Abstract
The perpetual significance of the pharmaceutical industry in society necessitates ongoing research efforts to enhance the efficacy of its manufacturing processes. Given that drug product manufacturing typically involves powder processing, a thorough understanding of powder characterization is needed for optimal process performance. Powder rheology is commonly examined in pharmaceutical manufacturing to elucidate the relationship between powder properties and the performance of pharmaceutical processes. This paper provides a brief discussion of recent literature regarding the various powder properties and characterization techniques encompassed in powder rheology. The powder properties are categorized into particle size, particle morphology, friability, electrostatics, permeability, wettability, cohesion, bulk density, and agglomeration sections. A distinct focus is placed on the segment about powder wettability. This review informs readers about the fundamental properties of powders known to influence pharmaceutical processes. It discusses the interrelationships among these properties, powder characterization techniques, and ideal states of powder properties that lead to optimal process performance.
1. Introduction
The pharmaceutical industry plays a crucial role in safeguarding the well-being of billions of individuals. Despite its significant impact, the industry faces ongoing challenges, prompting continuous research and improvement efforts. In recent years, there has been a notable shift from traditional batch processing to continuous manufacturing methods, driven by the benefits of enhanced safety and product quality control [1,2,3,4,5]. This transition has garnered considerable attention, but an equally important focus remains on understanding and characterizing powders and granular materials used in pharmaceutical manufacturing.
Powders are integral to various unit operations in pharmaceutical processes. Given that tablets represent upwards of 70% of all marketed drugs, the unit operations used in tablet manufacturing are particularly prevalent [6]. Key unit operations, such as feeders, blenders, and tablet press machines, are essential components of this process [2]. These operations often encounter issues tied to powder behavior, leading to performance challenges [7,8,9,10,11]. Given the centrality of powders in pharmaceutical operations, a precise understanding of their behavior is vital for ensuring optimal product quality [12,13,14,15,16]. Nevertheless, powders remain a complex and inadequately understood subject [17,18,19]. This knowledge gap has given rise to a significant body of literature dedicated to the quantification and prediction of powder behavior, known as powder rheology. Describing powder flow is uniquely challenging, as it defies rigid adherence to a set of rules or equations. Powders can exhibit solid-like compaction, liquid-like flow, and even gaseous properties during manufacturing processes. Consequently, characterizing powders demands the use of multiple parameters rather than relying on a single metric to assess flow accurately [20,21,22].
The subsequent sections of this paper provide a comprehensive literature review of powder properties, with a particular emphasis on wettability, and the associated characterization techniques most pertinent to pharmaceutical manufacturing. This review aims to meticulously describe the components of this paper in a way that effectively informs readers about powder rheology due to its paramount importance and various applications.
2. Importance of Powder Flowability in Pharmaceutical Manufacturing
Powder characterization techniques are employed to assess flowability, defined as a powder’s ability to flow under specific conditions [17]. Each technique yields one or more parameters that describe a particular powder property and quantify how a powder behaves in various circumstances. Although these parameters are interrelated [23], their evaluation of flowability often hinges on the stress state of the powder bed [24,25]. This implies that the same powder may yield significantly different results when subjected to testing under motionless or flowing conditions. Therefore, selecting appropriate characterization techniques is vital to prevent conflicting results among parameters. A plethora of parameters are employed in the literature to evaluate powder flowability in pharmaceutical operations, with each parameter associated with properties such as particle size, particle morphology, friability, electrostatics, permeability, wettability, cohesion, bulk density, and agglomeration [26,27,28,29,30,31,32]. These properties are typically analyzed through multiple characterization techniques, and the effectiveness of these techniques is often compared in the literature [33,34,35].
Flowability assessments are commonly coupled with the objective of improving powder flow in pharmaceutical manufacturing. This is frequently achieved through techniques like dry coating, where active pharmaceutical ingredients (APIs) are coated with nanoparticles [36,37,38,39]. Other methods include adding lubricants to blend formulations and controlling humidity to manage moisture content and other powder parameters [40,41,42]. Flowability assessments may also be used in efforts to improve the efficacy of drug delivery methods, such as dry powder inhalation and orodispersible tablets [43,44,45]. Beyond enhancing flowability, these powder parameters also shed light on the relationship between powder properties and the performance of pharmaceutical manufacturing processes. As mentioned earlier, suboptimal powder behavior can lead to unsatisfactory operational performance in pharmaceutical processes such as tableting and mixing [46,47,48]. Accurately evaluating powder properties is critical for properly determining and predicting a powder’s flowability. This pursuit also elevates our understanding of the connections between powder flowability and pharmaceutical process performance, as well as the relationships between various powder properties.
3. Powder Flow Properties and Characterization Techniques
The literature search for this paper entailed investigating powder flow properties and the respective characterization techniques frequently utilized in recent pharmaceutical research. In this section, these properties and techniques are explained while highlighting the connections to pharmaceutical process performance and the interrelationships among different powder properties. The powder properties are discussed in order of the increasing scale of each property, beginning with particle properties (particle size and morphology) and ending with bulk powder properties (cohesion and bulk density).
3.1. Particle Size
Particle size profoundly influences powder flowability [49] and is closely tied to cohesion and other dynamic and shear cell testing results [30,40,50]. Cohesion is notably sensitive to particle size because, with changing particle size, the balance between interparticle forces (e.g., van der Waals, capillary, electrostatic) and gravitational forces shifts. Smaller particles, when they fall below a certain threshold, experience relatively stronger interparticle forces, leading to increased cohesion and poor flowability [17,51]. The strong connection between particle size and cohesion signifies particle size as an imperative parameter to measure to enhance our understanding of powder cohesion.
Particle size holds great significance in pharmaceutical process performance [52]. In particular, particle size significantly impacts blending, tableting, and wet granulation processes [21,52,53]. This influences product attributes like content uniformity and drug dissolution [20,54]. Numerous pharmaceutical powder rheology studies employ particle size as a key factor for assessing powder flowability, covering diverse topics such as overall flowability, moisture content, surface modification, and direct compression in pharmaceutical manufacturing [7,12,20,46,55]. The clear significance of particle size in pharmaceutical manufacturing reinforces the importance of measuring it because of its use as an essential indicator of powder flowability and its relationship to process performance.
The measurement of particle size entails creating a particle size distribution (PSD) to show the range of particle diameters and their respective volume percentages. Common PSD parameters include d10, d50, d90, and span values. These values represent the diameters below which 10%, 50%, and 90% of the powder sample falls, while span provides a quantitative measure of PSD width by calculating the ratio between the difference of d90 and d10 to d50. Dry laser diffraction is a prevalent method for assessing the PSD of pharmaceutical powders, utilizing light scattering to determine particle sizes [20]. Instruments like the Malvern Mastersizer, Sympatec Helos/Rodos system, and Beckman Coulter Laser Diffraction Analyzer are commonly employed for laser diffraction [7,12,20,40,56,57]. Dry laser diffraction is favored for its speed, robustness, and minimal material requirement [54]. The PSD parameters produced from dry laser diffraction instruments allow one to understand the relevant details of a powder’s particle size quickly and effectively.
Other particle size analysis techniques include sieving and image analysis, each with specific limitations. Sieving, while straightforward, primarily suits larger particles, which are not typical for active pharmaceutical ingredients (APIs) in pharmaceutical manufacturing [54]. Therefore, sieving often complements other techniques to separate particles into different size ranges [58,59]. Image analysis offers more accurate particle size descriptions by accommodating for nonspherical particle shapes [30,59]. However, it demands significantly more time to generate a PSD representing a significant number of particles, making it a valuable tool for particle morphology analysis within powder rheology. The limitations of these other techniques result in them being less applicable than dry laser diffraction instruments for measuring a PSD; however, they can still be utilized advantageously for specific purposes when measuring particle size [30,58,59].
3.2. Particle Morphology
Particle morphology encompasses the shape and surface characteristics of powder particles, including surface roughness and energy [60,61,62,63]. Particle morphology, akin to particle size, plays a pivotal role in determining powder flowability [30,34,40]. The ideal morphology for optimal powder flowability consists of spherical particles with smooth surfaces [49]. However, in pharmaceutical manufacturing, API particles rarely exhibit this ideal shape [20]. Instead, their irregular shapes and rough surfaces adversely affect bulk powder flowability. The poor flowability of amorphous particles arises from interactions like mechanical interlocking and friction [13,21,50], ultimately impacting the performance of pharmaceutical manufacturing processes. Given the certainty of imperfect particle shapes and surfaces and their impact on powder flowability, particle morphology is another essential powder property to consider.
Particle Shape
In particle rheology studies, particle shape is often analyzed in conjunction with particle size due to their shared influence on powder flow properties [30]. Particle shape is typically assessed through optical or scanning electron microscopy (SEM). SEM produces high-definition particle images by scanning a surface with a focused electron beam [52,54,64]. Image analysis then yields various shape parameters as a single parameter is insufficient to quantify particle shape adequately [51]. Parameters commonly used include aspect ratio, roundness, and irregularity [51]. Aspect ratio (AR) quantifies particle elongation by calculating the ratio between the length (longest diameter) and width (shortest diameter) of a particle [40]. Roundness, expressed as the ratio between the square of the particle perimeter and the product of 4π and the particle image area, approaches 1 for particles close to a perfect circular shape. Irregularity measures the ratio between the particle perimeter and its longest diameter, with a value of π denoting a perfect circle [51]. Evaluating the deviation of particle shapes from an ideal sphere using parameters such as AR, roundness, and irregularity allows one to develop a better understanding of the effects of particle shape on powder flowability and other powder properties. A better understanding of these relationships can be leveraged to enhance powder flowability and, consequently, pharmaceutical process performance.
