl amphotericin B (Figure 4). This forms the basis for production of MFAME, a water-soluble amphotericin analogue with reduced haemolytic activity [34,35,36]. This reaction with glucose proceeds efficiently with many other polyene macrolides. Disaccharides, such as lactose, that can make a free aldehyde group available may also be used.
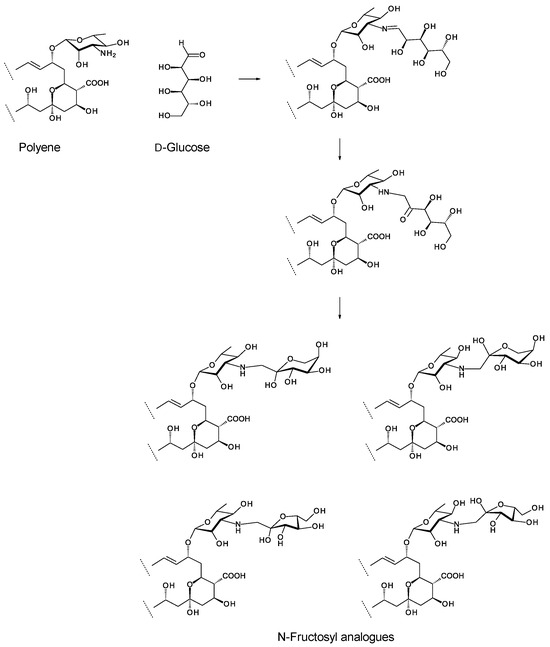
Figure 4.
Overview of chemical glycosylation of a polyene with D-glucose. The rearrangement reaction gives a linear fructosyl chain that can cyclize to give α or β anomers of pyranosyl and furanosyl forms. Preobrazhenskaya and co-workers [37] found that the linear open chain fructosyl form exists in equilibrium with the four cyclized forms.
Here, we set out to exploit old and new knowledge to develop new analogues of glycosylated polyenes (Figure 5). We aimed to investigate whether enzymatic glycosylation of the C4′ OH of mycosamine can be combined with chemical glycosylation of the C3′ amino group to give branched trisaccharides or tetrasaccharides. We made the first attempts to glycosylate the C35 hydroxyl of amphotericin B by enzymatic methods (Figure 5). We also gain new insights into the glycosyltransferases that add the mycosamine residue to polyene macrolactones.
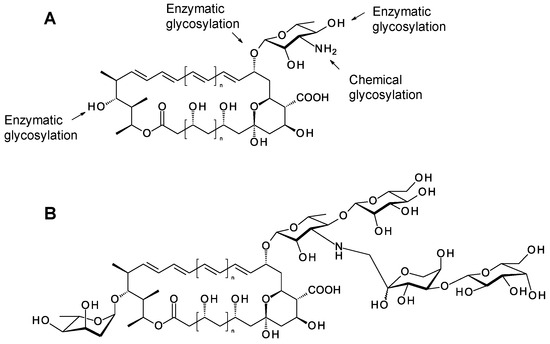
Figure 5.
Opportunities for generating polyene glycoanalogues by synthetic biology. (A) Polyene macrolactones can be modified at three points by enzymatic glycosylation, chemical glycosylation of sugar amino groups is also practicable. (B) Hypothetical example of a glycoanalogue that might be obtained by combining these methods.
2. Results
2.1. Studies on Extending Glycosyltransferases That Function in Biosynthesis of Disaccharide-Containing Polyenes
The PegA enzyme catalyses the incorporation of the second sugar residue into the disaccharide chain of 67-121C. Three homologues of PegA have been identified by genome mining [2]. Here, PegA was characterized further to increase yields of genetically engineered disaccharide-containing polyenes.
PegA gives mannosylated forms of all three components of the candicidin complex, candicidins D, A1 and A3. These candicidins result from variable β-ketone processing by PKS modules 17, 18, 20 and 21 [2]. The correct start codon for the pegA gene was not known at the start of this project. One possible initiation site specifies the N-terminal amino acid sequence MRVSLQGTG, yet the true open reading frame may begin with a downstream ATG codon to give a protein that starts with the sequence MTSHRPILFCC (Figure 6). Both possible start codons are preceded by plausible ribosome-binding sites. In the previous work presented in [31,38], pIAGO-pegA1 was designed to allow the use of the upstream start codon. If the downstream one is correct then this construct contains unnecessary upstream sequence that might impair high-level expression of the gene. Here, a second construct was made that uses the downstream start codon (Supplementary Figure S1). This was named pIAGO-pegA2.

Figure 6.
Alignment of N-terminal sequences of PegA and NypY showing methionines encoded by two plausible start codons.
The two plasmids were transformed into Streptomyces albidoflavus and polyene products were analysed by HPLC (Figure 7). This showed that pIAGO-pegA2 gave higher total levels of disaccharide-candicidins than pIAGO-pegA1. The extracts from S. albidoflavus pIAGO-pegA2 were analysed by LCMS to confirm that these compounds are synthesised (Figure 8, Supplementary Figures S2–S7). These results indicate that translation of pegA starts from the downstream start codon.
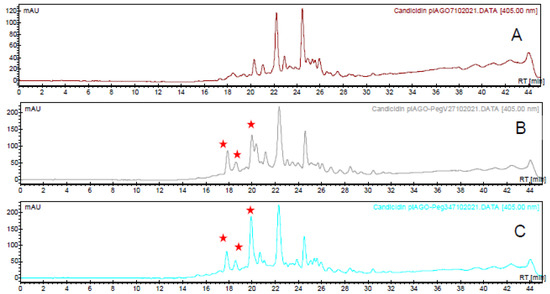
Figure 7.
HPLC analysis of candicidins. (A) Candicidin complex from S. albidoflavus containing the empty pIAGO vector. (B) Candicidins from S. albidoflavus containing pIAGO-pegA1. (C) Candicidins from S. albidoflavus containing pIAGO-pegA2. New heptaene peaks resulting from enzymatic mannosylation are marked with red stars.
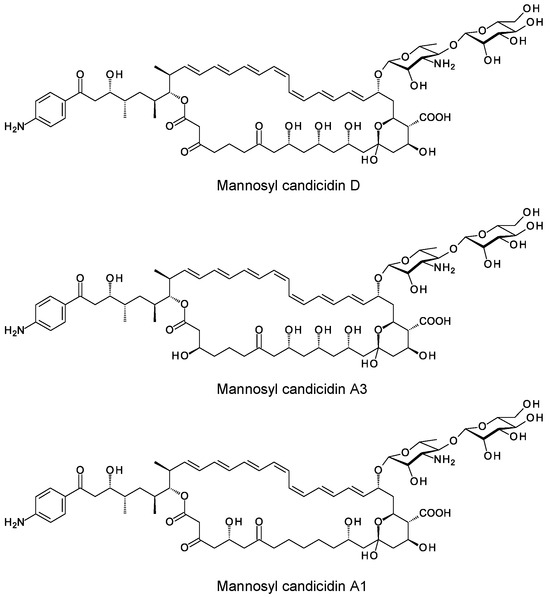
Figure 8.
Structures of mannosyl candidicidins.
Genes encoding PegA homologues have been identified in three micro-organisms, Amycolatopsis suaedae (WP_130478880.1), Cryptosporangium arvum (WP_035851739) [2] and Crossiella SN42.1 (WP_252478641.1). Amyc. suaedae is predicted to encode a methyltetraene and Cryp. arvum encodes an octaene. The genome sequence of Crossiella SN42.1 is incomplete. The gene for the PegA homologue is linked to a terpene cyclase rather than a polyene PKS. The unsequenced region of the genome might contain a polyene cluster.
The Cryp. arvum extending glycosyltransferase was the most promising for attempted production of new disaccharide-containing amphotericins and candicidins. We amplified the gene from genomic DNA of Cryp. arvum using primers CrypF and CrypR. The gene was cloned into the streptomycete expression plasmid pIAGO downstream from a strong ermE promoter. The resulting construct pIAGO-CrypEGT was verified by re-sequencing and transformed into S. nodosus and the candicidin producer S. albidoflavus. No disaccharide-containing analogues were produced. Further work will be required to exploit new extending glycosyltransferases.
2.2. Modifying Mycosamine Residues by Combining Enzymatic Glycosylation of the C4′ Hydroxyl with Chemical Glycosylation of the C3′ Amino Group
Attempts were made to generate trisaccharide- and tetrasaccharide-modified aromatic heptaenes by chemical glucosylation of 67-121C. C. caeruleus was grown on polyene production medium then sedimented cells and Amberlite XAD16 resin were extracted with methanol. The extracts were concentrated by rotary evaporation until polyenes precipitated from the residual water. The precipitates were washed extensively with purified water, diethyl ether, then chloroform and freeze-dried to give a yellow powder. Re-analysis by HPLC revealed two major heptaenes, the leading peak with a retention time (RT) of 18 min is 67-121C (Figure 9). The smaller second peak (RT = 21 min) is 67-121A.
