Abstract
Here, we demonstrate that nano–sized Mn–Ni–Co–O powder can be prepared at a low temperature via a co–precipitation method. In this work, Mn2+ was partially oxidized to Mn3+ ions in an aqueous solution by adding an oxidizing agent (H2O2). The co-presence of Mn2+ and Mn3+ cations enabled the precipitated products to be well-crystallized at a calcining temperature as low as 650 °C, forming a pure cubic spinel structure. The pellets fabricated from this calcined powder showed a relative density of up to 97.1% at a moderate sintering temperature of 1100 °C. Moreover, these ceramics exhibited electrical performance suitable for use in industrial thermistors, i.e., a room temperature resistivity (ρ25) of 1232 Ω cm, a thermistor constant (B25/85) of 3676 K, and an aging coefficient (ΔR/R) of 1.43%. High sintering activity as well as the excellent electrical properties of the ceramics was attributed to the fine-sized particles of the synthesized powder.
1. Introduction
Negative temperature coefficient of resistance (NTCR) nickel manganite oxide ceramics have been widely used for the production of thermistors owing to their excellent temperature sensitivity and cost-effectiveness [1,2,3]. These materials are absolutely lead–free, and are thus friendly to humans and environment, making them eligible for full RoHS compliance. With a general formula AB2O4 (A: tetrahedral and B: octahedral sites), the electrical conductivity of nickel manganite ceramics is commonly assigned to the electron hopping mechanism between the Mn3+ and Mn4+ states at the octahedral sites, described by the Arrhenius equation [2,3,4,5,6,7]: ρ(T) = ρ0 exp(Ea/kBT), where ρ0 is the resistivity at an infinite temperature, T is the absolute temperature, kB is the Boltzmann constant, and Ea is the activation energy for the hopping process. In practice, the NTCR thermistor response is typically determined by room temperature (RT) resistivity (ρ25) and thermal sensitivity (usually defined as B25/85).
The electrical properties of the spinel nickel manganite ceramics are strongly affected by imperfections, that cause changes in the distribution of cations in the spinel lattices. Therefore, to alter ρ25 and B25/85, the ceramic compositions have been modified (doping/substituting) with various transition metal ions [1,3,5]. Previous investigations have stated that cobalt (Co) is one the most effective ions for fine-tuning the conductivity without much degradation of temperature sensitivity [1,4,5,6,7,8,9]. Furthermore, incorporating Co was also demonstrated to improve the electrical stability of the nickel manganites [10,11]. Accordingly, Mn–Ni–Co–O ceramics have attracted intensive attention for industrial thermistors.
NTCR thermistors are typically fabricated by sintering powders at high temperatures, which is known as a grain-growth-induced densification process, driven by atomic diffusion in the solid state under thermal energy. Therefore, the characteristics of the powder particles, such as their morphology, crystal structure, chemical homogeneity, and uniformity, play important roles in sintering, as well as the output performance of the resulting ceramics. It has been reported that the use of coarse particles results in a network of pores, while fine-sized particles promote a much better sintering activity, owing to an increase in their surface free energy [12]. For nickel manganite-based compounds, high performance thermistors are commonly achieved by the use of powders with: (i) fine-sized particles and (ii) cubic spinel phase [13].
To prepare spinel powders, a heat treatment process known as calcination is required, as a prerequisite for crystallization. Although nickel manganite-based powders have been prepared by different methods with various experimental conditions [14,15,16], the pure cubic spinel phase typically occurs only at temperatures above 750 °C [17]. However, at such high temperatures, the calcination commonly causes the primary particles to become oxide particles of a much larger size, up to several micrometers [8,18,19]. To reduce the particle size, most researchers have employed ball milling as an additional mechanical activation step, after calcinations [13,14,18,19,20,21,22]. They were successful in the fabrication of small-sized particles which enhances the sinterability. However, the use of a mechanical process has disadvantages, notably with controlling the morphology, uniformity, as well as the size distribution and reproducibility. Thus, synthesizing nickel manganite powders with a single spinel phase, while imposing the requirement of keeping fine particles, remains a challenge.
In this light, we considered that the main reason for the large-sized powders is the growth driven by thermal energy during calcination. Therefore, a reduction in calcining temperature may offer a possible approach to achieving fine particles. Recently, we demonstrated that nano-sized cubic spinel Mn–Ni–Cu–O powders can be synthesized by a co-precipitation method [23]. The key point is that Mn3+ cations were created during preparation, which were then absorbed into the solid materials. The pre-existence of Mn3+ cations enabled the crystallization at a temperature as low as 300 °C. The aim of the present study is to prepare new NTCR materials with relatively low resistivities and high sensitivities. We thus sought to extend the co-precipitation technique to synthesize Mn–Ni–Co–O powder in a stepwise manner. Particularly, the targeted Mn1.5Co0.6Ni0.9O4 powder was produced from a chloride solution. The phase and morphology of the collected powder were characterized with respect to the calcining temperature. Moreover, the sintering activity and electrical property of the resulting bulk ceramics were reported.