3.3. Friability
Friability refers to a powder’s susceptibility to deformation or attrition under shear or other various processing stresses. In pharmaceutical manufacturing, certain processes, notably milling, can significantly affect friable powders, potentially compromising product quality [17]. Thus, it is essential to identify and handle powders with high friability carefully to avoid adverse manufacturing impacts. Friability assessment typically involves dynamic testing or the continuous monitoring of particle size during processing. The details of dynamic testing are presented in the following subsection.
Dynamic Testing
Dynamic testing examines powder properties during a state of flow, with the FT4 Powder Rheometer’s dynamic test (stability and variable flow rate test) being a focus due to its established correlation with powder friability [1,17]. This test employs a cylindrical vessel to contain the powder bed. After an initial conditioning step, a blade moves to the bed’s bottom and returns in seven cycles while rotating at a constant speed. Subsequently, four additional cycles are conducted at different blade rotational speeds. Each cycle records the energy required for the blade to descend to the bed’s bottom and return, known as flow energy. These flow energy values are then used to calculate various characteristic values indicative of a powder’s friability and other important powder properties.
The values derived from the FT4 Powder Rheometer dynamic test include the Basic Flowability Energy (BFE), Stability Index (SI), Specific Energy (SE), and Flow Rate Index (FRI). The BFE quantifies the flow energy needed for the blade to descend during the seventh test cycle at a constant blade rotational speed. By measuring the energy required to descend through the powder bed, the BFE is largely affected by a powder bed’s consolidation state (or compressibility) [17]. It is particularly useful for evaluating processes aimed at adjusting a powder’s flowability, such as lubrication or milling [1]. The SI measures the ratio between the downward flow energy required during the seventh and first test cycles, indicating a powder’s change in flow energy over time due to factors like agglomeration, aeration, or electrostatic charging [8,57]. A Stability Index close to 1 signifies a non-friable powder, while significant deviations indicate pronounced friability.
The SE is determined by calculating the average upward flow energy during the sixth and seventh test cycles and dividing it by the total mass of the powder bed. Unlike BFE, SE assesses a powder’s unconfined state by measuring upward flow energy and is highly dependent on a powder bed’s cohesive forces rather than its consolidation state [17,21]. The FRI is derived by comparing the downward flow energy of the final test cycle to the eighth test cycle and highlights a powder’s sensitivity to blade rotational speed. FRI values typically exceed 1, reflecting easier flow at higher blade speeds [21]. An FRI above 3 often signifies a cohesive powder, where the considerable increase in flow energy with decreasing blade speed is attributed to increased air retention within the powder bed [17]. Additionally, the conditioned bulk density (CBD) of the powder is recorded after the test. This parameter can be used to calculate the normalized BFE (NBFE) by dividing the BFE by the powder bed’s mass (determined from the CBD and the powder bed volume) and, importantly, considers the powder density in its value [17].
By subjecting a powder bed to a dynamic state of flow for a prolonged period, dynamic testing effectively assesses a powder’s friability by measuring changes in flow energy. The FT4 Powder Rheometer dynamic test specifically provides parameters indicative of a powder’s friability, cohesion, and compressibility. Due to the importance of preserving powder particle integrity to maintain consistent powder behavior, dynamic testing is widely incorporated in pharmaceutical powder research to evaluate powder flowability and promote its uniformity throughout processing [21,30,40,51,57].
3.4. Electrostatic Charging
Electrostatic charging refers to a powder’s propensity to accumulate electric charge due to particle–particle and particle–surface interactions. This property has been a subject of extensive research in recent decades due to its inherent complexity [65]. While there are instances (such as dry coating) where electrostatic charging can be harnessed to enhance powder flow [66], it often poses challenges, hindering optimal flow. Electrostatic charging leads to issues like segregation, agglomeration, and jamming, significantly affecting powder flow, particularly in pharmaceutical manufacturing processes prone to charge accumulation such as blending, milling, and transport [32,67,68,69,70]. These issues negatively impact pharmaceutical process performance and the quality of solid-dose manufacturing products and dry powder inhaler formulations [71,72]. The complex nature of electrostatic charging coupled with its detrimental effects on pharmaceutical process performance make it a significant property to consider.
Electrostatic charging results from electric charge transfer between powder particles, typically regarded as being carried by electrons [69]. Two principal mechanisms of charge transfer are contact and triboelectric charging [65]. Contact charging occurs when different materials (e.g., powders or surfaces) come into contact, transfer charge, and are subsequently isolated. The phenomenon is influenced by various factors, including material properties and environmental conditions [66]. Triboelectric charging, on the other hand, arises from the collision, sliding, rolling, or frictional interaction between powder particles and/or unit operation surfaces. Peter Ireland has comprehensively explored triboelectric charging between particles and surfaces in both experimental and theoretical studies [73,74]. Pharmaceutical powders are especially prone to electrostatic charging due to their small size and low bulk density [67,72]. The relationship between a powder’s electrostatic properties and other powder properties further necessitates the understanding of the mechanisms that cause electrostatic charging.
The accumulation of charge in a powder is quantified as charge density, which is the ratio of accumulated charge to powder mass [52]. Typically, this is measured using an experimental setup involving a Faraday cup (or cage) connected to an electrometer [60,67,70,75]. The Faraday cup acts as a protective enclosure to isolate powder particles from external electric fields following induced charging and the electrometer measures the total charge. Additionally, simulation and modeling techniques, validated against experimental data, are employed to evaluate electrostatic charging [67,74]. Some studies also utilize inductive charge sensors to measure charge levels and polarity in powders [68,72]. For experimental purposes, corona charging is an additional electrostatic charging method used to intentionally charge neutral particles [69,76,77]. Overall, there is a multitude of pharmaceutical powder studies that aim to understand the mechanisms of powder electrostatic charging and quantify the charge in a powder bed so that powder flowability and pharmaceutical process performance may be improved.
3.5. Permeability
Permeability quantifies the ease with which air can traverse a powder bed, a property profoundly influenced by physical characteristics like particle shape, size, and surface texture, as well as bulk properties including bulk density and cohesion [17,21]. In pharmaceutical manufacturing, permeability holds immense importance, as it is a fundamental requirement for inducing flow and preventing process failure [1,17]. Processes such as tablet die filling, compaction, and hopper flow hinge upon the powder’s permeability [1,17,21,57]. Thus, the measurement of permeability becomes indispensable during process development, warranting its inclusion in flow behavior analysis studies [52,78].
Contemporary literature frequently employs the FT4 Powder Rheometer’s permeability test as a standardized technique for assessing powder permeability [8,22,29,30]. In this methodology, a cylindrical container is used to house the powder bed, featuring a porous base. After an initial conditioning phase, an aeration unit affixed to the base initiates a constant air flow through the base, while a vented piston consolidates the powder bed from above. The piston exerts increasing normal stresses incrementally as the pressure drop across the bed is monitored. The permeability under each applied normal stress is computed using Darcy’s Law (Equation (1)),
where k is the permeability (cm2), q is the flow rate (cm/s), γ denotes air viscosity (Pa·s), L is the height of the powder bed (cm), and ΔP indicates the pressure drop (Pa). Larger pressure drop values during the permeability test signify that air encounters resistance while passing through the powder bed. This results in lower permeability values, indicating cohesive and poorly flowing powder.
Powder permeability can be further explored using the aeration test, also offered by the FT4 Powder Rheometer [20,64,79,80]. This procedure resembles the permeability test, with the vented piston replaced by a blade that was initially used in the conditioning step. It measures permeability under dynamic conditions, differing from the static conditions of the permeability test. It records the energy required for the blade to traverse the powder bed as various air flow rates are applied. This energy required for the blade to pass through the powder bed at a certain flow rate is referred to as Aeration Energy (AE). The Aeration Ratio (AR), calculated by comparing AE values under no air flow and at maximum air flow rate, can also be represented as an Aeration Index (AI), as elaborated in [64]. A higher AR indicates the powder’s ability to experience more robust aeration, signifying improved flowability. In summation, the permeability and aeration tests from the FT4 Powder Rheometer are frequently observed in pharmaceutical powder literature to evaluate powder permeability because of the parameter’s association with many powder characteristics and its relation to pharmaceutical process performance.
3.6. Wettability
Wettability, the capacity of a powder to absorb liquids, is a crucial property in the pharmaceutical industry. It significantly influences various processes like wet granulation, lubrication, and tablet coating, playing a pivotal role in determining final product attributes, including tablet strength and dissolution performance [31,61,81,82]. Given its clear significance in pharmaceutical processing, powder wettability is another property that requires the utmost accuracy when measuring it to properly characterize powders.
3.6.1. Methods of Characterization
There are several methods available to characterize the wettability of powders. This paper primarily focuses on experimental methods found in pharmaceutical powder literature, which are used in conjunction with theoretical equations to calculate the contact angle (an indicator of powder wettability) between a test liquid and a powder bed. Simulation methods for examining the interaction between droplets and powders exist in other areas of powder literature [83] but are not discussed here. A few experimental methods observed in the literature and the droplet mechanisms that influence their results are presented in the following subsections.
Experimental Methods
Experimental methods represent the most prevalent wettability characterization approach found in pharmaceutical powder literature. These methods primarily revolve around quantifying the contact angle formed between the powder bed and a droplet of the test liquid. This measurement can be obtained directly or indirectly by assessing another parameter associated with the contact angle, such as penetration time [82]. A reduced contact angle indicates greater wettability, signifying increased droplet absorption into the powder bed. This paper delves into the sessile drop, Washburn, and drop penetration methods. Alghunaim et al. [84] comprehensively review additional experimental wettability measurement techniques. Before delving into these methods, it is crucial to comprehend the various droplet mechanisms and factors influencing droplet impact and penetration, especially in methods reliant on droplet dynamics like the sessile drop and drop penetration techniques.