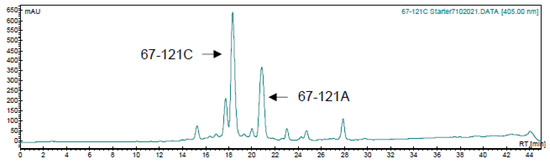
Figure 9.
HPLC of 67-121 polyenes extracted from C. caeruleus.
A 1 mg dry weight sample of the 67-121 polyene was dissolved in 1 mL dimethylformamide containing 20 mg/mL glucose and left at 37 °C for 16 h. The reaction mixture was then analysed by LC-MS to determine whether the 67-121C disaccharide was converted to a trisaccharide and whether the 67-121A monosaccharide was converted to a disaccharide (Figure 10 and Figure 11). The di- and trisaccharide-containing forms were not resolved by HPLC, but the trisaccharide-containing analogue was easily detected by LCMS (Figure 11).
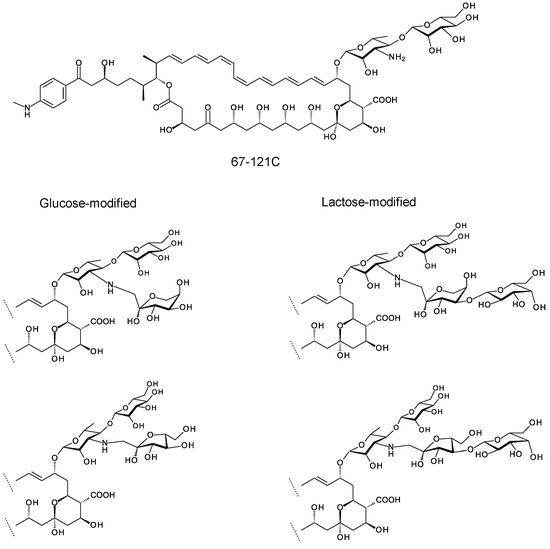
Figure 10.
Trisaccharides and tetrasaccharides formed by chemical glycosylation of 67-121C.
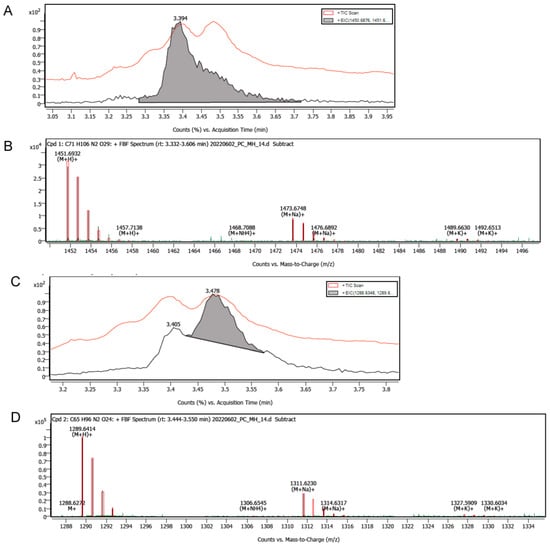
Figure 11.
Analysis of chemically glucosylated 67-121 polyenes by LC-MS. The chromatography step partially resolved the heptaene complex into two peaks containing trisaccharide- and disaccharide-modified polyenes, respectively. (A) The red trace shows total ion count (TIC) versus time. The more polar leading peak (shaded grey) has an RT of 3.394 min. (B) Mass spectrum of leading peak showing ions with a mass appropriate for a trisaccharide-modified 67-121 polyene, (M + H)+ = 1451.6932, (M + Na)+ = 1473.6748. (C) The red trace shows TIC versus time. The less polar trailing peak (shaded grey) has an RT of 3.478 min. (D) Mass spectrum of second peak showing ions with masses appropriate for disaccharide-containing 67-121 polyenes (M + H)+ = 1289.6414, (M + Na)+ = 1311.6230. In this analysis it is not possible to distinguish between unmodified 67-121C and glucose-modified 67-121A.
Attempts were then made to modify 67-121C with lactose. The same procedure was used except that lactose replaced glucose in the reaction mixture. The reaction with lactose generated more polar heptaenes that were easily separated from the starting materials (Figure 12). Analysis of the mixture by LC-MS confirmed that 67-121A monosaccharide was converted to a trisaccharide and 67-121C disaccharide was converted to a tetrasaccharide (Figure 13, Supplementary Figure S8, Table 1).
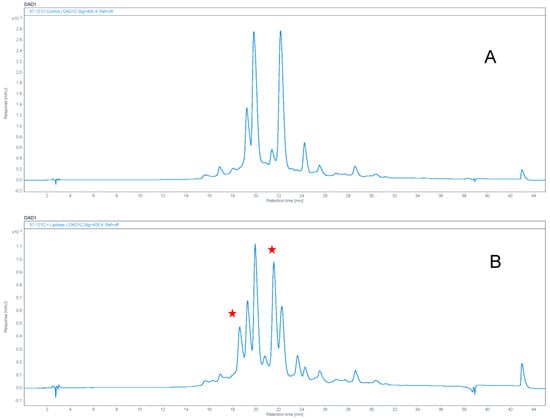
Figure 12.
Chemical modification of 67−121 complex with lactose. Panel (A) shows analysis of control unmodified 67−121 polyenes. Panel (B) shows material modified with lactose. New heptaene peaks appearing after chemical modification are marked with stars.
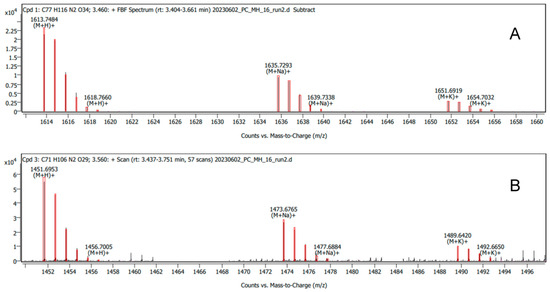
Figure 13.
Mass spectra of tetrasaccharide-containing (A) and trisaccharide-containing (B) aromatic heptaenes after modification of the 67−121C/67−121A complex with lactose.

Table 1.
Molecular ions detected for 67-121A monosaccharide, 67-121C disaccharide, glucose-modified 67-121C (trisaccharide), lactose modified 67-121A (trisaccharide), lactose-modified 67-121C (tetrasaccharide).
2.3. Attempts to Glycosylate the C35 Hydroxyl Group of Amphotericin B and Analogues
The SelSV glycosyltransferase (accession number WP_068800853.1) is thought to catalyse glycosylation of the C27 hydroxyl group of the selvamicin macrolactone with 4-O-methyl-L-digitoxose. A closely related glycosyltransferase PenSV (accession number WP_165922095.1) is encoded by a gene within a silent nystatin biosynthetic gene cluster in Pseudonocardia endophytica. This cluster includes genes for biosynthesis of dTDP-β-L-digitoxose and is thought to specify nystatin A3 [2]. In this section we aimed to find out whether SelSV or PenSV can transfer L-mycarose or L-digitoxose to the C35 hydroxyl of amphotericin B in S. nodosus cells.
Synthetic forms of the selSV and penSV genes were synthesised by BaseClear and cloned into the expression vector pET28 (Supplementary Figures S9–S11). The genes were excised from these constructs and cloned between the NdeI and Hin dIII sites of the integrating streptomycete plasmid pIJ10572, downstream from a strong ermE promoter. These pIJ-SelSV and pIJ-PenSV constructs were checked by re-sequencing.
pIJ-SelSV was introduced into S. nodosus ΔamphNM [39] and transformants were selected with hygromycin. The presence of the integrated selSV gene was confirmed by PCR analysis of genomic DNA. S. nodosus ΔamphNM pIJ-SelSV was further transformed with the Salas plasmid pFL942, which contains the biosynthetic genes for dTDP-β-L-mycarose [40]. This plasmid also directs biosynthesis of dTDP-β-L-digitoxose [40]. Transformants containing both plasmids were selected with hygromycin and thiostrepton. The strain was grown on fructose-dextrin-soya medium and polyenes were analysed by HPLC and LCMS. A schematic diagram illustrating the glycosylation engineering strategy is shown in the Supplementary Figure S12. No evidence for mycarosylated or digitoxosylated forms was obtained. Possibly SelSV cannot accommodate the amphotericin heptaene macrolactones that are larger than its normal acceptor substrate. Attention was then focused on PenSV, which is predicted to act on nystatin macrolactones that are more closely related to amphotericins. Plasmids pIJ-PenSV and pFL942 were transformed into S. nodosus, S. nodosus ΔamphNM and S. nodosus ΔamphDI-DII-NM. These strains produce amphotericins A and B, 16-descarboxyl-16-methyl-amphotericin B and 8-deoxy-16-descarboxyl-16-methyl-amphotericin A, and aglycones 8-deoxy-16-descarboxyl-16-methyl-amphoteronolides A and B, respectively. Polyenes were extracted from the transformants and analysed by HPLC and LCMS. Searching for ions with the calculated molecular masses of hypothetical mycarosylated or digitoxosylated products revealed no convincing matches (Tables S1 and S2). This indicates a failure in synthesis or attachment of the extra sugars. It is also possible that amphotericins are rapidly exported from producer cells before any non-native late modifications can occur.