2. Materials and Methods
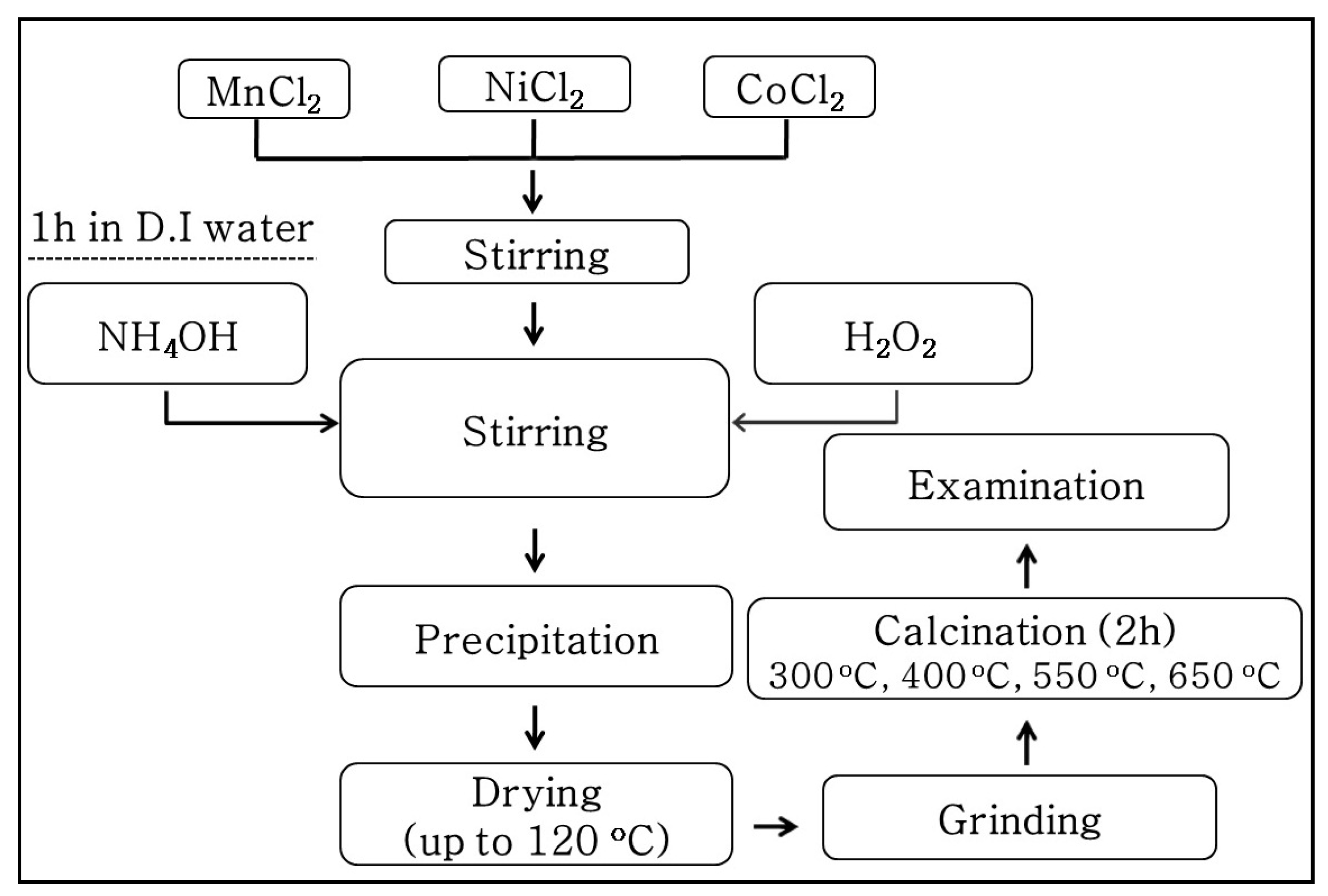
A schematic of the co-precipitation route used to synthesize thermistor materials in the present work is shown in Figure 1. The solution was prepared in a way similar to that used for the preparation of Mn–Ni–Cu–O powder [23]. MnCl2.4H2O, NiCl2.6H2O, and CoCl2.6H2O (99.99%, Sigma–Aldrich, MA, USA) were used as starting materials. After weighing, a mixed compound with a molar ratio of Mn:Ni:Co = 1.5:0.6:0.9 was dissolved in D.I water to form a 0.1 M solution in a flat bottom flask. NH4Cl was added and the mixture was stirred using a magnetic stirrer for 1 h. Then, ammonia (NH3.H2O) was slowly added to obtain a pH of 7.05. When the pH value was stable, a H2O2 (5 mM) solution was introduced, while the solution was continuously stirred for another 1 h. The pH value was further increased up to 9.00 and this state was maintained for an extra 30 min. The resulting brown solution was transferred into a petri dish and was then heated to 120 °C at 10 °C/h in an oven. The solid product was collected and ground, following by calcination in air for 2 h.
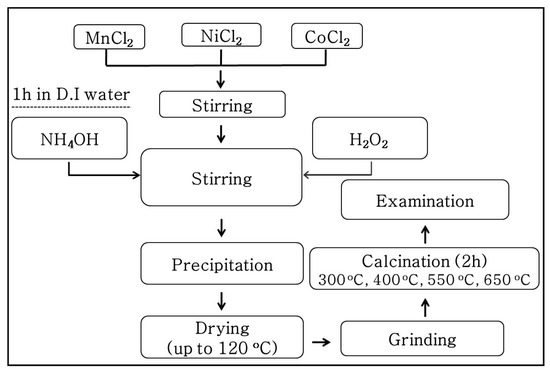
Figure 1.
Flowchart for Mn–Ni–Co–O powder preparation from a chloride solution.
The product (in powder form) calcined at 650 °C was selected to blend with polyvinyl alcohol (PVA), followed by cold pressing into circular disks with 12 mm diameter and 2 mm thickness, under a pressure of 200 MPa. After de-binding at 650 °C (2 h), the disks were sintered in air at various temperatures (900–1100 °C) in covered alumina crucibles for 2 h.