Droplet Mechanisms
In total, four distinct mechanisms characterize droplet behavior upon impact on a powder bed: tunneling, spreading, crater formation, and liquid marbling. While tunneling, spreading, and crater formation involve eventual droplet penetration into the powder bed, liquid marbling does not entail any penetration. For mechanisms involving penetration, additional processes (retraction, splashing, pinning, etc.) play a role in the penetration process [85,86]. By increasing the knowledge of droplet mechanisms, the results of experimental methods (which depend on droplet dynamics) are better understood and powder wettability can be more accurately evaluated.
Tunneling occurs when a droplet encounters a nonuniform, cohesive powder bed, subsequently rolling and bouncing until penetration transpires [87]. This mechanism arises due to loose aggregates formed in a powder bed, resulting from its high cohesion. Instead of flowing between aggregate gaps, the droplet flows into the aggregate pores, absorbing the aggregates from all sides, significantly increasing its weight, and enabling it to “tunnel” into the powder bed, as shown in Figure 1.
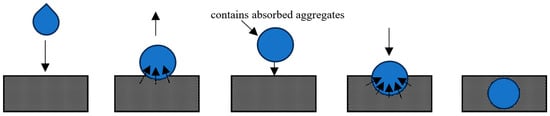
Figure 1.
Depiction of tunneling (arrows show the direction of droplet movement).
Spreading typically unfolds when a droplet interacts with a homogeneously packed powder bed, displacing a small amount of surface powder upon impact. As the droplet spreads, it accumulates a small number of powder particles and gradually penetrates the bed concurrently [87]. This behavior is observed with droplets released at low drop heights onto powder beds with a wide particle size distribution (PSD). The low drop height prevents significant crater formation, and the wide PSD ensures a densely packed bed resistant to other droplet mechanisms. Further insights into this mechanism are explored elsewhere [88,89,90]. Figure 2 provides a visual of the spreading phenomenon.
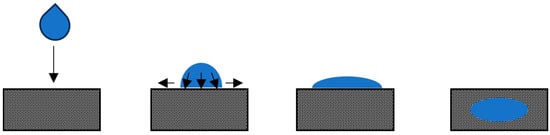
Figure 2.
Depiction of spreading (arrows show the direction of droplet movement).
Crater formation represents an intensified version of the spreading mechanism, resulting from a significantly higher droplet release point. This elevated release point imparts greater impact velocity and momentum, leading to the displacement of a larger amount of powder (a crater) on the powder bed surface [87]. The droplet accumulates more powder particles than observed in spreading and is forced to occupy a deeper crater; this causes reduced spreading over the powder bed surface as the droplet penetrates the bed. This process is visualized below in Figure 3.
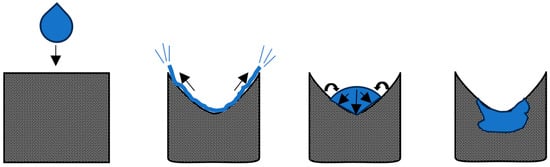
Figure 3.
Depiction of crater formation (arrows show the direction of droplet movement).
In the absence of droplet penetration, a liquid marble forms. Liquid marbles, completely covered by a powder particle layer, maintain a spherical shape held together by the powder shell. The process, known as solid spreading nucleation [91], is prevalent when a droplet impacts a predominantly hydrophobic powder bed [92]. Although the liquid marble may initially contain a droplet, evaporation may occur, leaving behind a hollow marble. Purposefully crafted hollow marbles find advantageous applications in pharmaceutical manufacturing [93,94]. The process of liquid marbling is displayed below in Figure 4.

Figure 4.
Depiction of marbling.
With the knowledge of these different droplet mechanisms, one can more easily distinguish when they occur during experimental methods used to evaluate powder wettability. Recognition of these droplet mechanisms then allows for one to make inferences about other powder properties (e.g., cohesion) or the experimental apparatus (e.g., droplet release point). With these inferences, a greater understanding of a powder’s wettability and its relationship to powder flowability can be achieved.
Factors Affecting Droplet Impact and Penetration
The impact and subsequent penetration of a liquid droplet into a powder bed are influenced by various factors, primarily stemming from the properties of both the powder bed and the liquid itself. Understanding the intricate relationship between these properties and the dynamics of droplet impact and penetration is crucial as it sheds light on the complex interactions between powders and liquids, ultimately aiding in a more comprehensive characterization of a powder’s wettability. This understanding, in turn, facilitates endeavors to modify powder wettability for specific manufacturing purposes.
The literature extensively documents numerous powder properties that are correlated with droplet impact and penetration dynamics. The hydrophobicity of a powder is widely investigated due to its significance in pharmaceutical processes like wet granulation or lubrication [81,95]. Notably, research indicates a direct relationship between hydrophobicity and droplet penetration. For instance, an experiment demonstrated that the drop penetration time increases as the hydrophobicity of a powder increases [94]. Similarly, particle size is implicated in droplet penetration dynamics, with decreasing particle size being correlated with longer drop penetration times [94]. Additionally, variations in particle size between hydrophobic and hydrophilic components in a powder blend contribute to substantial variability in drop penetration times [94,95]. Another powder bed property, packing fraction—calculated as the difference in mass between an empty and filled powder bed—is reported by Marston et al. [85] to significantly affect a droplet’s impact behavior and the ensuing mechanisms. It is important to be aware of these additional powder properties that affect droplet penetration in order to fully comprehend the results of powder wettability experimental methods and accurately assess powder wettability.
Equally imperative are the liquid properties of droplets, as highlighted by Marston et al. [85]. Their study explores the effects of liquid properties, such as surface tension, viscosity, and impact velocity, on droplet impact behavior when interacting with a powder bed. The experimentation involves the utilization of characteristic numbers, including the Weber number and Ohnesorge number, to quantify these effects. Furthermore, Mundozah et al. [96] delve into the competing mechanisms of droplet spreading and penetration upon impact with a powder bed and underscore the crucial role of viscosity in these processes. A further understanding of these liquid properties, which may affect experimental results, will similarly enable one to properly assess a powder’s wettability.
Sessile Drop Method
The sessile drop method, a direct approach used to measure the contact angle, is a commonly used technique for powder wettability characterization, visualized in Figure 5. In this method, a sessile drop of the test liquid is carefully placed on a conditioned powder bed’s surface. Subsequently, the contact angle between the droplet and the powder bed is directly measured using a contact angle goniometer or a high-speed camera [31,84]. A sessile drop, by definition, does not move laterally after deposition, ensuring accurate contact angle measurement. While the method is straightforward and requires minimal material, challenges arise from variations in powder surface properties, irregular droplet shapes, and rapid liquid penetration, contributing to inconsistencies [31,84]. The variety of complications that can negatively affect the accuracy of the results of this method often overshadow the method’s simplicity, which has led to researchers questioning its practicality in many scenarios [84].
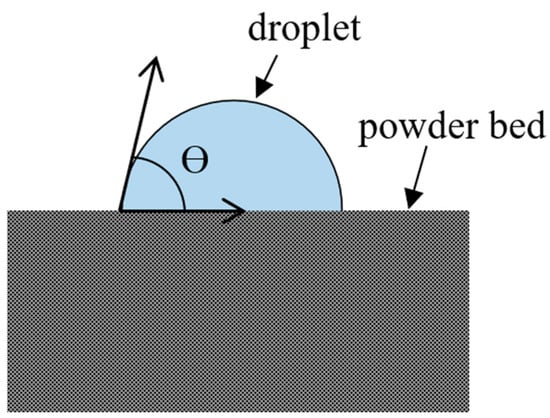
Figure 5.
Image of sessile drop method.
Washburn Method
The Washburn method indirectly gauges powder wettability by linking liquid penetration through capillary rise to the contact angle. It uses Darcy’s law (Equation (1)) to describe the flow of liquid within the powder bed along with a set of assumptions and boundary conditions to establish the relationship between the liquid penetration and contact angle. This method entails filling a cylindrical vessel with powder and submerging its base slightly in the test liquid, as shown in Figure 6. The top of the vessel is sealed to maintain constant air pressure and the bottom of the vessel is designed to hold the powder in place, enabling the liquid to ascend through the powder via capillary pressure. The Washburn method similarly follows a simple procedure and is often used for powder wettability measurements [84,97]. Despite its widespread use, this method faces challenges such as achieving reproducible packing and selecting an appropriate reference liquid, both noted in the literature [31,84,98]. Although this method has limitations, they are not as severe as those noted for the sessile drop method, enabling the Washburn method to still be utilized as a reliable indicator of powder wettability [84].
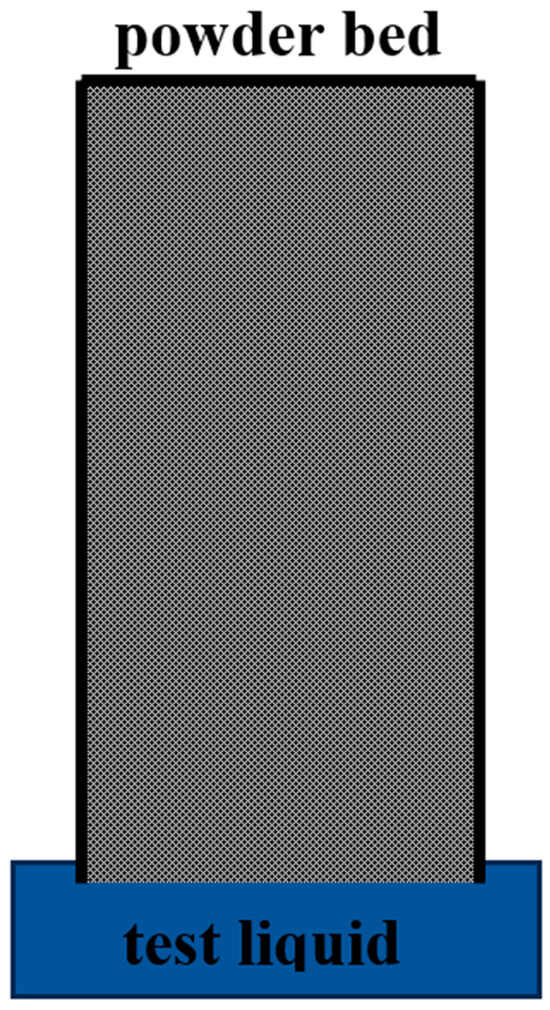
Figure 6.