2.4. Aglycone Feeding
In many cases, streptomycetes equipped with genes for biosynthesis and attachment of deoxysugars can glycosylate exogenously supplied aglycones. This aglycone feeding approach has been successful with erythronolide, tylactone and tetracenomycin acceptors [41]. S. lividans was transformed with pIJ-SelSV and pFL942 or with pIJ-SelSV and pLNBIV, which directs biosynthesis of dTDP-β-L-digitoxose only [42]. This gave biotransformation strains with the potential to mycarosylate or digitoxosylate exogenous aglycones (Supplementary Figure S13). Amphotericin B, M57 pentaene (see below) and other polyenes were fed to these strains but polyenes were not stable and the chromophores disappeared from the biotransformation flasks within 24 h after addition.
A control strain was constructed to assess the biotransformation process. The gene for the PerDIII GDP-mannose 4, 6 dehydratase was amplified from Streptomyces cacaoi genomic DNA with oligonucleotides PerD3F and PerD3R. The amplified DNA was digested with HindIII and cloned into the expression plasmid pIAGO-Hap2 [43]. Clones containing the perDIII gene in the same orientation as the Hap2 glycosyltransferase and PerDII perosamine synthase genes were identified by PCR. The final construct contained the genes required for biosynthesis of GDP-α-D-perosamine from GDP-α-D-mannose, preceded by a gene for an AmphDI-PerDI hybrid glycosyltransferase capable of perosaminylating amphotericin aglycones. S. lividans, transformed with the plasmid, was assessed as a biotransformation strain (Supplementary Figure S14). This strain would allow sensitive detection of perosaminylated products on the basis of antifungal activity. However, inactive amphoteronolide aglycones also disappeared from S. lividans cultures and no antifungal activity could be detected. AmphJ PKS-deficient S. nodosus mutants produce no polyenes but retain the genes for biosynthesis and attachment of mycosamine. These were also tested as biotransformation strains but no antifungal activity was detected. The instability of polyene aglycones was apparently insurmountable. In polyene production cultures Amberlite XAD16 resin stabilizes polyene products and dramatically increases yields. Amberlite was not used in biotransformations because it would sequester the substrate.
2.5. Expression of gloDI and eurDI-DII-N-M Genes in S. nodosus ΔamphDI-NM and S. nodosus ΔamphDI-DII-NM
Genome mining previously uncovered a pentaene BGC in the genome of Saccharopolyspora gloriosae [2]. The predicted structure is shown in Figure 14. This is similar to selvamicin except that it contains mycosamine at C15, an exocyclic carboxyl group at C12 and a 2,6-dideoxy-D-sugar, possibly D-olivose, at C-27. One way to access these hyperglycosylated polyenes would be to activate the silent clusters, isolate the products, and verify the structures. An alternative approach would be engineer the amphotericin polyketide synthase (PKS) to make ring-contracted pentaene macrolactones that might act as acceptor substrates for pentaene glycosyltransferases. We previously constructed S. nodosus M57 which makes a pentaene analogue that is not efficiently recognized by the native AmphDI mycosaminyltransferase [44]. Here we investigated mycosaminyltransferases from Streptomyces eurocidicus, producer of the known pentaene eurocidin, and Sacc. gloriosae, producer of the hypothetical pentaene.
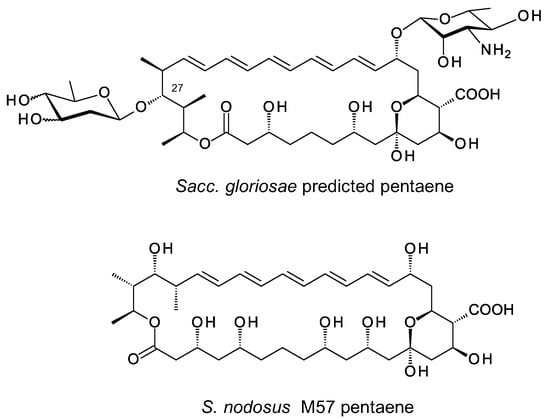
Figure 14.
Structures of pentaene predicted for Sacc. gloriosae and S. nodosus M57 pentaene.
In amphotericin biosynthesis, the polyketide synthase synthesizes the heptaene 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide B, and the tetraene 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide A in which the C28–C29 double bond is reduced [45]. Both macrolactones undergo the same series of reactions to form amphotericins B and A (Figure 15). The AmphN P450 and AmphM ferredoxin form the exocyclic group at C16. The AmphDI glycosyltransferase transfers mycosamine to the aglycone. Finally, the AmphL P450 hydroxylates C8. Other late genes encode the AmphDIII GDP-mannose 4,6 dehydratase and AmphDII GDP-mycosamine synthase that function in synthesis of GDP-α-D-mycosamine from GDP-α-D-mannose [45].
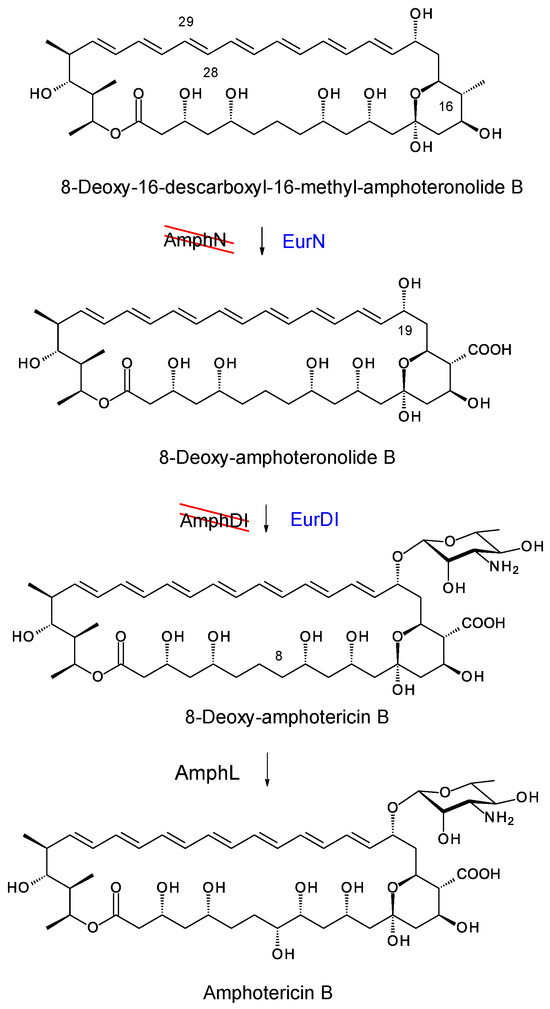
Figure 15.
EurN and EurDI replace AmphN and AmphDI in S. nodosus ΔamphDI-NM. The red lines indicate that the chromosomal amphN and amphDI genes have been deleted. Enzymes in blue text are encoded by a complementing pIAGO construct. This convention is used in Figures 18, 20, 22 and 24.
Here we investigated EurDI and EurN, the Streptomyces eurocidicus counterparts of AmphDI and AmphN. The eurDI-DII-N-M genes were amplified by PCR with EurD1-F and EurM-R and cloned into pIAGO. The gene for GloDI, the Sacc. gloriosae counterpart of AmphDI, was amplified with SGDF1 and SGDR and also cloned into pIAGO. The resulting constructs pIAGO-EurDI-DII-N-M and pIAGO-GloDI were transformed into derivatives of S. nodosus M57 but no glycosylated pentaenes were detected. The plasmids were then tested in other aglycone-producing mutants of S. nodosus.
The pIAGO-EurDI-DII-N-M and pIAGO-GloDI plasmids were transformed into S. nodosus ΔamphDI-NM. This strain normally produces a series of isoforms of 8-deoxy-16-descarboxyl-16-methyl-amphoteronolides B and A [45] (Figure 16A and Figure 17A). Introduction of pIAGO-EurDI-DII-N-M restored production of amphotericin B (Figure 15 and Figure 16B,C), indicating that EurN and EurDI can recognize 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide B.