According to the Archimedes method, an object will be buoyed up when immersed in fluid by a force that is equal to the weight of the fluid displaced. Therefore, the density of the sintered ceramics can be determined by the following equation: Density (g/cm3) = (W0 × rf)/(W0 – Wi), where W0 and Wi are the weight of the sample in air and in fluid, and rf is the density of the fluid. The powder surface morphology was examined by field-emission scanning electron microscopy (FE-SEM, 6700F, JEOL, Tokyo, Japan) and transmission electron microscopy (TEM, JEM 2100, JEOL, Tokyo, Japan). The crystal structure of the calcined powders and sintered pellets was analyzed by X–ray diffractometer (XRD, Rigaku, Japan). Inductively coupled plasma optical emission spectroscopy (5110 ICP–OES, Agilent, Santa Clara, CA, USA) was used to characterize chemical composition. Change in valence states of manganese was confirmed by X-ray photoelectron spectroscopy (XPS) using a VG ESCA 3000 system (Microtech, Maidehead, UK). To measure electrical properties, the disks were polished to 1.0 mm in thickness, and silver paste was printed to both surfaces. Afterwards, they were cured at 700 °C for 10 min. These electroded specimens were held in a silicon oil bath, where a digital thermometer was used to measure temperature. Change in electrical resistance with temperature was detected by a digital multimeter (Fluke 45, 8808A, WA, USA). The B25/85 constant was calculated by the equation B25/85 = 1778 × (lnR25 − lnR85), where R25 and R85 are the bulk specific resistances (Ω) at 25 °C (298 K) and 85 °C (358 K), respectively. The aging coefficient was examined by the relative change in resistance ΔR/R = (R – Ro)/Ro × 100%, where Ro and R are the resistances at 298 K before and after aging 500 h in air at 150 °C. The values of ρ25, B25/85, and ΔR/R were the averages of four tested pellets.
3. Results and Discussion