Image of Washburn method.
Drop Penetration Method
The drop penetration method is another commonly employed technique for wettability measurement [82,84,99]. It calculates powder wettability by using the time it takes for a droplet to penetrate the powder bed to determine the contact angle. This method has a similar appearance to the sessile drop method seen in Figure 5 and, like the Washburn method, utilizes Darcy’s law (Equation (1)) to determine the relationship between the droplet penetration time and contact angle. The procedure involves applying a liquid droplet to a conditioned powder bed’s surface and observing its penetration dynamics using a high-speed camera. The total time for complete droplet absorption into the powder bed, known as the total penetration time, is used to compute the contact angle [99]. During the analysis, it is assumed that either the droplet contact area remains constant, causing a change in contact angle during penetration, or the contact angle remains constant, resulting in a reduction in the contact area as penetration progresses. This method offers the advantages of speed and minimal powder usage [99], making it particularly advantageous in pharmaceutical manufacturing, where API powders are often expensive and available only in limited quantities. Potential drawbacks include exceptionally fast liquid penetration and contact angle hysteresis due to surface roughness [84]. Similar to the Washburn method, the drawbacks for the drop penetration method are minor and allow the method to be utilized in pharmaceutical powder research as well as various other areas of powder research [99].
3.7. Cohesion
Powder cohesion, also termed powder cohesivity [100], refers to the tendency of powder particles to cling together, often due to the dominance of external forces over gravity-induced particle flow. These external forces encompass interparticle interactions like van der Waals, capillary, and electrostatic forces [63]. These forces are frequently influenced by various powder properties discussed in this review. For instance, van der Waals forces become more pronounced as particle size decreases [51]; capillary forces arise from powder wettability, leading to the formation of liquid bridges or lubrication within the powder bed [101], and both particle size and wettability contribute to electrostatic forces [102]. The relationships between cohesion and numerous other powder properties make it a crucial property to consider when assessing and predicting a powder’s flowability.
The intensity of interparticle forces significantly affects cohesion and, consequently, flowability. Cohesive powders exhibit poor flowability, characterized by issues such as powder agglomeration, which adversely impacts manufacturing processes [51]. In the pharmaceutical industry, challenges arise due to the inherent properties of active pharmaceutical ingredients (APIs), which are notorious for their cohesive nature and poor flowability [34]. These challenges manifest as difficulties in feeding, blending, granulation, and die filling processes, and can affect final product attributes, including content uniformity and weight variability [12,34,36,38,103]. In recognizing the strong connection between powder cohesion and pharmaceutical process performance, it becomes necessary to accurately assess cohesion for comprehensive powder characterization.
3.7.1. Shear Cell
The shear cell is a prevalent method for powder cohesion characterization because of its high repeatability [54,104,105]. Nevertheless, the absence of a universally accepted shear cell design does introduce some ambiguity when comparing data across different shear cells for the same powder. Koynov et al. conducted a study comparing results from three modern commercially available shear cells: the Schulze ring shear tester (RST), Freeman Technology powder rheometer (FT4), and Brookfield powder flow tester (PFT) [106]. Salehi et al. similarly compared outcomes from the Schulze RST, Brookfield PFT, and Jenike shear cell [107]. While a universally accepted shear cell is still lacking in the field of powder research, all of these instruments share a common testing procedure that has proven to be effective.
In a typical test, a powder bed undergoes pre-consolidation, pre-shearing, and shearing steps. The test cell is initially filled with powder in a gentle manner to prevent undesirable consolidation due to loading. Subsequently, the powder bed is compressed to a user-defined preliminary consolidation stress. Then, an equivalent normal stress is applied to the bed along with a rotational shear stress to reach the pre-shear point. The pre-shear point is denoted as the point where a user-defined normal stress is reached and the steady-state shear stress, also known as the yield stress, is achieved [17,54]. The yield stress occurs when the shear stress becomes constant, and the powder no longer resists the shear stress such that it begins to flow. Following these initial steps, four to five additional normal stresses, lower than the pre-consolidation stress, are applied to the powder bed as shearing occurs until it yields. These yield points, along with the pre-shear point, together construct a yield locus. Mohr circle analysis derives various critical values from this locus, primarily serving to quantify a powder’s cohesion and, consequently, flowability [54].
Beyond providing a cohesion value, Mohr circle analysis offers other valuable parameters such as the angle of internal friction, unconfined yield strength, major principal stress, and the flow function coefficient. The procedure involves plotting the yield locus points and fitting a best-fit line that extends to the y-axis, as shown in Figure 7.
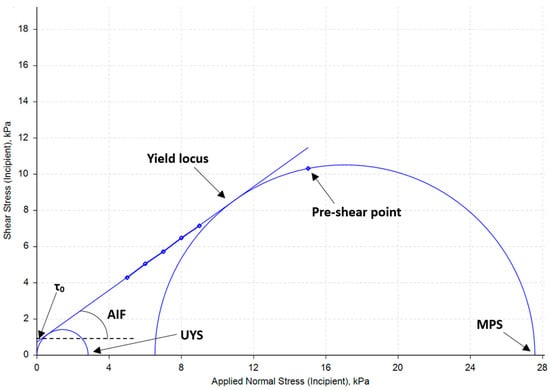
Figure 7.
Mohr circle analysis diagram.
The point of intersection with the y-axis represents cohesion (τ0), defined as the yield stress under zero normal applied stress. The angle of internal friction (AIF) is discerned by the angle between the best-fit line and the x-axis. Two circles are then drawn: one includes the origin point and remains tangential to the best-fit line, revealing the unconfined yield strength (UYS) at the point of intersection with the x-axis. UYS represents the yield stress of the powder under free surface (e.g., low stress) conditions [1,17]. The second circle is similarly drawn tangential to the best-fit line and includes the pre-shear point, revealing the major principal stress (also known as major consolidation stress) at the point where the circle intersects the x-axis. Major principal stress (MPS) characterizes the compacting stress in the powder bed under steady-state conditions. The flow function coefficient (FFC) can be calculated by taking the ratio of major principal stress and unconfined yield stress using Equation (2):
The flow function coefficient serves as a widely adopted metric for assessing powder flowability [104,105]. Higher FFC values indicate superior flowing capabilities, with typical values ranging from 1 to 10 and associated with various levels of flow behaviors [1,37]. The flow function, based on MPS and UYS values at different initial consolidation stresses, is a plot that also provides insights into a powder’s flowability [106]. In summation, the results of the shear cell test are represented by multiple parameters that depend on the cohesive forces in a powder bed. These parameter values allow one to assess the magnitude of a powder’s cohesion and the quality of a powder’s flowability.
3.7.2. Avalanche Testers
Avalanche testing offers an alternative approach to evaluating a powder’s cohesion and overall flowability that is employed in the pharmaceutical industry. There are various types of avalanche testers with their own unique mechanism for assessing avalanche properties. Every avalanche tester utilizes a rotating drum, which simulates a dynamic flow setting for a powder. This dynamic powder flow state resembles the type of flow that is observed during solid dosage production [103]. The following sections provide definitions of the avalanche properties measured and their relevance to cohesion and powder flowability. The different mechanisms of each tester for evaluating powder avalanches vary based on their approach to analyzing avalanche behavior during rotational powder flow. While some testers assess individual avalanche properties, others use alternative powder bed properties to quantify avalanche behavior. Regardless of the approach, each tester ultimately serves as a method of measuring a powder’s cohesion.
3.7.3. Revolution Powder Analyzer
The Revolution Powder Analyzer is one of the most frequently encountered testers in the literature, specializing in evaluating powder flow within a rotating drum using a camera and integrated software [40]. This instrument provides data on avalanche properties, including the avalanche angle, avalanche time, avalanche energy, and avalanche break energy [40,103,108]. The avalanche angle is determined just before an avalanche occurs, measuring the angle of the powder bed. Avalanche time records the duration between successive avalanches, while avalanche energy quantifies the energy released during each avalanche. An unconventional parameter, avalanche break energy, reflects the energy required to initiate an avalanche. Trpělková et al. investigate the efficacy of this parameter for evaluating powder cohesion [103]. Larger values for these properties suggest stronger cohesive forces within the powder, indicative of reduced flowability. Additional parameters like avalanche surface fractal and volume expansion ratio have been detailed by Spierings et al. [108]. Overall, the primary objective of the Revolution Powder Analyzer is to assess several properties of individual avalanches during rotational powder flow to quantify powder cohesion.
3.7.4. Gravitational Displacement Rheometer
The Gravitational Displacement Rheometer (GDR) characterizes avalanche behavior by employing a load cell beneath the rotating drum to monitor fluctuations in the drum’s center of gravity during avalanches. The methodology is further elaborated in the literature [109,110]. The resultant avalanche properties include dilation and the GDR Flow Index [109]. Dilation measures the percentage change in volume recorded for a pre-consolidated powder upon rotation of the drum. Greater dilation percentages signify the presence of larger avalanches, which, in turn, imply stronger cohesion and, consequently, poorer flowability. The GDR Flow Index quantifies the magnitude of a powder’s avalanching behavior by measuring the relative standard deviation of the powder bed volume, influenced by changes in the center of mass of the powder bed, at different rotational speeds. A higher GDR Flow Index similarly reflects the occurrence of larger avalanches, indicative of a powder with reduced flowability. Rather than measuring individual avalanche properties, the GDR evaluates powder cohesion by tracking the powder bed volume throughout rotational powder flow.