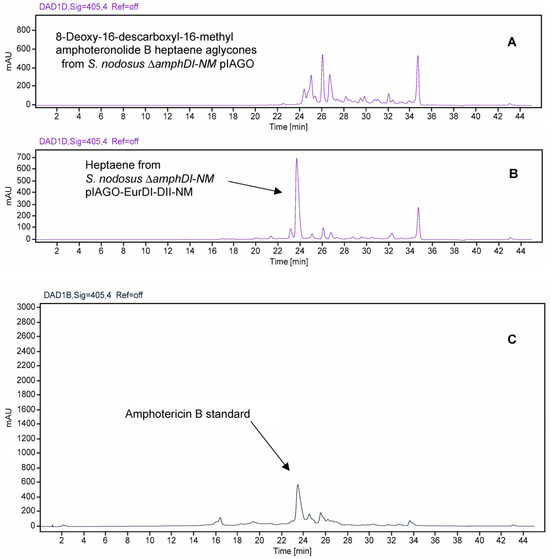
Figure 16.
HPLC evidence for modification of amphotericin heptaene aglycones by EurN and EurDI in S. nodosus ΔamphDI-NM. Panel (A) Heptaenes from S. nodosus ΔamphDI-NM pIAGO (empty vector control). Panel (B) Heptaenes from S. nodosus ΔamphDI-NM pIAGO-EurDI-DII-NM. Panel (C) amphotericin B standard isolated from S. nodosus. Heptaenes were detected by monitoring at A405.
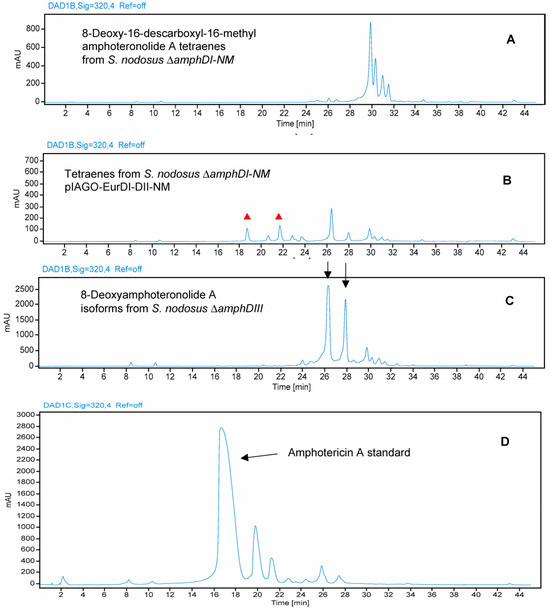
Figure 17.
HPLC evidence for modification of amphotericin tetraene aglycone by EurN and EurDI in S. nodosus ΔamphDI-NM. Panel (A) Tetraenes from S. nodosus ΔamphDI-NM pIAGO (empty vector control). Panel (B) Tetraenes from S. nodosus ΔamphDI-NM pIAGO-EurDI-DII-NM. Red triangles highlight polar, possibly glycosylated, tetraenes with retention times different from that of amphotericin A. Panel (C) 8-deoxyamphoteronolide A isoforms isolated from S. nodosus ΔamphDIII. Panel (D) Amphotericin A standard isolated from S. nodosus. Tetraenes were detected by monitoring at A320.
Analysis of tetraene products revealed that EurN converted 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide A to 8-deoxyamphoteronolide A, but this was not converted to amphotericin A (Figure 17). Two polar tetraenes were detected as minor peaks. These may be new glycosylated forms but the main product was tetraene 8-deoxyamphoteronolide A. EurDI did not act efficiently on 8-deoxyamphoteronolide A (Figure 18).
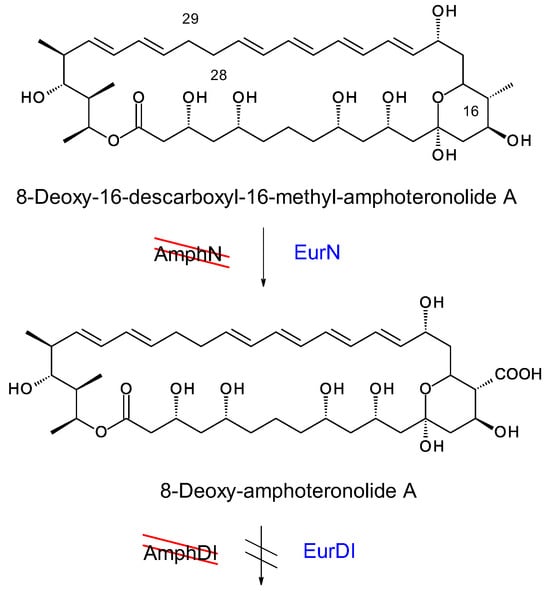
Figure 18.
EurN P450 acts on 8-deoxy-16-descarboxyl-16-methylamphoteronolide A tetraene but EurDI does not efficiently mycosaminylate 8-deoxy-amphoteronolide A.
In S. nodosus ΔamphDI-NM, pIAGO-GloDI restored production of mycosaminylated 16-descarboxyl-16-methyl-amphotericin B and 8-deoxy-16-descarboxyl-16-methyl-amphotericin A (Figure 19, Figure 20, Figure 21 and Figure 22).
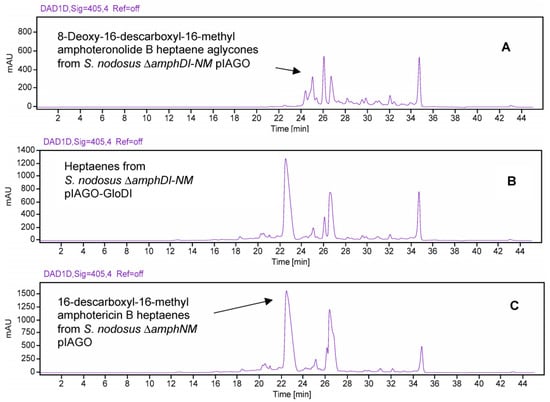
Figure 19.
HPLC evidence for glycosylation of amphotericin heptaene aglycones by GloDI in S. nodosus ΔamphDI-NM. Panel (A) Heptaenes from S. nodosus ΔamphDI-NM pIAGO (empty vector control). Panel (B) Heptaenes from S. nodosus ΔamphDI-NM pIAGO-GloDI. Panel (C) Mycosaminylated 16-descarboxyl-16-methyl-amphotericin B standard isolated from S. nodosus ΔamphNM. Heptaenes were detected by monitoring at A405.
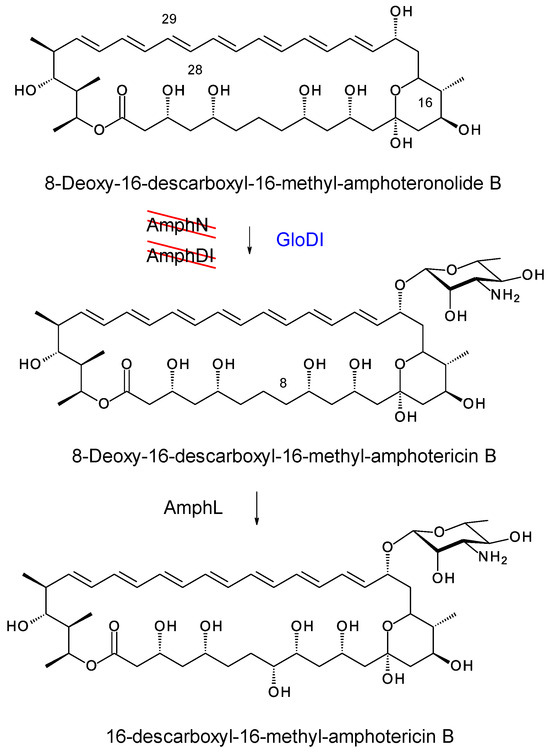
Figure 20.
GloDI catalyses glycosylation of 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide B in S. nodosus ΔamphDI-NM.
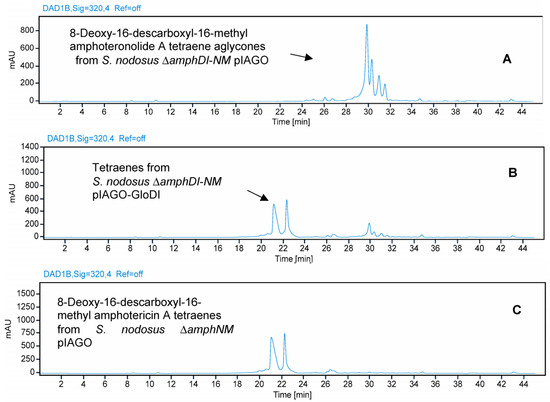
Figure 21.