Figure 2 shows an FE-SEM image of the as-synthesized powder, observed after drying at 120 °C. It is seen that the primary particles of the powder are quite similar, with tens of nanometers in size. However, some of them have agglomerated and form large, densified aggregates with a larger size up to a few hundred nanometers. This can be understood as a common phenomenon that occurs to minimize the surface free energy of these particles [13].

Figure 2.
FE-SEM photograph of the as-prepared powder.
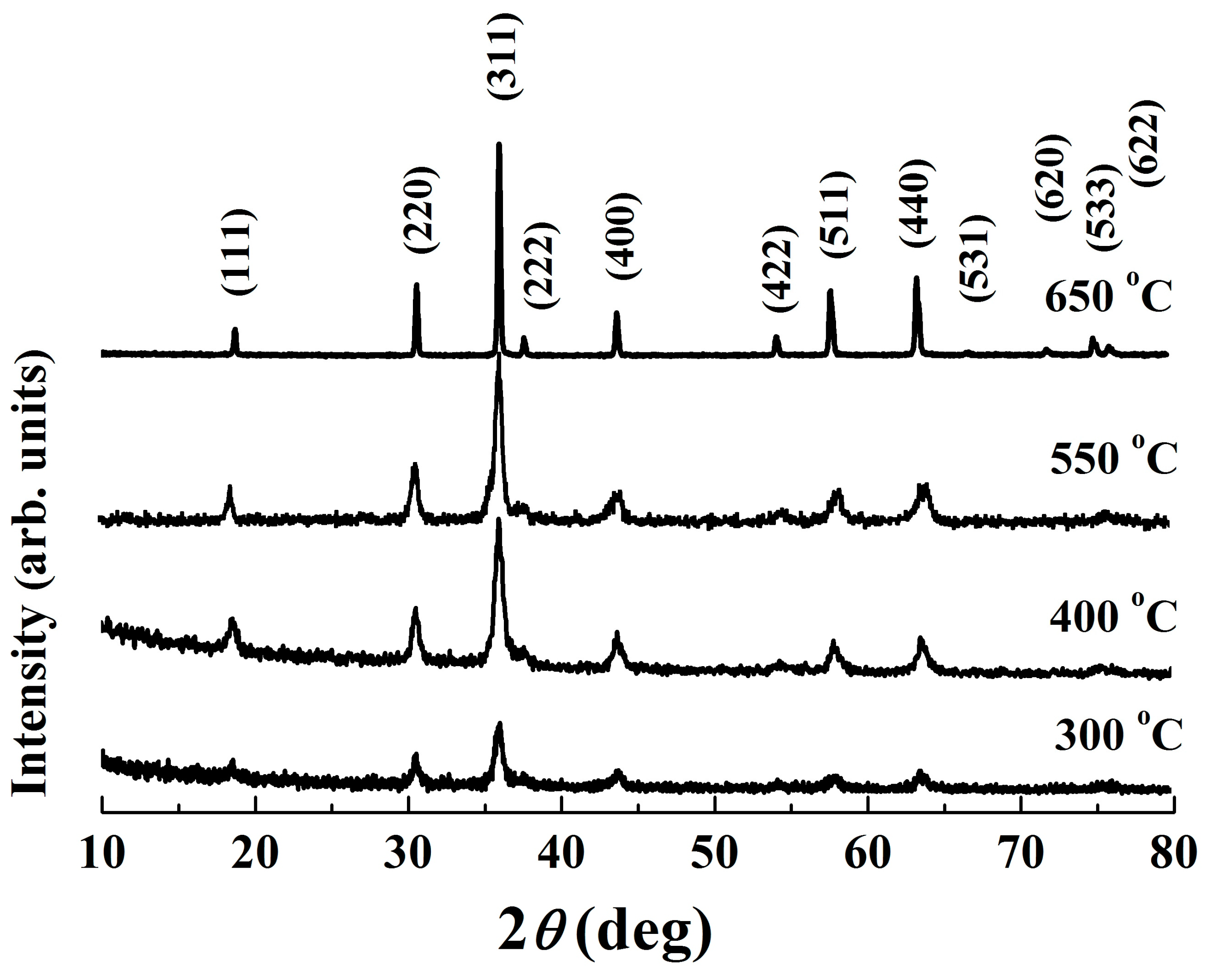
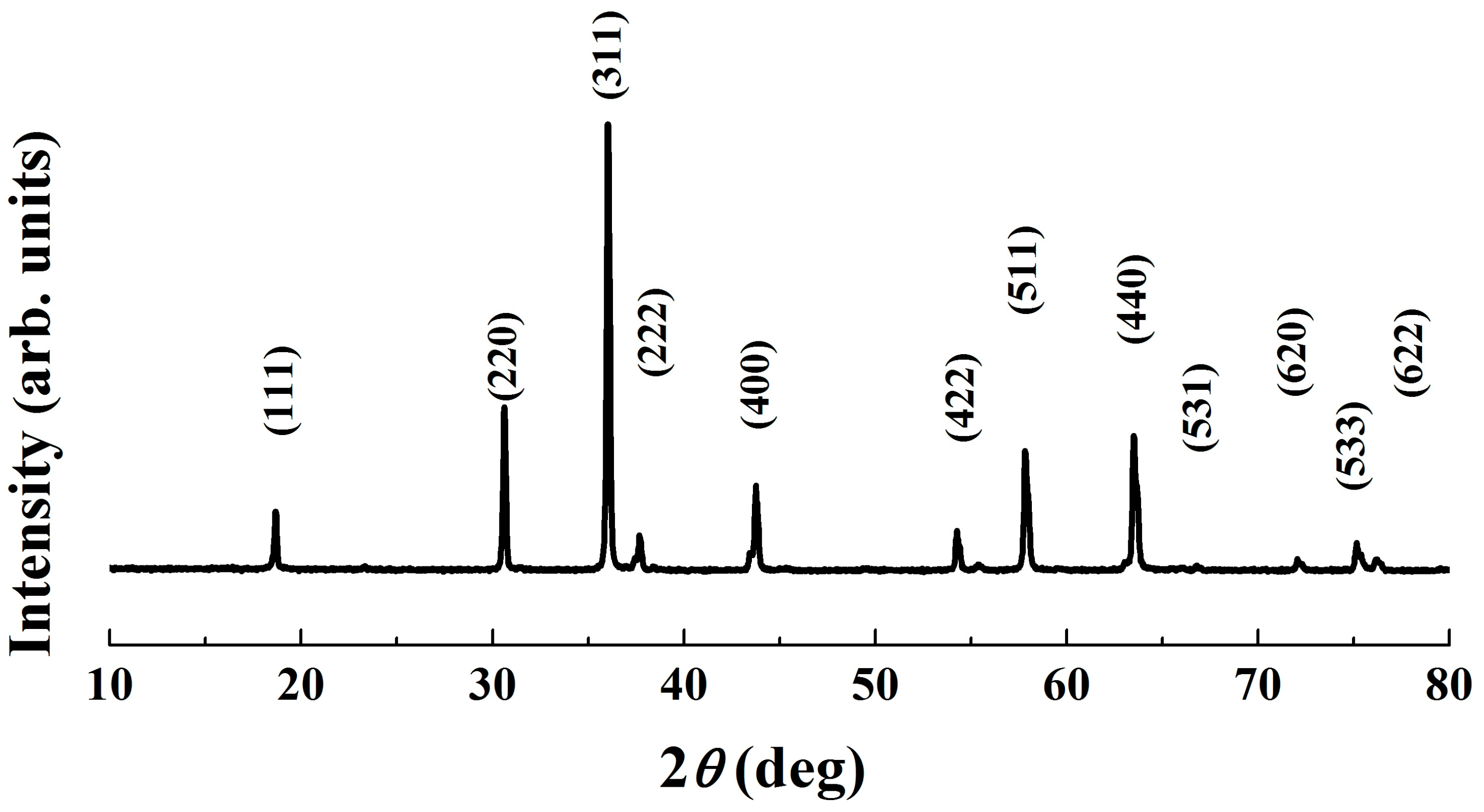
The XRD patterns of the powder calcined at various temperatures are depicted in Figure 3. As expected, distinct peaks appear in the powder calcined at 300 °C, which are in accordance with those of the standard Fd3m space group cubic spinel NiMn2O4 ceramics (JCPDS card No. 84–0542). The broadening of the preferential orientation (311) peak implies that the material exhibits a low crystallinity degree [24]. As the calcining temperature increases, sharper peaks with higher intensity and less humps are observed. These changes in XRD patterns demonstrate the growth of crystallites, which is further confirmed by an increase in crystallite size, shown in Table 1. Notably, at 650 °C, a well–crystallized pure single phase was found, indicating the completion of phase formation. It has been reported that there is a phase decomposition into NiMnO3 and Mn2O3 in the temperature range 400–750 °C, and therefore stoichiometric spinel NiMn2O4-based compounds can be achieved only at temperatures higher than 750 °C [15,25]. Nevertheless, except for an increase in intensity, Figure 3 shows an unchanged XRD characteristic within the studied temperature range (300–650 °C). No dissociation and/or secondary phases are detected, indicating an unchanged cubic spinel structure phase for the present Mn–Ni–Co–O solid products.
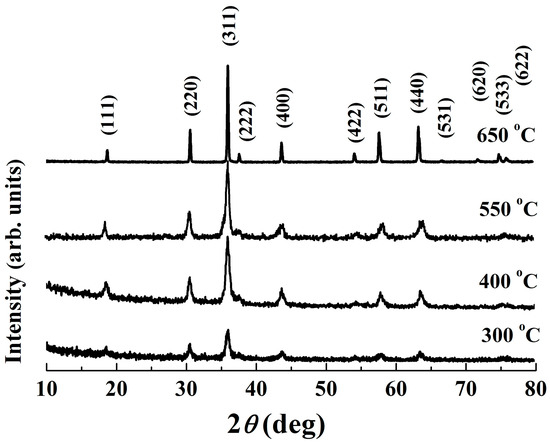
Figure 3.
XRD patterns of calcined powders.

Table 1.
Crystallite size of calcined powders, calculated by using Scherrer’s formula [26,27].