3.7.5. API AeroFlow Avalanche Tester
The API AeroFlow avalanche tester records both avalanche time and the total number of avalanches within a rotating drum using a light absorption sensor [59]. Avalanche time holds a synonymous definition to that seen for the Revolution Powder Analyzer. The total number of avalanches counts the number of avalanches during a defined test interval. An increased number of avalanches signifies a powder with lower cohesion, indicating improved flowability due to the presence of multiple smaller avalanches rather than a few larger ones, which are indicative of weaker cohesive forces. The unique utilization of a light absorption sensor in the API AeroFlow avalanche tester allows for the number of avalanches to be precisely recorded and implemented as an indicator of powder cohesion.
3.7.6. GranuDrum Instrument
The GranuDrum tester evaluates avalanche properties by analyzing the powder–air interface within a rotating drum using image analysis [52]. The primary measurements include the dynamic flow angle and dynamic cohesive index [52]. The dynamic flow angle is calculated based on the mean position of the powder–air interface. A larger dynamic flow angle corresponds to higher cohesive forces and, consequently, reduced flowability. The dynamic cohesive index is derived from changes in the interface. A more dynamic interface suggests poorer flow capabilities, as the powder struggles to avalanche and collapse upon itself as the drum rotates. Therefore, a higher dynamic cohesive index indicates greater cohesion and decreased flowability. The GranuDrum tester differs in its assessment of powder cohesion by considering the powder–air interface at the surface of a powder bed as it endures through rotational flow.
3.7.7. Bond Number
The Bond number serves as a quantitative measure of powder cohesion that is observed in the literature on powder rheology [34,36,58,62,100]. Rather than relying on experimental apparatus, the Bond number calculates cohesion as the ratio of cohesive forces to gravitational forces between powder particles, as shown in Equation (3):
where Bog is the Bond number, Fc represents interparticle cohesive forces, and Wg represents the interparticle gravitational forces induced by each particle’s weight. A Bond number exceeding 1 signifies a cohesive powder, as cohesive forces overpower gravitational forces, preventing flow. Conversely, a Bond number less than 1 identifies a non-cohesive powder. The Bond number uses a simple ratio between the primary forces present among powder particles to accurately calculate the magnitude of cohesion in a powder bed. However, there are numerous complex aspects of each interparticle force that may be considered before this simple ratio calculation.
Interparticle cohesive forces often encompass a combination of van der Waals, capillary, and electrostatic forces [34,111]. While the relative contribution of each force may vary with manufacturing conditions, van der Waals (vdW) forces are generally considered the dominant force [111]. Accordingly, considerable effort has been invested in accurately predicting vdW forces between particles. The Rumpf model is primarily referenced in the Bond number literature concerning cohesion in pharmaceutical powders. This model initially calculated vdW forces by accounting for non-contact and contact forces between a spherical particle and particle asperities [111]. Over time, various versions of this model have emerged to address particle surface roughness more accurately. One common variant, the “multi-asperity” model, factors in particle surface roughness, surface energy, and non-contact/contact forces [34,36,58]. This modified model considers these factors using particle diameter, the diameter of particle asperities, separation distance between particles, the Hamaker constant, and equilibrium separation distance. Recent research has adapted the original Bond number equation to assess powder cohesion more accurately, by incorporating factors like agglomeration [36], multi-component powder blends [34], and interactions between particles of different sizes in the same powder [58]. Studies comparing the original or modified Bond number equations with experimental parameters for assessing powder cohesion and flowability consistently demonstrate a strong correlation, confirming its efficacy in predicting cohesion [58,62,100]. It is noteworthy that the Bond number is considered by some authors as a superior predictor for ranking cohesive powders compared to non-cohesive ones [34,36]. Although the Bond number is proven to be an effective predictor of powder bed cohesion, the ample amount of research that aims to expand the models used for the Bond number calculation demonstrates the consensus that there is significant potential for improvement in the accuracy of the parameter.
3.8. Bulk Density
Bulk density is another fundamental powder property instrumental in evaluating powder flowability. It is defined as the mass of powder divided by the volume of the powder bed and serves as an indicator of both particle density and the consolidation state of the powder bed [17]. Techniques for measuring particle density, often referred to as true density [12], include helium or liquid pycnometry to assess a fluid’s penetration into particle pores [50,112]. Another method, detailed by Kalman et al. [113], entails counting and weighing a specific number of particles, with density calculated based on the average particle size. Due to the influence of consolidation state on bulk density, the literature often references aerated and tapped bulk densities for a more precise characterization of a powder’s density and its ability to pack and consolidate. Aerated bulk density represents the density without intentional consolidation by measuring the powder bed volume after it is carefully loaded into its container. Tapped bulk density reflects a highly consolidated state by inducing a specified number of taps to the powder bed before recording its volume. These values are often used to calculate the Carr Index and Hausner Ratio discussed in the latter subsections. Bulk density is an especially important parameter to consider in the assessment of powder flowability because it represents both small-scale (particle density) and large-scale (consolidation state) powder bed properties.
Bulk density is closely linked to powder flowability and pharmaceutical manufacturing processes [57]. In pharmaceutical manufacturing, powders undergo various processes that alter their packing state and, consequently, their bulk density [7,54,113]. Given the dynamic nature of bulk density during pharmaceutical manufacturing and its reflection of powder flowability, it is a vital property for characterization and continuous monitoring to ensure proper process performance [7].
3.8.1. Compressibility
Compressibility is a measure of how a powder’s bulk density changes under applied stress. In the literature, it is often assessed using a compressibility index, primarily involving aerated and tapped bulk density or volume values [7,28,109,114]. Alternatively, compressibility can be measured as the percent change in the volume of a powder bed under applied normal stress, typically using instruments such as the FT4 Powder Rheometer [30,115]. Complementing bulk density, compressibility is a prevalent parameter used to assess powder flowability in pharmaceutical processes [52]. The ability of a powder to consolidate under compressive stresses is particularly critical for processes like direct compression tableting, where successful compression is essential for producing solid products [14,21]. While compressibility alone does not solely predict powder flowability, it correlates with various other flowability indices. Highly compressible powders tend to pack poorly without applied stress, typically indicating cohesive behavior [21,51]. Therefore, a highly compressible powder is assumed to have poor flowability [8]. The relevance of compressibility to pharmaceutical manufacturing processes and its relationship to other powder properties explains the prevalence of this parameter in pharmaceutical powder research.
3.8.2. Carr Index
The Carr Index (CI) quantifies the ability of a powder’s bulk density to change in response to stress. It is often used analogously to the compressibility index in the literature and can be calculated using Equation (4) [20]:
Here, ρT represents the tapped bulk density and ρA is the aerated bulk density. An alternative approach involves using initial and final volume measurements of the powder bed during tapping [23]. Typically, a CI below 25% indicates acceptable to excellent powder flowability, while values exceeding this threshold suggest poor flowability [20,101]. The CI proficiently measures a powder’s compressibility by evaluating changes in bulk density and is in incorporated in extensive studies of powder flowability [39,51,59].
3.8.3. Hausner Ratio
The Hausner Ratio (HR) is another parameter used to estimate a powder’s flowability based on its bulk density. It is often employed in conjunction with the Carr Index and can be expressed by Equations (5) and (6) [24,116]:
where V0 is the volume of the aerated powder bed and Vf is the volume of the tapped powder bed. A Hausner Ratio exceeding 1.4 typically indicates cohesive behavior, while a value below 1.25 suggests that the powder is non-cohesive and free flowing [20,108]. Powders with HR values between 1.25 and 1.4 are in a transitional state, exhibiting slight cohesive qualities [46]. Similar to the CI, the HR effectively evaluates compressibility by examining a powder bed’s change in bulk density or volume. Both the Hausner Ratio and Carr Index are closely linked and are used separately or concurrently in the study of powder rheology [51,116].
3.9. Agglomeration
The propensity of powder particles to agglomerate and potentially segregate during processing is another powder property to consider. This property strongly links to powder flow characteristics, particularly cohesion and particle size [1,3,17,117]. In pharmaceutical manufacturing, agglomeration is a recurrent challenge, primarily due to APIs’ high cohesion and small particle sizes [13,48]. Agglomeration presents issues in various pharmaceutical processes, including drying and the formation of dry powder inhaler formulations [117,118,119]. The frequency and severity of agglomeration in these processes have direct consequences on blend uniformity and drug bioavailability, critical attributes of the final product [117,120]. Agglomeration is highly important to quantify because of its correlations to other significant powder properties as well as its widespread occurrence in pharmaceutical manufacturing, which causes performance and product issues.
Assessing the extent of powder agglomeration during manufacturing often involves the visual inspection of agglomerates at different processing stages. Techniques like near-infrared or ultraviolet spectroscopy provide the visualization of agglomerates [117,121]. Sieving is another method used to observe agglomerates of various sizes [118,120]. Spectroscopy and sieving techniques monitor agglomeration by measuring agglomerate concentration in powder blends during processing. This concentration is frequently compared to the shear rate and strain that a powder experiences, emphasizing the importance of proper blending and mixing processes to reduce agglomeration [117,119,120]. Simulation and modeling approaches are also employed in the literature to gain a deeper understanding of the mechanisms governing powder deagglomeration [48,122]. Employing these characterization techniques enhances the understanding of agglomeration within a powder bed, facilitating a deeper comprehension of its impact on pharmaceutical processes and the interrelationships between agglomeration and other powder properties.
4. Conclusions
Powder rheology is a captivating research area, with diverse industries constantly seeking to optimize their powder-centric manufacturing processes. The complex nature of powder flow, defying simplification into a single parameter, has sparked a continuous collaboration between industry leaders and researchers to unravel the intricate relationship between powder flowability and process performance. This review has specifically focused on powder rheology within the pharmaceutical industry, highlighting key powder properties relevant to powder flowability in manufacturing processes.