HPLC evidence for glycosylation amphotericin tetraene aglycones by GloDI in S. nodosus ΔamphDI-NM. Panel (A) Tetraenes from S. nodosus ΔamphDI-NM pIAGO (empty vector control). Panel (B) Tetraenes from S. nodosus ΔamphDI-NM pIAGO-GloDI. Panel (C) Mycosaminylated 8-deoxy-16-descarboxyl-16-methyl-amphotericin A standard isolated from S. nodosus ΔamphNM. Tetraenes were detected by monitoring at A320.
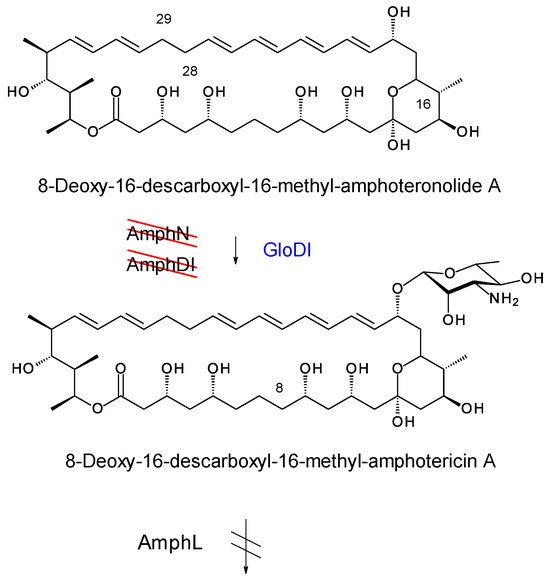
Figure 22.
GloDI catalyses glycosylation of the tetraene 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide A in the S. nodosus ΔamphDI-NM mutant. AmphL does not C8 hydroxylate 8-deoxy-8-deoxy-16-descarboxyl-16-methyl-amphotericin A [39].
The pIAGO-GloDI plasmid was also transformed into S. nodosus ΔamphDI-DII-NM, which lacks AmphDII GDP-mycosamine synthase as well as AmphN and AmphDI. This mutant produces the same 8-deoxy-16-descarboxyl-16-methyl-amphoteronolides B and A as S. nodosus ΔamphDI-NM. In S. nodosus ΔamphDI-DII-NM, this mutant GloDI converted the heptaene aglycones to a more polar species. This is most likely 19-O-rhamnosyl-16-descarboxyl-16-methyl-amphoteronolide B which is produced by AmphDI-proficient S. nodosus ΔamphDII-NM strains (Figure 23A,B and Figure 24). GloDI did not glycosylate the tetraene aglycone with a neutral sugar (Figure 23C,D and Figure 24).
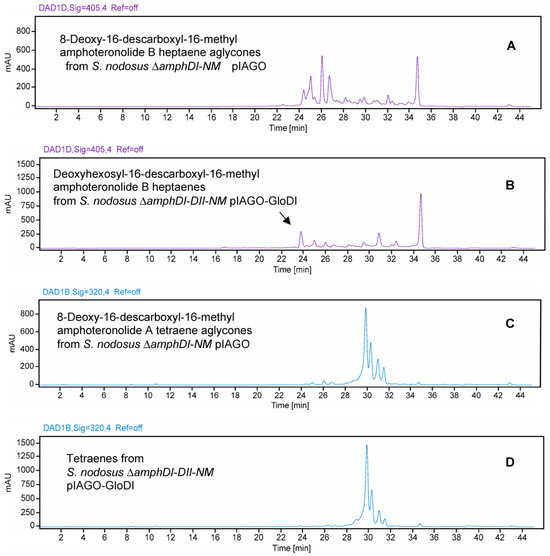
Figure 23.
HPLC evidence for activity of GloDI in S. nodosus ΔamphDI-DII-NM. Heptaenes and tetraenes from S. nodosus ΔamphDI-DII-NM pIAGO-GloDI are shown in Panels (B,D). Panels (A,C) show heptaenes and tetraenes from S. nodosus ΔamphDI-NM pIAGO control.
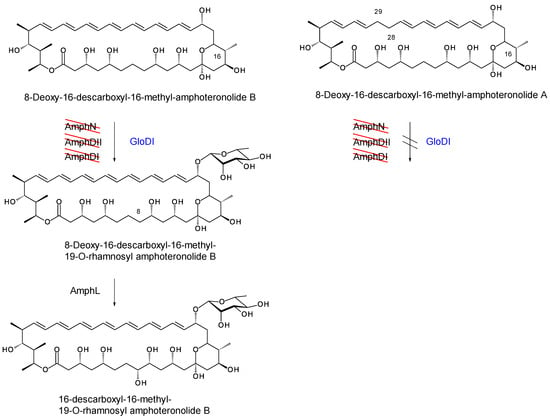
Figure 24.
In the mycosamine-deficient S. nodosus ΔamphDI-DII-NM, GloDI transfers a neutral deoxyhexose to 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide B heptaene but not to 8-deoxy-16-descarboxyl-16-methyl-amphoteronolide A tetraene.
Bioassays against Candida albicans were carried out on the polyene extracts of S. nodosus ΔamphDI-NM containing empty pIAGO vector, pIAGO-EurDI-DII-N-M, or pIAGO-GloDI. The extract of S. nodosus ΔamphDI-DII-NM pIAGO-GloDI was also tested. Large inhibition zones were observed for extracts of S. nodosus ΔamphDI-NM containing either pIAGO-GloDI or pIAGO- EurDI-DII-N-M (Figure 25). This restoration of antifungal activity confirmed that both GloDI and EurDI can substitute for AmphDI to give mycosaminylated amphoteronolides. Extracts of S. nodosus ΔamphDI-DII-NM pIAGO-GloDI gave a slight inhibition zone. This indicated that in the absence of AmphDII mycosamine synthase, GloDI modifies the aglycone with a neutral deoxysugar, as does AmphDI. This accounts for the weak antifungal activity. The biological activity testing results were consistent with the HPLC results.

Figure 25.
Testing for antifungal activity in methanol extracts of S. nodosus ΔamphDI-NM containing pIAGO, pIAGO-GloDI, pIAGO-EurDI-DII-N-M, and S. nodosus ΔamphDI-DII-NM pIAGO-GloDI.
3. Discussion
Aromatic heptaenes have potential as drugs for treatment of systemic mycoses and leishmania infections. MePartricin (partricin A methyl ester) is used to treat benign prostatic hyperplasia [46]. Aromatic heptaenes have a higher antifungal activity than amphotericin B but are not as widely used clinically because of toxic adverse effects. Glycosylation engineering could help to reduce this toxicity.
We improved the yields of mannosyl-candicidins by using the correct start codon when overexpressing the pegA gene in S. albidoflavus. Purification of these new analogues could be achieved using a preparative HPLC system.
A complex containing 60% 67-121C and 40% 67-121A can be isolated from production cultures of C. caeruleus DSM43634. This crude material can be purified by washing with water, chloroform and diethyl ether. This work shows that 67-121C can be converted to trisaccharide- or tetrasaccharide-containing forms by the simple chemical glycosylation method developed by Borowski et al. This process could be optimised in future work. The chemical process starts with polyenes dissolved in organic solvents and is more easily scaled up than enzymatic glycosylation. Enzyme-catalysed reactions require aqueous buffers, in which polyenes are poorly soluble. In future work, the chemical process will be optimised, scaled up, and tri- and tetrasaccharide analogues will be purified for assessment of biological activities.
The aromatic heptaene hamycin is synthesised by Streptomyces pimprina and is identical to 67-121B, which is 67-121A that is not N-methylated. Liposomal hamycin is an effective treatment for visceral leishmaniasis [47]. Leishmania parasites are taken up by liver macrophages but they resist destruction in phagolysosomes and eventually overwhelm the host. Macrophages have cell surface receptors that recognise glycosides with terminal residues of mannose, galactose, or fucose. Mannose-coated liposomal hamycin is more effective than conventional liposomal hamycin or free hamycin, possibly because it is more readily internalised by liver macrophages [48]. Mannose-coated liposomes were superior to galactose- or glucose-coated liposomes for delivery of pentamidine isethionate antileishmanial drugs [49].
67-121C has a terminal mannose and might be effective against leishmaniases. Reaction with lactose is of interest because it converts a polyene disaccharide to a branched tetrasaccharide, improving water-solubility, and providing terminal mannose and galactose residues that might aid internalisation by macrophages. The non-toxic amphotericin glycoanalogue MFAME is superior to liposomal amphotericin B. Liposomal encapsulation of MFAME gave no further improvement in its selective toxicity [50]. This suggests that polyene glycosylation engineering might replace costly liposomal formulation.