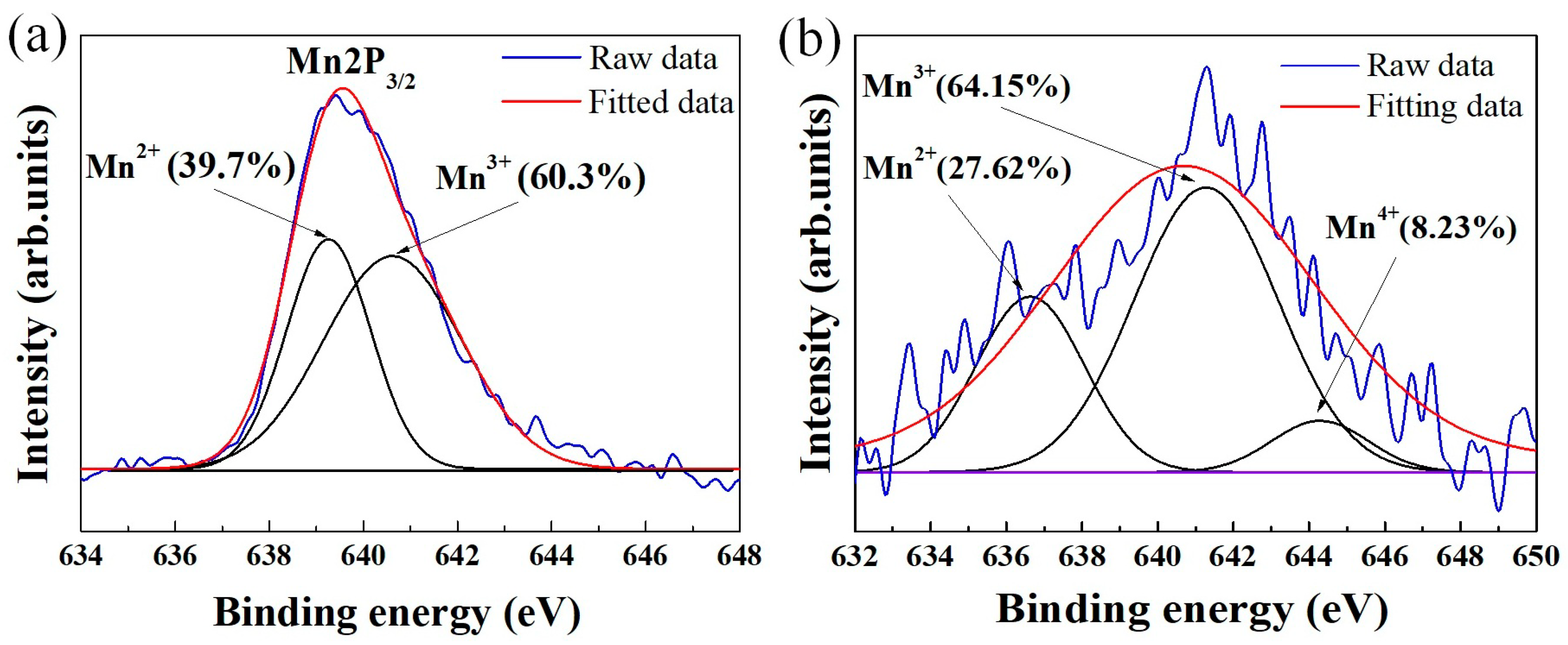
It has been demonstrated that the use of solution routes such as co-precipitation can reduce the crystallization temperature of powders due to the homogeneous mixing of the starting components [28]. Nevertheless, we think that chemical homogeneity alone cannot sufficiently account for a crystallization temperature as low as 300 °C. Figure 4a presents the results of high-resolution XPS Mn2p3/2 analysis of the pristine powder (Figure 2). Through Gaussian–Lorentzian curve fitting, the Mn2p3/2 spectra can be split into two peaks in the binding energy range 634–648 eV, which can be assigned to the Mn2+ and Mn3+ oxidation states [29]. As expected, both 2+ and 3+ valence states of manganese are found, implying that Mn2+ was partially oxidized to Mn3+ during synthesis. Commonly, due to the occurrence of Mn2+ → Mn3+ oxidation reactions (driven by heat energy), there is a difference in the Mn3+/Mn2+ ratio between the pristine and heated states. However, the XPS analysis for powder fired at 300 °C (Figure 4b) shows that the Mn3+/Mn2+ ratio does not change significantly after calcination. This is likely due to the high portion of Mn3+ ions (60.3%) pre-existing in the as-prepared material (Figure 4a), which causes only a few Mn2+ → Mn3+ conversions to happen for acquiring the spinel phase. Consequently, the required thermal energy budget for crystallization is lower. We therefore theorize that the major factors responsible for the formation of the spinel at the critical temperature of 300 °C are the co-existence of Mn2+ and Mn3+ and the chemical homogenous dispersion. We also suggest that due to the suppression of Mn2+ → Mn3+ conversion in the manganite, no secondary phases (NiMnO3 and α–Mn2O3) were formed [30], which may be a possible reason for the stable spinel phase shown in Figure 3. In addition, Figure 4b indicates that Mn4+ cations are found in the powder calcined at 300 °C. This result illustrates the conversion of Mn3+ to Mn4+ even at only 300 °C. For nickel manganite-based materials, the formation of Mn4+ ions is the critical factor which enables electrical conduction via the hoping mechanism [2,3,4,5,6,7,20,21,22,23].
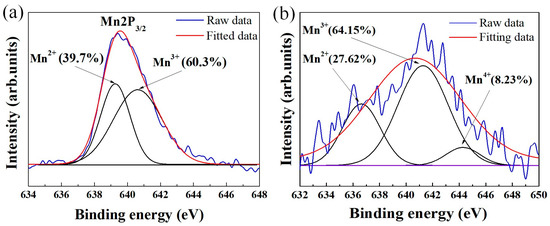
Figure 4.
XPS spectra of Mn2p3/2 for different powder states (a) as-prepared and (b) calcined at 300 °C.