The paper explored particle size, particle morphology, friability, electrostatic charging, permeability, wettability, cohesion, bulk density, and agglomeration and their impacts on pharmaceutical processing. It delved into the measurement techniques commonly used to assess these properties. Notably, each of these properties proved to influence at least one primary unit operation—feeders, blenders/mixers, or tableting—vital in pharmaceutical manufacturing. Interconnectedness among powder properties was evident, yet none stood alone as an accurate predictor of overall flowability. This exemplifies the necessity for employing multiple powder parameters for comprehensive powder characterization in pharmaceutical manufacturing.
The intricate nature of powder rheology demands ongoing research to understand powder flowability, improve existing characterization techniques, and devise more precise modern methods to categorize powders for manufacturing purposes. By advancing research in these areas of powder rheology, it is possible to improve both the simplicity and accuracy of measuring powder flowability, thereby optimizing pharmaceutical manufacturing processes. This review functions as an introduction to the complex field of powder rheology and, consequently, fosters a broader comprehension of the subject. Given its considerable relevance to various industries, a basic knowledge of powder characterization is essential. A deeper grasp of the topic can also serve to significantly enhance the efficacy of pharmaceutical and other critical industry processes by enriching process understanding.
Author Contributions
Conceptualization, supervision, and editing, S.M.; writing—original draft. preparation: J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors gratefully acknowledge the support of the CSULB Research, Scholarly, and Creative Activity Program.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, Z. Understanding Powder Behavior in Pharmaceutical Manufacturing to Enhance Drug Productivity and Therapeutic Performance. Ph.D. Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Moghtadernejad, S.; Escotet-Espinoza, M.S.; Oka, S.; Singh, R.; Liu, Z.; Román-Ospino, A.D.; Li, T.; Razavi, S.; Panikar, S.; Scicolone, J.; et al. A training on: Continuous Manufacturing (Direct Compaction) of Solid Dose Pharmaceutical Products. J. Pharm. Innov. 2018, 13, 155–187. [Google Scholar] [CrossRef]
- Ervasti, T.; Niinikoski, H.; Mäki-Lohiluoma, E.; Leppinen, H.; Ketolainen, J.; Korhonen, O.; Lakio, S. The comparison of two challenging low dose APIs in a continuous direct compression process. Pharmaceutics 2020, 12, 279. [Google Scholar] [CrossRef]
- Plumb, K. Continuous Processing in the Pharmaceutical Industry: Changing the Mind Set. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- Oka, S.S.; Escotet-Espinoza, M.S.; Singh, R.; Scicolone, J.V.; Hausner, D.B.; Ierapetritou, M.; Muzzio, F.J. Design of an Integrated Continuous Manufacturing System; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Patel, D.B.; Dave, R.H. Predicting lubricants effect on tablet sticking using ketoprofen as model drug and evaluating sticking propensity using different metals and powder rheology. Int. J. Pharm. 2021, 606, 913. [Google Scholar] [CrossRef]
- Garg, V.; Mallick, S.S.; Garcia-Trinanes, P.; Berry, R.J. An investigation into the flowability of fine powders used in pharmaceutical industries. Powder Technol. 2018, 336, 375–382. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Muzzio, F.J.; Glasser, B.J. Predicting feeder performance based on material flow properties. Powder Technol. 2017, 308, 135–148. [Google Scholar] [CrossRef]
- Portillo, P.M.; Ierapetritou, M.G.; Muzzio, F.J. Characterization of continuous convective powder mixing processes. Powder Technol. 2008, 182, 368–378. [Google Scholar] [CrossRef]
- Saddik, J.S.; Dave, R.H. Evaluation of powder rheology as a potential tool to predict tablet sticking. Powder Technol. 2021, 386, 298–306. [Google Scholar] [CrossRef]
- Parekh, B.V.; Saddik, J.S.; Patel, D.B.; Dave, R.H. Evaluating the effect of glidants on tablet sticking propensity of ketoprofen using powder rheology. Int. J. Pharm. 2023, 635, 2710. [Google Scholar] [CrossRef]
- Janssen, P.H.M.; Depaifve, S.; Neveu, A.; Francqui, F.; Dickhoff, B.H.J. Impact of powder properties on the rheological behavior of excipients. Pharmaceutics 2021, 13, 1198. [Google Scholar] [CrossRef]
- Majerová, D.; Kulaviak, L.; Růžička, M.; Štěpánek, F.; Zámostný, P. Effect of colloidal silica on rheological properties of common pharmaceutical excipients. Eur. J. Pharm. Biopharm. 2016, 106, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Macho, O.; Gabrišová, Ľ.; Brokešová, J.; Svačinová, P.; Mužíková, J.; Galbavá, P.; Blaško, J.; Šklubalová, Z. Systematic study of paracetamol powder mixtures and granules tabletability: Key role of rheological properties and dynamic image analysis. Int. J. Pharm. 2021, 608, 1110. [Google Scholar] [CrossRef] [PubMed]
- Worku, Z.A.; Kumar, D.; Gomes, J.V.; He, Y.; Glennon, B.; Ramisetty, K.A.; Rasmuson, Å.C.; O’Connell, P.; Gallagher, K.H.; Woods, T.; et al. Modelling and understanding powder flow properties and compactability of selected active pharmaceutical ingredients, excipients and physical mixtures from critical material properties. Int. J. Pharm. 2017, 531, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Chendo, C.; Pinto, J.F.; Paisana, M.C. Comprehensive powder flow characterization with reduced testing. Int. J. Pharm. 2023, 642, 3107. [Google Scholar] [CrossRef] [PubMed]
- Koynov, S. Using Statistical Methods to Optimize Powder Flow Measurements and to Predict Powder Processing Performance. Ph.D. Thesis, The State University of New Jersey, New Brunswick, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Navaneethan, C.V.; Missaghi, S.; Fassihi, R. Application of powder rheometer to determine powder flow properties and lubrication efficiency of pharmaceutical particulate systems. AAPS PharmSciTech 2005, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Freeman, T.; Brockbank, K.; Sabathier, J. Characterising powder flow properties—the need for a multivariate approach. EPJ Web Conf. 2017, 140, 3008. [Google Scholar] [CrossRef]
- Ghoroi, C.; Gurumurthy, L.; McDaniel, D.J.; Jallo, L.J.; Davé, R.N. Multi-faceted characterization of pharmaceutical powders to discern the influence of surface modification. Powder Technol. 2013, 236, 63–74. [Google Scholar] [CrossRef]
- Narang, A.S.; Badawy, S.I.F. An Introduction to Powder Characterization. In Handbook of Pharmaceutical Wet Granulation; Elsevier: Amsterdam, The Netherlands, 2019; pp. 569–611. [Google Scholar] [CrossRef]
- Divya, S.; Ganesh, G. Characterization of Powder Flowability Using FT4—Powder Rheometer. J. Pharm. Sci. Res. 2019, 11, 25–29. [Google Scholar]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Davé, R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef]
- Lumay, G.; Boschini, F.; Traina, K.; Bontempi, S.; Remy, J.C.; Cloots, R.; Vandewalle, N. Measuring the flowing properties of powders and grains. Powder Technol. 2012, 224, 19–27. [Google Scholar] [CrossRef]
- Krantz, M.; Zhang, H.; Zhu, J. Characterization of powder flow: Static and dynamic testing. Powder Technol. 2009, 194, 239–245. [Google Scholar] [CrossRef]
- Colbert, M.J.; Grandbois, M.; Abatzoglou, N. Identification of inter-particular forces by atomic force microscopy and how they relate to powder rheological properties measured in shearing tests. Powder Technol. 2015, 284, 396–402. [Google Scholar] [CrossRef]
- Mullarney, M.P.; Beach, L.E.; Davé, R.N.; Langdon, B.A.; Polizzi, M.; Blackwood, D.O. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011, 212, 397–402. [Google Scholar] [CrossRef]
- Sarraguça, M.C.; Cruz, A.V.; Soares, S.O.; Amaral, H.R.; Costa, P.C.; Lopes, J.A. Determination of flow properties of pharmaceutical powders by near infrared spectroscopy. J. Pharm. Biomed. Anal. 2010, 52, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Cordts, E.; Steckel, H. Capabilities and limitations of using powder rheology and permeability to predict dry powder inhaler performance. Eur. J. Pharm. Biopharm. 2012, 82, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of particle shape and size on flow properties of lactose powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- Susana, L.; Campaci, F.; Santomaso, A.C. Wettability of mineral and metallic powders: Applicability and limitations of sessile drop method and Washburn’s technique. Powder Technol. 2012, 226, 68–77. [Google Scholar] [CrossRef]
- Jallo, L.J.; Dave, R.N. Explaining Electrostatic Charging and Flow of Surface-Modified Acetaminophen Powders as a Function of Relative Humidity Through Surface Energetics. J. Pharm. Sci. 2015, 104, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Blanco, D.; Antikainen, O.; Räikkönen, H.; Mah, P.T.; Healy, A.M.; Juppo, A.M.; Yliruusi, J. Image-based characterization of powder flow to predict the success of pharmaceutical minitablet manufacturing. Int. J. Pharm. 2020, 581, 9280. [Google Scholar] [CrossRef]