Engineering enzymatic glycosylation of amphotericin B at C35 remains challenging. Future work will focus on activating the pentaene cluster in Sacc. gloriosae. This organism is predicted to add a dideoxy-D-hexose to the C27 position of a macrolactone similar to selvamicin. Genes for enzymes involved in this pathway will be transformed into S. nodosus and S. nodosus strains engineered to make ring-contracted pentaene macrolactones. Biosynthesis of a D-sugar from dTDP-4-keto-6-deoxyglucose is somewhat simpler than synthesis of L-sugars.
Chemical studies on amphotericin B have shown that ergosterol specificity can be increased by replacing the mycosamine sugar with 2-deoxymycosamine or 2-epi-mycosamine. Enzymes synthesizing dTDP-linked forms of these sugars operate in the biosyntheses of the mycaminose, desosamine and angolosamine sugars of macrolides such as tylosin, erythromycin A and angolomycin [51]. This suggests that 2′-deoxy or 2′ epi amphotericins could be generated by synthetic biology. In vitro studies on AmphDI suggest that it has highly restricted tolerance for alternative GDP-sugars and does not accept dTDP-sugars [52]. Protein engineering will be required to change this narrow NDP-sugar specificity. Here we have shown that the EurDI and GloDI pentaene mycosaminyltransferases catalyse efficient mycosaminylation of the amphotericin heptaene aglycone, which is larger than their natural substrates. EurDI and GloDI show 66% and 71% sequence identity with AmphDI. We previously found that the 67-121 mycosaminyltransferase AceDI does not act on amphotericin aglycones even though it shares 68% identity with AmphDI [38]. In the biosynthesis of perimycin, another aromatic heptaene, the PerDI glycosyltransferase catalyses transfer of perosamine (4,6-dideoxy-4-amino-D-mannose) to the aglycone in place of mycosamine (3,6-dideoxy-3-amino-D-mannose). PerDI does not perosaminylate amphoteronolides despite sharing 62% sequence identity with AmphDI [43]. In the absence of a crystal structure for a polyene glycosyltransferase, these observations should assist attempts to redesign the substrate specificity of AmphDI.
4. Materials and Methods
4.1. Microbial Strains
The microbial strains used in this work are listed in Supplementary Table S3.
4.2. DNA Methods
PCR was carried out using Phusion DNA polymerase. Amplified DNA was purified using a QIAquick PCR purification kit (QIAGEN, Manchester, UK). Restriction enzyme digestions and ligations were carried out by standard methods. Plasmids were purified using QIAGEN (Manchester, UK) or GeneJET (ThermoFisher Scientific, Dublin, Ireland) miniprep kits. Resequencing of plasmid constructs was carried out by Source BioScience. The selSV and penSV genes were synthesized and cloned into pET28 by BaseClear BV. Oligonucleotide primer sequences are given in Supplementary Table S4.
Streptomyces transformations were carried out as described in the John Innes Institute manual [53]. Genomic DNA was isolated as described [53].
Plasmids pFL942 and pLNBIV [40,42] were kind gifts from Professor José A. Salas, University of Oviedo, Spain.
4.3. Polyene Extractions
Strains were grown at 30 °C with shaking (150 rpm) in TS broth medium for 40 h. These starter cultures were used to inoculate flasks of production medium fructose dextrin soya medium (2 g fructose, 6 g dextrin, 3 g soya flour, 1 g CaCO3, 1 g glycerol, 5 g Amberlite XAD16 per 100 mL H2O). Flasks were incubated at 30 °C with shaking at 150 rpm for 5 days. Mycelial cells and Amberlite XAD16 resin beads were sedimented by centrifugation at 10,000 rpm for 10 min at 4 °C. Polyenes were detected in the pellet fraction. Polyenes were extracted twice with volumes of methanol equal to the original culture volume. The extracts were concentrated by rotary evaporation to remove the methanol. Polyenes precipitating from the residual water were sedimented by centrifugation and washed extensively with water. The polyene precipitate was then washed once with diethyl ether and once with chloroform and dried in a Savant SPD111V vacuum centrifuge (ThermoFisher Scientific, Dublin, Ireland).
4.4. Aglycone Feeding
Amphotericin B and other possible acceptors were fed to cultures of S. nodosus transformants containing genes for biosynthesis and attachment of mycarose and digitoxose, perosamine or mycosamine. For each experiment, a sample of crude extract containing 5 to 20 mg of total polyene aglycone was added to a 50 mL TS broth culture of the biotransformation strain that had been grown for 40 h at 30 °C with shaking. The flask was then returned to the Innova 42R orbital incubator (ThermoFisher Scientific, Dublin, Ireland). for a further 48 h to allow modification to occur. At intervals, 1 mL samples of the culture were extracted with butanol and the extracts were checked for the presence of polyenes by UV-vis spectrophotometry. At the end of the “biotransformation” period, the culture was centrifuged and any remaining polyene was extracted from the pellet with methanol. The methanol was concentrated by rotary evaporation and products analysed by UV-vis spectrophotometry and HPLC. All polyene aglycones were unstable and there was >90% loss of the chromophore from the biotransformation culture within one to two days of addition.
4.5. HPLC
HPLC was carried out using Varian Prostar (SpectraLab, Markham, Ontario, Canada) or Agilent (Agilent Technologies Ireland, Cork, Ireland) systems using a semi-preparative Agilent Zorbax SB-C18 column (9.4 × 150 mm, 5 mm). Solvent A was 0.1% (v/v) formic acid in H2O, solvent B was 0.1% (v/v) formic acid in methanol. Samples of 500 μL volume were applied to column equilibrated with 50% B. Polyenes were separated using a standard gradient was 50% to 90% B. The flow rate was 4 mL per min.
4.6. Mass Spectrometry
Mass spectrometry was carried out using an Agilent 6500 series Q-TOF LC/MS system (Agilent Technologies Ireland, Cork, Ireland).
4.7. Chemical Glycosylation of 67-121C
1 mg of partially purified 67-121 polyene complex was dissolved in 1 mL dimethylformamide containing 20 mg/mL glucose or 20 mg/mL lactose and left at 37 °C for 16 h. In control reactions commercially available amphotericin B (>80% pure) was treated in the same way. Glycosylation was assessed by HPLC and mass spectrometry.
4.8. Tests for Antifungal Activity
Candida albicans was grown overnight at 30 °C on yeast medium (3 g malt extract, 3 g yeast extract, 5 g peptone, 10 g glucose per litre H2O). A 1 mL volume of this overnight culture was added to 100 mL of molten cooled (~50 °C) autoclaved yeast medium containing 1.5% agar. The agar was poured into 4 Petri dishes and allowed to set. Wells were punched in the agar using a sterilized cork borer. Test samples were pipetted into the wells and the plate was incubated base down in a 30 °C incubator (LTE Scientific, Oldham, United Kingdom). A lawn of C. albicans appeared after overnight incubation. Samples containing active antifungals gave a clear inhibition zone.
5. Conclusions
The disaccharide containing aromatic heptaene 67-121C is abundantly produced by C. caeruleus. This polyene can be converted to analogues containing branched trisaccharide or tetrasaccharide chains by a straightforward chemical reaction with glucose or lactose. These new polyenes are of interest for testing as anti-Leishmania drugs.
In our in vivo system, the SelSV and PenSV glycosyltransferases did not transfer L-digitoxose or L-mycarose sugars to the C35 hydroxyl group of amphotericin B in S. nodosus. Three enzymatic glycosylations of amphotericin macrolactones should be possible: attachment of mycosamine at C19, addition of a hexose to C4′ of mycosamine, and addition of a deoxyhexose at C35. Further work will be required to develop S. nodosus strains that perform all three glycosylations on a single heptaene macrolactone.
Chemical methods exist for synthesis of amphotericin B derivatives in which mycosamine is replaced with C2′-modified alternatives that improve pharmacological properties. These compounds could be obtained by enzymatic methods. Here we show that pentaene mycosaminyltransferases EurDI and GloDI act efficiently on amphotericin heptaene aglycones. This information will assist protein engineering designed to change the strict substrate specificity of AmphDI mycosaminyl transferase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/synbio2010003/s1, Figure S1: Difference between pIAGO-pegA1 and pIAGO-pegA2; Figure S2: Detection of candicidin A1; Figure S3: Detection of candicidin A3; Figure S4: Detection of candicidin D; Figure S5: Detection of mannosyl candicidin D; Figure S6: Detection of mannosyl candicidin A3; Figure S7: Detection of mannosyl candicidin A1; Figure S8: Detection of tetrasaccharide-, trisaccharide-, disaccharide- and monosaccharide-containing aromatic heptaenes after modification of the 67-121C/67-121A complex with lactose; Figure S9: Alignment of SelSV and PenSV amino acid sequences; Figure S10: DNA sequence of synthetic selSV gene; Figure S11: DNA sequence of synthetic penSV gene; Figure S12: Strategy for engineering biosynthesis of a mycarosylated or digitoxosylated amphotericin analogue; Figure S13: Schematic diagram illustrating strategy for aglycone feeding; Figure S14: Schematic diagram illustrating strategy for biotransformation of inactive amphoteronolides to active perosaminyl-amphoteronolides; Table S1: S. nodosus transformants tested for production of new glycoanalogues; Table S2: Molecular formulae and masses of hypothetical 35-O-glycosylated amphotericins; Table S3: Microbial strains; Table S4: Oligonucleotides.