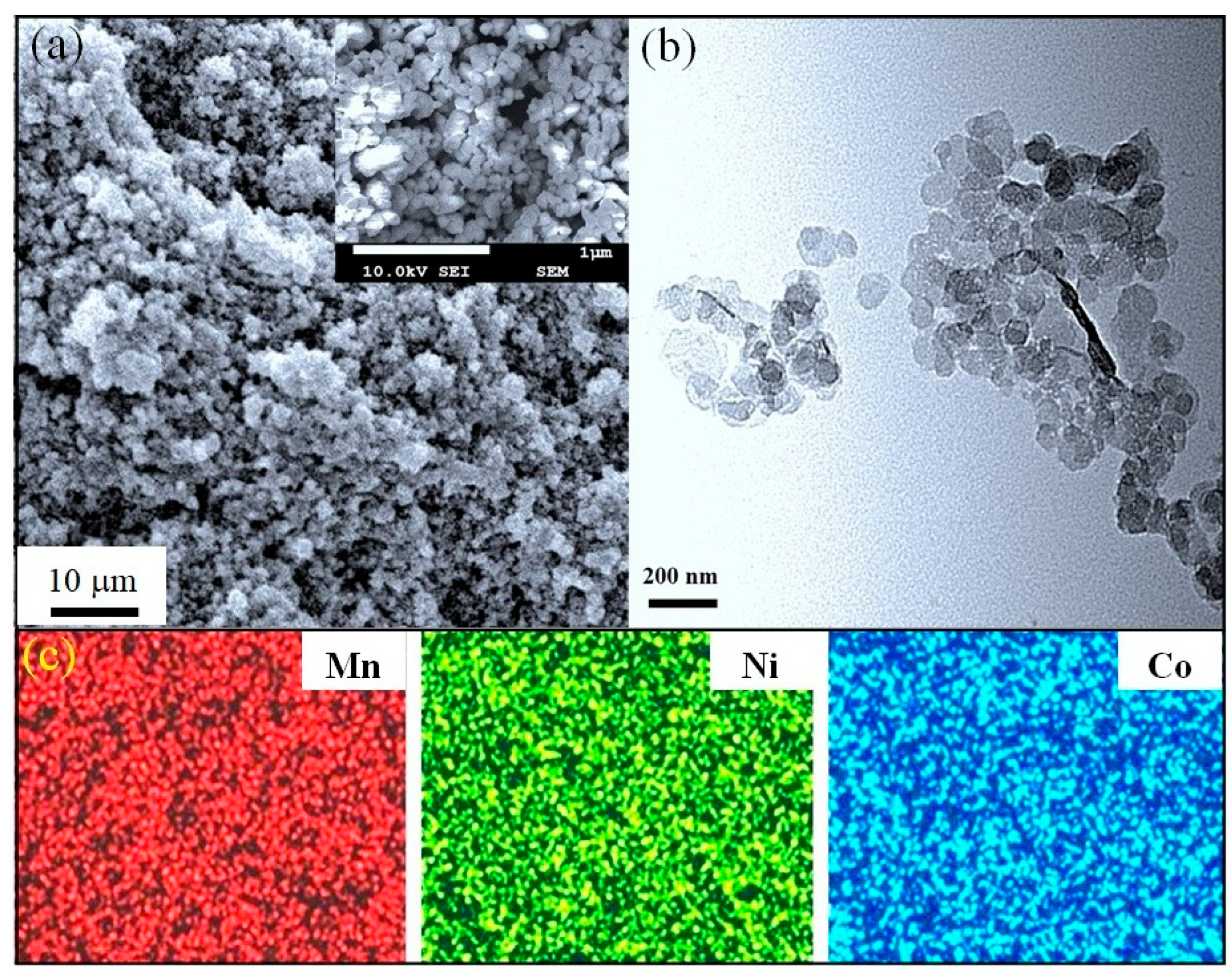
The calcination process commonly causes a growth and/or a morphological change in the synthesized particles. In our case, the powder fired at 650 °C shown in Figure 5a looks quite uniform over the FE-SEM scan area. The magnified FE-SEM image (insert of Figure 5a) shows a spherical shape for particles, with a majority at approximately 100–120 nm in size. TEM observation (Figure 5b) confirmed that these particles are not larger than 150 nm. Clearly, the particles are enlarged after the heat treatment; however, such particles are much smaller than those prepared by most other recent techniques [8,12,13,14,21,22,26,31]. The fine size of the calcined powder can be attributed to the moderate firing temperature of only 650 °C. Otherwise, the uniformity and narrow particle size distribution may relate to the fact that no mechanical impacts were involved in the proposed process.
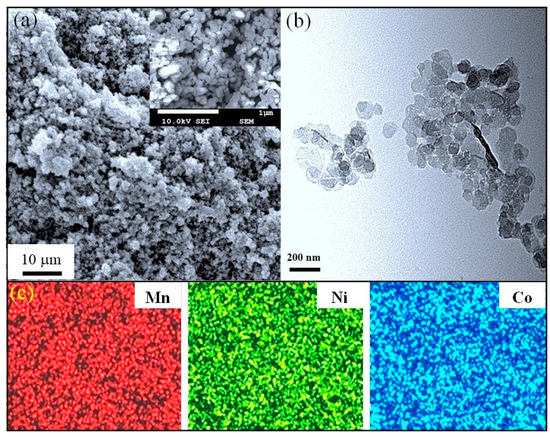
Figure 5.
(a) FE-SEM, (b) TEM and (c) EDS mapping (Mn, Ni, and Co) images of the powder calcined at 650 °C. Inset in (a): magnified FE-SEM image shows quite uniform particles with a spherical shape.
The chemical compositions of the powder in the as-prepared and 650 °C calcined states were characterized using inductively coupled plasma (ICP) and the results are shown in Table 2. It is found that the ratios of the constituent elements (Mn:Ni:Co) measured in these different states are similar to those in the initial solution. The ICP results thus suggest that both the precipitate collection and calcining processes did not noticeably affect the chemical composition. Moreover, all elements were homogeneously distributed over the powder, as can be observed from the elemental mapping SEM image in Figure 5c. This result is reasonable due to the fact that a homogeneous mixture of starting materials can be easily obtained in solution processes. Due to the combined advantages of a cubic spinel structure, fine-sized particles, and uniformity, it is possible to expect good sintering behavior and electrical performance for the resulting ceramics.