- Capece, M.; Ho, R.; Strong, J.; Gao, P. Prediction of powder flow performance using a multi-component granular Bond number. Powder Technol. 2015, 286, 561–571. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.I.; Zamudio-Flores, P.B.; Ortega-Rivas, E.; Perez-Vega, S.; Sepulveda, D.R. Effect of water content on the flowability of hygroscopic powders. J. Food Eng. 2017, 205, 12–17. [Google Scholar] [CrossRef]
- Huang, Z.; Scicolone, J.V.; Gurumuthy, L.; Davé, R.N. Flow and bulk density enhancements of pharmaceutical powders using a conical screen mill: A continuous dry coating device. Chem. Eng. Sci. 2015, 125, 209–224. [Google Scholar] [CrossRef]
- Huang, Z.; Scicolone, J.V.; Han, X.; Davé, R.N. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int. J. Pharm. 2015, 478, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Setia, G. Mechanical dry particle coating on cohesive pharmaceutical powders for improving flowability—A review. Powder Technol. 2019, 356, 458–479. [Google Scholar] [CrossRef]
- Zhou, Q.T.; Qu, L.; Larson, I.; Stewart, P.J.; Morton, D.A.V. Effect of mechanical dry particle coating on the improvement of powder flowability for lactose monohydrate: A model cohesive pharmaceutical powder. Powder Technol. 2011, 207, 414–421. [Google Scholar] [CrossRef]
- Goh, H.P.; Heng, P.W.S.; Liew, C.V. Comparative evaluation of powder flow parameters with reference to particle size and shape. Int. J. Pharm. 2018, 547, 133–141. [Google Scholar] [CrossRef]
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Hausmann, A.; Buck, B.; Shaw, L.; Simons, T.; Jäger, F.K.; Williams, D. The importance of humidity control in powder rheometer studies. Powder Technol. 2023, 421, 8425. [Google Scholar] [CrossRef]
- Li, J.; Ma, S.; He, X.; Sun, Y.; Zhang, X.; Guan, J.; Mao, S. Exploring the influence of magnesium stearate content and mixing modality on the rheological properties and in vitro aerosolization of dry powder inhaler. Int. J. Pharm. 2023, 642, 3179. [Google Scholar] [CrossRef]
- Almansour, K.; Alfagih, I.M.; Shalash, A.O.; Brockbank, K.; Ali, R.; Freeman, T.; Elsayed, M.M.A. Insights into the potential of rheological measurements in development of dry powder inhalation formulations. Int. J. Pharm. 2022, 614, 121407. [Google Scholar] [CrossRef]
- Tranová, T.; Macho, O.; Loskot, J.; Mužíková, J. Study of rheological and tableting properties of lubricated mixtures of co-processed dry binders for orally disintegrating tablets. Eur. J. Pharm. Sci. 2022, 168, 106035. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, C.; Sun, C.C.; Rantanen, J. Analytical method development for powder characterization: Visualization of the critical drug loading affecting the processability of a formulation for direct compression. J. Pharm. Biomed. Anal. 2016, 128, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Hsiau, S.S.; Huang, T.Y. The effect of vibrating conditions on the electrostatic charge in a vertical vibrating granular bed. Powder Technol. 2011, 208, 1–6. [Google Scholar] [CrossRef]
- Kale, K.; Hapgood, K.; Stewart, P. Drug agglomeration and dissolution—What is the influence of powder mixing? Eur. J. Pharm. Biopharm. 2009, 72, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Schulze, D. Powders and Bulk Solids: Behavior, Characterization, Storage and Flow, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Shi, H.; Mohanty, R.; Chakravarty, S.; Cabiscol, R.; Morgeneyer, M.; Zetzener, H.; Ooi, J.Y.; Kwade, A.; Luding, S.; Magnanimo, V. Effect of particle size and cohesion on powder yielding and flow. KONA Powder Part. J. 2018, 2018, 226–250. [Google Scholar] [CrossRef]
- Tay, J.Y.S.; Liew, C.V.; Heng, P.W.S. Powder Flow Testing: Judicious Choice of Test Methods. AAPS PharmSciTech 2017, 18, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di Pretoro, G.; Remon, J.P.; Vervaet, C.; De Beer, T.; et al. A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef]
- Pingali, K.; Mendez, R.; Lewis, D.; Michniak-Kohn, B.; Cuitiño, A.; Muzzio, F. Evaluation of strain-induced hydrophobicity of pharmaceutical blends and its effect on drug release rate under multiple compression conditions. Drug Dev. Ind. Pharm. 2011, 37, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Schoenwald, D.R.D.; Guillory, J.K.; Matheson, L.E.; Parrott, E.L.; Flanagan, D.R.; Wurster, D.E.; Veng-Pedersen, P.; Grant, D.J.W. Particle, powder, and compact characterization. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2009; pp. 163–183. [Google Scholar]
- Kalman, H. Effect of moisture content on flowability: Angle of repose, tilting angle, and Hausner ratio. Powder Technol. 2021, 393, 582–596. [Google Scholar] [CrossRef]
- Juarez-Enriquez, E.; Olivas, G.I.; Ortega-Rivas, E.; Zamudio-Flores, P.B.; Perez-Vega, S.; Sepulveda, D.R. Water activity, not moisture content, explains the influence of water on powder flowability. LWT—Food Sci. Technol. 2019, 100, 35–39. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Scicolone, J.; Wang, Y.; Pereira, G.; Schäfer, E.; Vigh, T.; Klingeleers, D.; Ierapetritou, M.; Muzzio, F.J. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018, 339, 659–676. [Google Scholar] [CrossRef]
- Capece, M.; Silva, K.R.; Sunkara, D.; Strong, J.; Gao, P. On the relationship of inter-particle cohesiveness and bulk powder behavior: Flowability of pharmaceutical powders. Int. J. Pharm. 2016, 511, 178–189. [Google Scholar] [CrossRef]
- Allenspach, C.; Timmins, P.; Sharif, S.; Minko, T. Characterization of a novel hydroxypropyl methylcellulose (HPMC) direct compression grade excipient for pharmaceutical tablets. Int. J. Pharm. 2020, 583, 119343. [Google Scholar] [CrossRef]
- Lapčík, L.; Otyepka, M.; Otyepková, E.; Lapčíková, B.; Gabriel, R.; Gavenda, A. Prudilová, Surface heterogeneity: Information from inverse gas chromatography and application to model pharmaceutical substances. Curr. Opin. Colloid. Interface Sci. 2016, 24, 64–71. [Google Scholar] [CrossRef]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef]
- Jallo, L.J.; Chen, Y.; Bowen, J.; Etzler, F.; Dave, R. Prediction of inter-particle adhesion force from surface energy and surface roughness. J. Adhes. Sci. Technol. 2011, 25, 367–384. [Google Scholar] [CrossRef]
- Zafar, U.; Vivacqua, V.; Calvert, G.; Ghadiri, M.; Cleaver, J.A.S. A review of bulk powder caking. Powder Technol. 2017, 313, 389–401. [Google Scholar] [CrossRef]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Matsusaka, S.; Maruyama, H.; Matsuyama, T.; Ghadiri, M. Triboelectric charging of powders: A review. Chem. Eng. Sci. 2010, 65, 5781–5807. [Google Scholar] [CrossRef]
- Naik, S.; Mukherjee, R.; Chaudhuri, B. Triboelectrification: A review of experimental and mechanistic modeling approaches with a special focus on pharmaceutical powders. Int. J. Pharm. 2016, 510, 375–385. [Google Scholar] [CrossRef]
- Naik, S.; Sarkar, S.; Gupta, V.; Hancock, B.C.; Abramov, Y.; Yu, W.; Chaudhuri, B. A combined experimental and numerical approach to explore tribocharging of pharmaceutical excipients in a hopper chute assembly. Int. J. Pharm. 2015, 491, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Garg, V.; Bradley, M.S.A. Electrostatic Charging of Fine Powders and Assessment of Charge Polarity Using an Inductive Charge Sensor. Nanomanufacturing 2023, 3, 281–292. [Google Scholar] [CrossRef]
- Kaialy, W. A review of factors affecting electrostatic charging of pharmaceuticals and adhesive mixtures for inhalation. Int. J. Pharm. 2016, 503, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Šupuk, E.; Zarrebini, A.; Reddy, J.P.; Hughes, H.; Leane, M.M.; Tobyn, M.J.; Timmins, P.; Ghadiri, M. Tribo-electrification of active pharmaceutical ingredients and excipients. Powder Technol. 2012, 217, 427–434. [Google Scholar] [CrossRef]
- Pingali, K.C.; Shinbrot, T.; Hammond, S.V.; Muzzio, F.J. An observed correlation between flow and electrical properties of pharmaceutical blends. Powder Technol. 2009, 192, 157–165. [Google Scholar] [CrossRef]
- Hussain, T.; Deng, T.; Bradley, M.S.A.; Armour-Chélu, D.; Gorman, T.; Kaialy, W. Evaluation studies of a sensing technique for electrostatic charge polarity of pharmaceutical particulates. IET Sci. Meas. Technol. 2016, 10, 442–448. [Google Scholar] [CrossRef]
- Ireland, P.M. Triboelectrification of particulate flows on surfaces: Part I—Experiments. Powder Technol. 2010, 198, 189–198. [Google Scholar] [CrossRef]
- Ireland, P.M. Triboelectrification of particulate flows on surfaces: Part II—Mechanisms and models. Powder Technol. 2010, 198, 199–210. [Google Scholar] [CrossRef]
- Zarrebini, A.; Ghadiri, M.; Dyson, M.; Kippax, P.; McNeil-Watson, F. Tribo-electrification of powders due to dispersion. Powder Technol. 2013, 250, 75–83. [Google Scholar] [CrossRef]
- Biegaj, K.W.; Rowland, M.G.; Lukas, T.M.; Heng, J.Y.Y. Surface Chemistry and Humidity in Powder Electrostatics: A Comparative Study between Tribocharging and Corona Discharge. ACS Omega 2017, 2, 1576–1582. [Google Scholar] [CrossRef]
- Intra, P.; Tippayawong, N. Development and evaluation of a faraday cup electrometer for measuring and sampling atmospheric ions and charged aerosols. Part. Sci. Technol. 2015, 33, 257–263. [Google Scholar] [CrossRef]