Author Contributions
Conceptualization, P.C.; Mass spectrometry, J.M.; Investigation and experimental work, M.H., Y.S., J.M. and P.C.; data curation, P.C.; writing—original draft preparation, M.H., Y.S. and P.C.; writing—review and editing, P.C.; supervision, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
M.H. received a Ph.D. studentship from the Irish Research Council for Science Engineering and Technology (IRCSET). Y.S. received a Ph.D. studentship from the China Scholarship Scheme. Mass spectrometry was carried out in the UCD Chemistry Mass Spectrometry facility, which was funded as part of the Comprehensive Molecular Analysis Platform (CMAP) initiative under The SFI Research Infrastructure Programme in 2019, reference 18/RI/5702, and Biorbic—the SFI Bioeconomy Research Centre, and with the support of the School of Chemistry and UCD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Any data not available in the article or the supporting information are available from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Omura, S.; Tanaka, H. Production, Structure and Antifungal Activity of Polyene Macrolides. In Macrolide Antibiotics, Chemistry, Biology and Practice; Omura, S., Ed.; Academic Press: New York, NY, USA, 1986; pp. 351–404. [Google Scholar]
- Caffrey, P.; Hogan, M.; Song, Y. New Glycosylated Polyene Macrolides: Refining the Ore from Genome Mining. Antibiotics 2022, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Jin, Y.; Yang, S.; Joachim, A.M.; Ning, Y.; Mori-Quiroz, L.M.; Fromm, J.; Perera, C.; Zhang, K.; Werbovetz, K.A.; et al. Sterol profiling of Leishmania parasites using a new HPLC-tandem mass spectrometry-based method and antifungal azoles as chemical probes reveals a key intermediate sterol that supports a branched ergosterol biosynthetic pathway. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 27–42. [Google Scholar] [CrossRef]
- Abu-Salah, K.M. Amphotericin B: An update. Br. J. Biomed. Sci. 1996, 53, 8757689. [Google Scholar]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol Sponge Mechanism Is Conserved for Glycosylated Polyene Macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Soutar, C.P.; Greenwood, A.I.; Nimerovsky, E.; De Lio, A.M.; Holler, J.T.; Hisao, G.S.; Khandelwal, A.; Zhang, J.; SantaMaria, A.M.; et al. Fungicidal amphotericin B sponges are assemblies of staggered asymmetric homodimers encasing large void volumes. Nat. Struct. Mol. Biol. 2021, 28, 972–981. [Google Scholar] [CrossRef]
- Rayens, E.; Norris, K.A. Prevalence and Healthcare Burden of Fungal Infections in the United States, 2018. Open Forum Infect. Dis. 2022, 9, ofab593. [Google Scholar] [CrossRef]
- Croatt, M.P.; Carreira, E.M. Probing the role of the mycosamine C2′-OH on the activity of amphotericin B. Org. Lett. 2011, 13, 1390–1393. Available online: https://pubs.acs.org/doi/10.1021/ol2000765 (accessed on 1 November 2023). [CrossRef]
- Wilcock, B.C.; Endo, M.M.; Uno, B.E.; Burke, M.D. C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc. 2013, 135, 8488–8491. [Google Scholar] [CrossRef]
- Maji, A.; Soutar, C.P.; Zhang, J.; Lewandowska, A.; Uno, B.E.; Yan, S.; Shelke, Y.; Murhade, G.; Nimerovsky, E.; Borcik, C.G.; et al. Tuning sterol extraction kinetics yields a renal-sparing polyene antifungal. Nature 2023, 623, 1079–1085. [Google Scholar] [CrossRef]
- Golenser, J.; Frankenburg, S.; Ehrenfreund, T.; Domb, A.J. Efficacious treatment of experimental leishmaniasis with amphotericin B-arabinogalactan water-soluble derivatives. Antimicrob. Agents Chemother. 1999, 43, 2209–2214. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Golenser, J.; Domb, A. New Formulations and Derivatives of Amphotericin B for Treatment of Leishmaniasis. Mini Rev. Med. Chem. 2006, 6, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.P.; Gurudevan, S.; Jayakrishnan, A. Synthetic polymannose as a drug carrier: Synthesis, toxicity and anti-fungal activity of polymannose-amphotericin B conjugates. J. Biomater. Sci. Polym. Ed. 2018, 29, 1529–1548. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.J.; Greeves, D.; Mallams, A.K.; Picker, D.H. Structural elucidation of heptaene macrolide antibiotics 67-121A and 67-121C. J. Chem. Soc. Chem. Commun. 1977, 1977, 710–712. [Google Scholar] [CrossRef]
- Barke, J.; Seipke, R.F.; Grüschow, S.; Heavens, D.; Drou, N.; Bibb, M.J.; Goss, R.J.; Yu, D.W.; Hutchings, M.I. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 2010, 8, 109. [Google Scholar] [CrossRef]
- Lee, M.J.; Kong, D.; Han, K.B.; Sherman, D.H.; Bai, L.; Deng, Z.; Lin, S.; Kim, E.S. Structural analysis and biosynthetic engineering of a solubility-improved and less-hemolytic nystatin-like polyene in Pseudonocardia autotrophica. Appl. Microbiol. Biotechnol. 2012, 95, 157–168. [Google Scholar] [CrossRef]
- Bruheim, P.; Borgos, S.E.; Tsan, P.; Sletta, H.; Ellingsen, T.E.; Lancelin, J.-M.; Zotchev, S.B.; Stocker, H.; Kruse, G.; Kreckel, P.; et al. Chemical Diversity of Polyene Macrolides Produced by Streptomyces noursei ATCC 11455 and Recombinant Strain ERD44 with Genetically Altered Polyketide Synthase NysC. Antimicrob. Agents Chemother. 2004, 48, 4148–4153. [Google Scholar] [CrossRef]
- Kotiuszko, D.M.; Wituch, K.M.; Siejko, D.J.; Morawska, H.; Porowska, N.; Horodecka, M.T.; Wolkowicz, M.W.; Nowecka, M.; Makarowska-Plociennik, Z.E.; Halski, L. Method for Preparation of a New Antibiotic. U.S. Patent 3,891,505, 24 June 1975. Available online: https://patents.google.com/patent/US3891505A/en (accessed on 28 January 2022).