Table 2.
Chemical compositions of synthesized powder in different states.
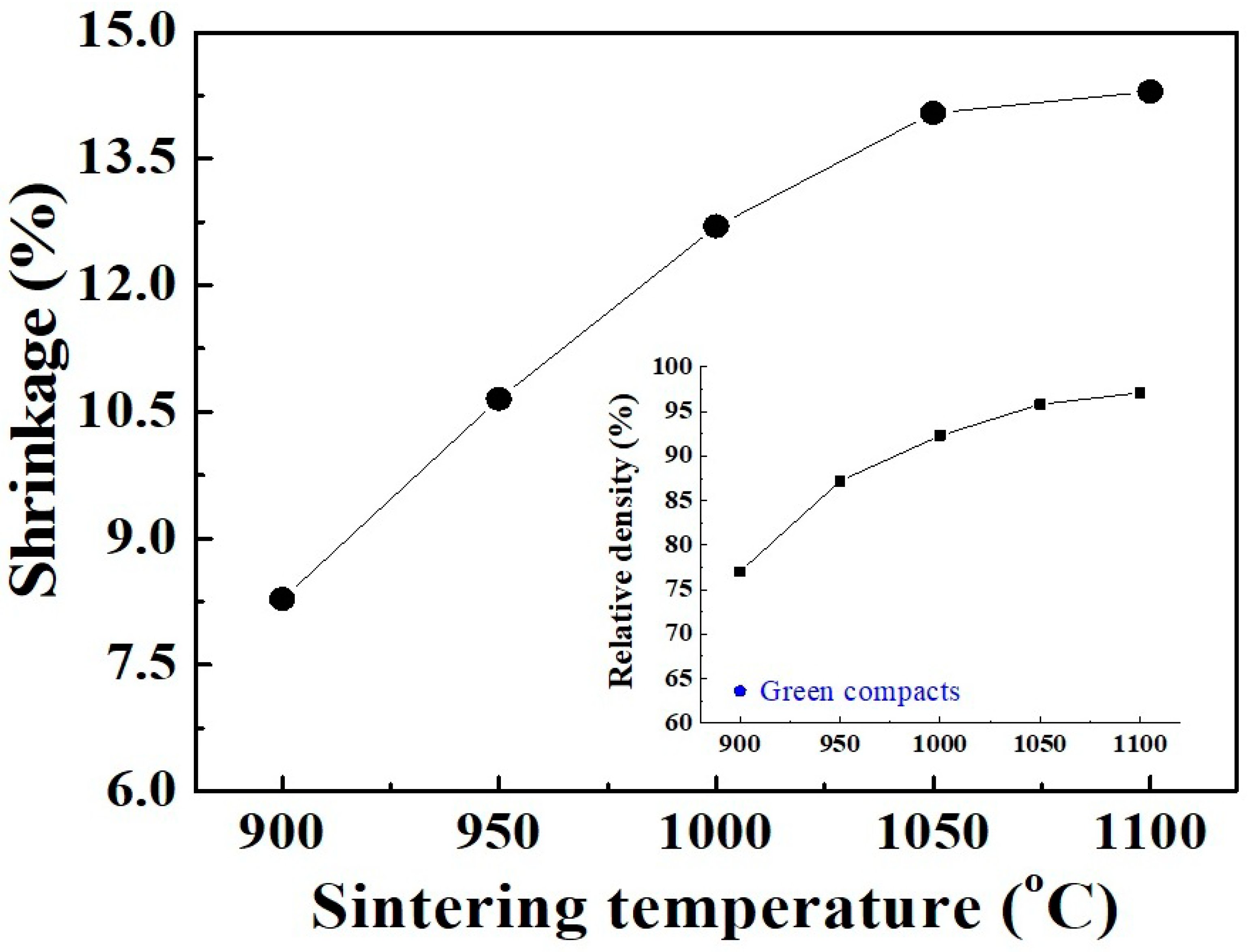
Generally, the crystallization degree of calcined powders strongly affects the electrical characteristics of the resulting ceramics. In the present work, although the prepared Mn–Ni–Co–O material was started to crystalize at 300 °C, the formation of the cubic spinel structure (from amorphous phase) was only completed at 650 °C (Figure 3). Therefore, for further investigations of sinterability and electrical properties, the powder calcined at 650 °C was selected to form into circular bulks and sintered in air for 2 h. Figure 6 shows the shrinkage of the ceramics observed as a function of sintering temperature. It was found that the linear shrinkage rapidly increased from 8.2% to 12.7% as the temperature increased from 900 °C to 1000 °C. This noticeable change in shrinkage is in agreement with the results of Vidales et al. [8] for Mn–Ni–Co–O ceramics prepared via the same route. Because of the large shrinkage rate, a remarkable increase in relative density (RD) of up to 92.3% is observed, as shown in Figure 6 (insert). At temperatures above 1000 °C, the shrinkage tendency is retained but with a reduced rate. A RD of 97.1% is achieved at 1100 °C, where the final linear shrinkage reaches 14.3%. In fact, the ceramics gave an RD value of nearly 97.5% when the sintering duration was held for 6 h.
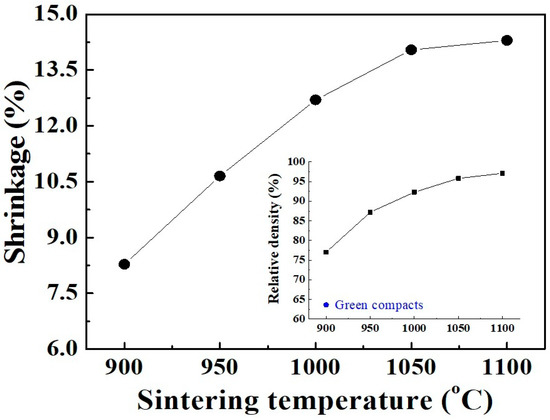
Figure 6.