- Freeman, R. Measuring the flow properties of consolidated, conditioned and aerated powders—A comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007, 174, 25–33. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, D.; Li, J.; Zhao, J.; Feng, Y.; Zhang, X.; Mao, S. Elucidation of lactose fine size and drug shape on rheological properties and aerodynamic behavior of dry powders for inhalation. Eur. J. Pharm. Biopharm. 2022, 179, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, S.; Sun, Y.; Song, R.; Cai, B.; Li, H.; Chen, Y.; Zhang, X.; Guan, J.; Mao, S. Predicting in vitro lung deposition behavior of combined dry powder inhaler via rheological properties. Eur. J. Pharm. Biopharm. 2022, 181, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Llusa, M.; Levin, M.; Snee, R.D.; Muzzio, F.J. Measuring the hydrophobicity of lubricated blends of pharmaceutical excipients. Powder Technol. 2010, 198, 101–107. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Muzzio, F.; Drazer, G. Callegari, A drop penetration method to measure powder blend wettability. Int. J. Pharm. 2018, 538, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Tan, H. Three-dimensional simulation of micrometer-sized droplet impact and penetration into the powder bed. Chem. Eng. Sci. 2016, 153, 93–107. [Google Scholar] [CrossRef]
- Alghunaim, A.; Kirdponpattara, S.; Newby, B.M.Z. Techniques for determining contact angle and wettability of powders. Powder Technol. 2016, 287, 201–215. [Google Scholar] [CrossRef]
- Marston, J.O.; Thoroddsen, S.T.; Ng, W.K.; Tan, R.B.H. Experimental study of liquid drop impact onto a powder surface. Powder Technol. 2010, 203, 223–236. [Google Scholar] [CrossRef]
- Mohammadi, M.; Tembely, M.; Dolatabadi, A. Supercooled water droplet impacting superhydrophobic surfaces in the presence of cold air flow. Appl. Sci. 2017, 7, 130. [Google Scholar] [CrossRef]
- Emady, H.N.; Kayrak-Talay, D.; Schwerin, W.C.; Litster, J.D. Granule formation mechanisms and morphology from single drop impact on powder beds. Powder Technol. 2011, 212, 69–79. [Google Scholar] [CrossRef]
- Marston, J.O.; Sprittles, J.E.; Zhu, Y.; Li, E.Q.; Vakarelski, I.U.; Thoroddsen, S.T. Drop spreading and penetration into pre-wetted powders. Powder Technol. 2013, 239, 128–136. [Google Scholar] [CrossRef]
- Charles-Williams, H.R.; Wengeler, R.; Flore, K.; Feise, H.; Hounslow, M.J.; Salman, A.D. Granule nucleation and growth: Competing drop spreading and infiltration processes. Powder Technol. 2011, 206, 63–71. [Google Scholar] [CrossRef]
- Werner, S.R.L.; Jones, J.R.; Paterson, A.H.J.; Archer, R.H.; Pearce, D.L. Droplet impact and spreading: Droplet formulation effects. Chem. Eng. Sci. 2007, 62, 2336–2345. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Farber, L.; Michaels, J.N. Agglomeration of hydrophobic powders via solid spreading nucleation. Powder Technol. 2009, 188, 248–254. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Properties of liquid marbles. Proc. R. Soc. A Math. Phys. Eng. Sci. 2006, 462, 973–999. [Google Scholar] [CrossRef]
- Hapgood, K.P.; Khanmohammadi, B. Granulation of hydrophobic powders. Powder Technol. 2009, 189, 253–262. [Google Scholar] [CrossRef]
- Nguyen, T.; Shen, W.; Hapgood, K. Drop penetration time in heterogeneous powder beds. Chem. Eng. Sci. 2009, 64, 5210–5221. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Shen, W.; Hapgood, K. Effect of formulation hydrophobicity on drug distribution in wet granulation. Chem. Eng. J. 2010, 164, 330–339. [Google Scholar] [CrossRef]
- Mundozah, A.L.; Cartwright, J.J.; Tridon, C.C.; Hounslow, M.J.; Salman, A.D. Hydrophobic/hydrophilic static powder beds: Competing horizontal spreading and vertical imbibition mechanisms of a single droplet. Powder Technol. 2018, 330, 275–283. [Google Scholar] [CrossRef]
- Li, M.; Callegari, G.; Drazer, G. Capillary rise in a closed column: Application to the characterization of powders. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 124822. [Google Scholar] [CrossRef]
- Thakker, M.; Karde, V.; Shah, D.O.; Shukla, P.; Ghoroi, C. Wettability measurement apparatus for porous material using the modified Washburn method. Meas. Sci. Technol. 2013, 24, 125902. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Muzzio, F.J.; Callegari, G.; Drazer, G. Capillary Drop Penetration Method to Characterize the Liquid Wetting of Powders. Langmuir 2017, 33, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Affleck, S.; Thomas, A.L.; Routh, A.F.; Vriend, N.M. Novel protocol for quantifying powder cohesivity through fluidisation tests. Powder Technol. 2023, 415, 118147. [Google Scholar] [CrossRef]
- Crouter, A.; Briens, L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech 2014, 15, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Karner, S.; Urbanetz, N.A. The impact of electrostatic charge in pharmaceutical powders with specific focus on inhalation-powders. J. Aerosol. Sci. 2011, 42, 428–445. [Google Scholar] [CrossRef]
- Trpělková, Ž.; Hurychová, H.; Kuentz, M.; Vraníková, B. Šklubalová, Introduction of the energy to break an avalanche as a promising parameter for powder flowability prediction. Powder Technol. 2020, 375, 33–41. [Google Scholar] [CrossRef]
- Wang, Y.; Koynov, S.; Glasser, B.J.; Muzzio, F.J. A method to analyze shear cell data of powders measured under different initial consolidation stresses. Powder Technol. 2016, 294, 105–112. [Google Scholar] [CrossRef]
- Leung, L.Y.; Mao, C.; Chen, L.P.; Yang, C.Y. Precision of pharmaceutical powder flow measurement using ring shear tester: High variability is inherent to powders with low cohesion. Powder Technol. 2016, 301, 920–926. [Google Scholar] [CrossRef]
- Koynov, S.; Glasser, B.; Muzzio, F. Comparison of three rotational shear cell testers: Powder flowability and bulk density. Powder Technol. 2015, 283, 103–112. [Google Scholar] [CrossRef]
- Salehi, H.; Barletta, D.; Poletto, M. A comparison between powder flow property testers. Particuology 2017, 32, 10–20. [Google Scholar] [CrossRef]
- Spierings, A.B.; Voegtlin, M.; Bauer, T.; Wegener, K. Powder flowability characterisation methodology for powder-bed-based metal additive manufacturing. Progress. Addit. Manuf. 2016, 1, 9–20. [Google Scholar] [CrossRef]
- Vasilenko, A.; Koynov, S.; Glasser, B.J.; Muzzio, F.J. Role of consolidation state in the measurement of bulk density and cohesion. Powder Technol. 2013, 239, 366–373. [Google Scholar] [CrossRef]
- Vasilenko, A.; Glasser, B.J.; Muzzio, F.J. Shear and flow behavior of pharmaceutical blends—Method comparison study. Powder Technol. 2011, 208, 628–636. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Dave, R.N.; Pfeffer, R. Fluidization of coated group C powders. AIChE J. 2008, 54, 104–121. [Google Scholar] [CrossRef]
- Kalman, H. Quantification of mechanisms governing the angle of repose, angle of tilting, and Hausner ratio to estimate the flowability of particulate materials. Powder Technol. 2021, 382, 573–593. [Google Scholar] [CrossRef]
- Kalman, H.; Portnikov, D. Analyzing bulk density and void fraction: A. the effect of archimedes number. Powder Technol. 2021, 381, 477–487. [Google Scholar] [CrossRef]
- Akseli, I.; Hilden, J.; Katz, J.M.; Kelly, R.C.; Kramer, T.T.; Mao, C.; Osei-Yeboah, F.; Strong, J.C. Reproducibility of the Measurement of Bulk/Tapped Density of Pharmaceutical Powders Between Pharmaceutical Laboratories. J. Pharm. Sci. 2019, 108, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.E.; Cooke, J.R.; Schneider, L.C.R. Measuring shear properties and normal stresses generated within a rotational shear cell for consolidated and non-consolidated powders. Powder Technol. 2009, 190, 65–69. [Google Scholar] [CrossRef]
- Saker, A.; Cares-Pacheco, M.G.; Marchal, P.; Falk, V. Powders flowability assessment in granular compaction: What about the consistency of Hausner ratio? Powder Technol. 2019, 354, 52–63. [Google Scholar] [CrossRef]
- Llusa, M.; Sturm, K.; Sudah, O.; Stamato, H.; Goldfarb, D.J.; Ramachandruni, H.; Hammond, S.; Smith, M.R.; Muzzio, F.J. Effect of high shear blending protocols and blender parameters on the degree of API agglomeration in solid formulations. Ind. Eng. Chem. Res. 2009, 48, 93–101. [Google Scholar] [CrossRef]
- Lim, H.L.; Hapgood, K.P.; Haig, B. Understanding and preventing agglomeration in a filter drying process. Powder Technol. 2016, 300, 146–156. [Google Scholar] [CrossRef]
- Le, V.N.P.; Robins, E.; Flament, M.P. Agglomerate behaviour of fluticasone propionate within dry powder inhaler formulations. Eur. J. Pharm. Biopharm. 2012, 80, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Llusa, M.; Levin, M.; Snee, R.D.; Muzzio, F.J. Shear-induced APAP de-agglomeration. Drug Dev. Ind. Pharm. 2009, 35, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Scherholz, M.L.; Wan, B.; McGeorge, G. A Rational Analysis of Uniformity Risk for Agglomerated Drug Substance Using NIR Chemical Imaging. AAPS PharmSciTech 2017, 18, 432–440. [Google Scholar] [CrossRef]
- FAlfano, O.; Di Maio, F.P.; Di Renzo, A. Deagglomeration of selected high-load API-carrier particles in swirl-based dry powder inhalers. Powder Technol. 2022, 408, 117800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).