- Zielinski, J.; Jereczek, E.; Sowinski, P.; Falkowski, L.; Rudowski, A.; Borowski, E. The structure of a novel sugar component of polyene macrolide antibiotics: 2,6-Dideoxy-L-ribohexopyranose. J. Antibiot. 1979, 32, 565–568. [Google Scholar] [CrossRef]
- Synak, R.; Zielinski, J.; Golik, J.; Borowski, E. The structure of candidoin a component of the candidin antibiotic complex. J. Antibiot. 1983, 36, 1415–1417. [Google Scholar] [CrossRef]
- Pawlak, J.; Sowinski, P.; Borowski, E.; Gariboldi, P. Stereostructure and NMR characterization of the antibiotic candidin. J. Antibiot. 1993, 46, 1598–1604. [Google Scholar] [CrossRef]
- Song, M.; He, W.; Cai, S.; Wang, F.; Xu, W.; Xu, W. Nysfungin production improvement by UV mutagenesis in Streptomyces noursei D-3-14. Catalysts 2023, 13, 247. [Google Scholar] [CrossRef]
- Szpilman, A.M.; Cereghetti, D.M.; Manthorpe, J.M.; Wurtz, N.R.; Carreira, E.M. Synthesis and biophysical studies on 35-deoxy amphotericin B methyl ester. Chemistry 2009, 15, 7117–7128. [Google Scholar] [CrossRef] [PubMed]
- Van Arnam, E.B.; Ruzzini, A.C.; Sit, C.S.; Horn, H.; Pinto-Tomás, A.A.; Currie, C.R.; Clardy, J. Selvamicin, an atypical antifungal polyene from two alternative genomic contexts. Proc. Natl. Acad. Sci. USA 2016, 113, 12940–12945. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Won, H.J.; Kim, H.J.; Choi, S.S.; Lee, H.S.; Kim, P.; Kim, E.S. Carboxyl-terminal domain characterization of polyene-specific P450 hydroxylase in Pseudonocardia autotrophica. J. Ind. Microbiol. Biotechnol. 2016, 43, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Kang, S.-H.; Choi, S.-S.; Kim, E.-S. Redesign of antifungal polyene glycosylation: Engineered biosynthesis of disaccharide-modified NPP. Appl. Microbiol. Biotechnol. 2017, 101, 5131–5137. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Han, C.-Y.; Park, J.-S.; Oh, S.-H.; Kang, S.-H.; Choi, S.-S.; Kim, J.-M.; Kwak, J.-H.; Kim, E.-S. Nystatin-like Pseudonocardia polyene B1, a novel disaccharide-containing antifungal heptaene antibiotic. Sci. Rep. 2018, 8, 13584. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Kim, H.-J.; Han, C.-Y.; Nah, H.-J.; Choi, S.-S.; Kim, E.-S. Stimulated Biosynthesis of an C10-Deoxy Heptaene NPP B2 via Regulatory Genes Overexpression in Pseudonocardia autotrophica. Front. Microbiol. 2020, 11, 19. [Google Scholar] [CrossRef]
- Park, J.H.; Park, H.S.; Nah, H.J.; Kang, S.H.; Choi, S.S.; Kim, E.S. Streptomyces BAC Cloning of a Large-Sized Biosynthetic Gene Cluster of NPP B1, a Potential SARS-CoV-2 RdRp Inhibitor. J. Microbiol. Biotechnol. 2022, 32, 911–917. [Google Scholar] [CrossRef]
- De Poire, E.; Stephens, N.; Rawlings, B.; Caffrey, P. Engineered Biosynthesis of Disaccharide-Modified Polyene Macrolides. Appl. Environ. Microbiol. 2013, 79, 6156–6159. [Google Scholar] [CrossRef]
- Walmsley, S.; De Poire, E.; Rawlings, B.; Caffrey, P. Engineered biosynthesis and characterisation of disaccharide-modified 8-deoxyamphoteronolides. Appl. Microbiol. Biotechnol. 2016, 101, 1899–1905. [Google Scholar] [CrossRef]
- Falkowski, L.; Golik, J.; Kolodziejczyk, P.; Pawlak, J.; Zielinski, J.; Ziminski, T.; Borowski, E. N-glycosyl derivatives of polyene macrolide antibiotics. J. Antibiot. 1975, 28, 244–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grzybowska, J.; Sowinski, P.; Gumieniak, J.; Zieniawa, T.; Borowski, E. N-Methyl-N-D-fructopyranosylamphotericin B Methyl Ester, New Amphotericin B Derivative of Low Toxicity. J. Antibiot. 1997, 50, 709–711. Available online: https://www.jstage.jst.go.jp/article/antibiotics1968/50/8/50_8_709/_article/-char/en (accessed on 1 November 2023). [CrossRef] [PubMed]
- Cybulska, B.; Gadomska, I.; Mazerski, J.; Borowski, J.G.E.; Cheron, M.; Bolard, J. N-Methyl-N-D-fructosyl amphotericin B methyl ester (MF-AME), a novel antifungal agent of low toxicity: Monomer/micelle control over selective toxicity. Acta Biochim. Pol. 2000, 47, 121–131. [Google Scholar] [CrossRef]
- Szlinder-Richert, J.; Mazerski, J.; Cybulska, B.; Grzybowska, J.; Borowski, E. MFAME, N-methyl-N-D-fructosyl amphotericin B methyl ester, a new amphotericin B derivative of low toxicity: Relationship between self-association and effects on red blood cells. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2001, 1528, 15–24. [Google Scholar] [CrossRef]
- Preobrazhenskaya, M.N.; Olsufyeva, E.N.; Solovieva, S.E.; Tevyashova, A.N.; Reznikova, M.I.; Luzikov, Y.N.; Terekhova, L.P.; Trenin, A.S.; Galatenko, O.A.; Treshalin, I.D.; et al. Chemical modification and biological evaluation of new semisynthetic derivatives of 28,29-Didehydronystatin A1 (S44HP), a genetically engineered antifungal polyene macrolide antibiotic. J. Med. Chem. 2009, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.; Rawlings, B.; Caffrey, P. Versatility of Enzymes Catalyzing Late Steps in Polyene 67-121C Biosynthesis. Biosci. Biotechnol. Biochem. 2013, 77, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Carmody, M.; Murphy, B.; Byrne, B.; Power, P.; Rai, D.; Rawlings, B.; Caffrey, P. Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J. Biol. Chem. 2005, 280, 34420–34426. [Google Scholar] [CrossRef]
- Lombó, F.; Gibson, M.; Greenwell, L.; Braña, A.F.; Rohr, J.; Salas, J.A.; Méndez, C. Engineering biosynthetic pathways for deoxysugars: Branched-chain sugar pathways and derivatives from the antitumor tetracenomycin. Chem. Biol. 2004, 11, 1709–1718. [Google Scholar] [CrossRef]
- Méndez, C.; Salas, J.A. Altering the glycosylation pattern of bioactive compounds. Trends Biotechnol. 2001, 19, 449–456. [Google Scholar] [CrossRef]
- Rodríguez, L.; Aguirrezabalaga, I.; Allende, N.; Braña, A.F.; Méndez, C.; Salas, J.A. Engineering deoxysugar biosynthetic pathways from antibiotic-producing microorganisms. A tool to produce novel glycosylated bioactive compounds. Chem. Biol. 2002, 9, 721–729. [Google Scholar] [CrossRef]
- Hutchinson, E.; Murphy, B.; Dunne, T.; Breen, C.; Rawlings, B.; Caffrey, P. Redesign of polyene macrolide glycosylation: Engineered biosynthesis of 19-(O)-perosaminyl-amphoteronolide B. Chem. Biol. 2010, 17, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Carmody, M.; Byrne, B.; Murphy, B.; Breen, C.; Lynch, S.; Flood, E.; Finnan, S.; Caffrey, P. Analysis and manipulation of amphotericin biosynthetic genes by means of modified phage KC515 transduction techniques. Gene 2004, 343, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.; Anderson, K.; Borissow, C.; Caffrey, P.; Griffith, G.; Hearn, J.; Ibrahim, O.; Khan, N.; Lamburn, N.; Lee, M.; et al. Isolation and characterisation of amphotericin B analogues and truncated polyketide intermediates produced by genetic engineering of Streptomyces nodosus. Org. Biomol. Chem. 2010, 8, 3758–3770. [Google Scholar] [CrossRef] [PubMed]
- Szczeblewski, P.; Andrałojć, W.; Polit, J.; Żabka, A.; Winnicki, K.; Laskowski, T. Ipertrofan Revisited-The Proposal of the Complete Stereochemistry of Mepartricin A and B. Molecules 2021, 26, 5533. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.T.; McQueen, T.J.; Keyhani, A.; Lopez-Berestein, G. Liposomal hamycin: Reduced toxicity and improved antifungal efficacy in vitro and in vivo. J. Infect. Dis. 1991, 164, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Bhaduri, A.N.; Basu, M.K. Mannose-coated liposomal hamycin in the treatment of experimental leishmaniasis in hamsters. Biochem. Med. Metab. Biol. 1994, 53, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Nandi, G.; Mahato, S.B.; Pakrashi, A.; Basu, M.K. Drug delivery system: Targeting of pentamidines to specific sites using sugar grafted liposomes. J. Antimicrob. Chemother. 1996, 38, 145–150. [Google Scholar] [CrossRef]
- Cybulska, B.; Kupczyk, K.; Szlinder-Richert, J.; Borowski, E. Comparative in vitro studies on liposomal formulations of amphotericin B and its derivative, N-methyl-N-D-fructosyl amphotericin B methyl ester (MFAME). Acta Biochim. Pol. 2002, 49, 67–75. [Google Scholar] [CrossRef]
- Schell, U.; Haydock, S.F.; Kaja, A.L.; Carletti, I.; Lill, R.E.; Read, E.; Sheehan, L.S.; Low, L.; Fernandez, M.J.; Grolle, F.; et al. Engineered biosynthesis of hybrid macrolide polyketides containing D-angolosamine and D-mycaminose moieties. Org. Biomol. Chem. 2008, 6, 3315–3327. [Google Scholar] [CrossRef]
- Zhang, C.; Moretti, R.; Jiang, J.; Thorson, J.S. The in vitro characterization of polyene glycosyltransferases AmphDI and NysDI. Chembiochem 2008, 9, 2506–2514. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics: A Laboratory Manual; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).