Shrinkage of ceramic pellets, examined as a function of the sintering temperature. The inset shows the relative density (RD) of the ceramics.
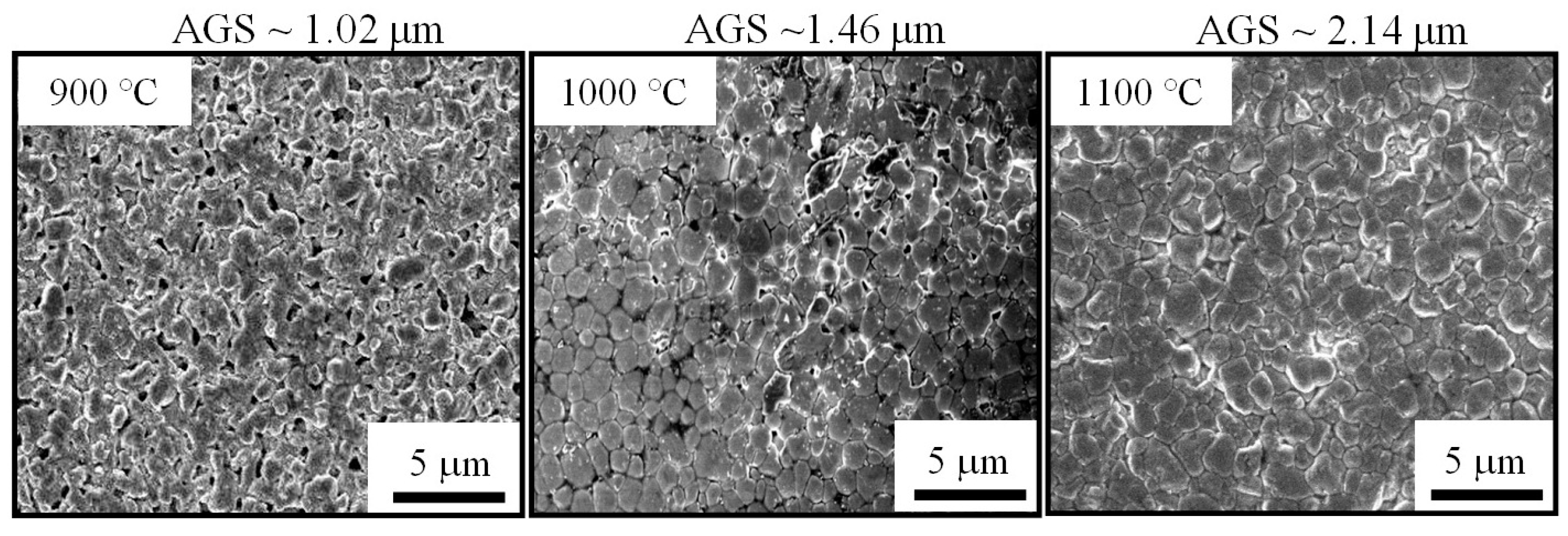
Figure 7 shows FE-SEM images of the surface of three pellets that were sintered at different temperatures. It can be clearly seen that the specimen fired at 900 °C exhibits many large pores. However, only small closed pores are visible on the surface of the one sintered at 1000 °C, revealing a denser structure with less porosity. In the sample heated at 1100 °C, the pores are almost eliminated. The bulk looks quite dense and homogenous. Otherwise, within the sintering temperature range, there is an increase in the average grain size (AGS) from 1.04 to 1.82 μm. These changes in microstructure explain the promotion of densification, as well as the RD increase with respect to sintering temperature shown in Figure 6. It should be noted that the sintering temperature of 1100 °C, which enabled an RD value larger than 97%, is lower than those reported for most conventional ceramics that possess the same RD level [32,33,34]. On the other hand, at the sintering temperature of 1100 °C, the pure cubic spinel structure is retained without any other secondary phase being detected, even though the dwell time was extended to 6 h (Figure 8). This means that no phase transformation and/or segregation took place during sintering. Therefore, it is worth noting that the sintering at such a low temperature (1100 °C) prevents decomposition reactions (into rock salt NiO phase), that are commonly observed at high temperatures [13,17,26,35]. These are consistent with the previous results [12,15,23], which indicate that the formation of the secondary phase can be suppressed by sintering at a low temperature. We suggest that the fine size of the calcined powder (Figure 5) is mainly responsible for the good densification of the ceramics because it allows a high possibility for particles to fill any spaces/voids in the bulk volume. This is confirmed by the result shown in Figure 6 (insert), where the RD of the green compact was found to be as high as 63.2%. In addition, because of having large free surface energy [12], more contacts and reactions among particles can be created, which enables the diffusion process in the solid state during sintering.
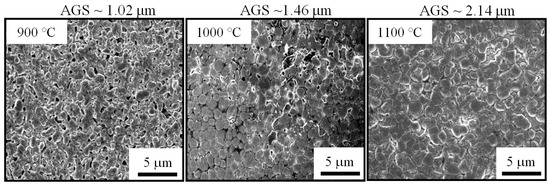
Figure 7.
Surface FE-SEM micrographs of sintered ceramics.
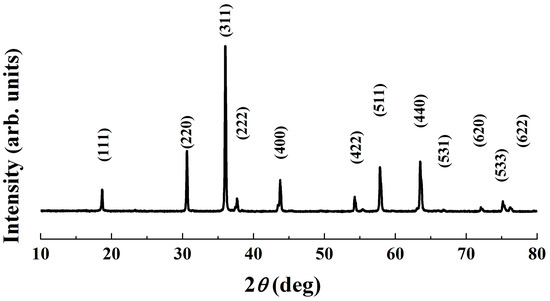
Figure 8.
XRD patterns of bulk ceramic sintered at 1100 °C for 6 h.
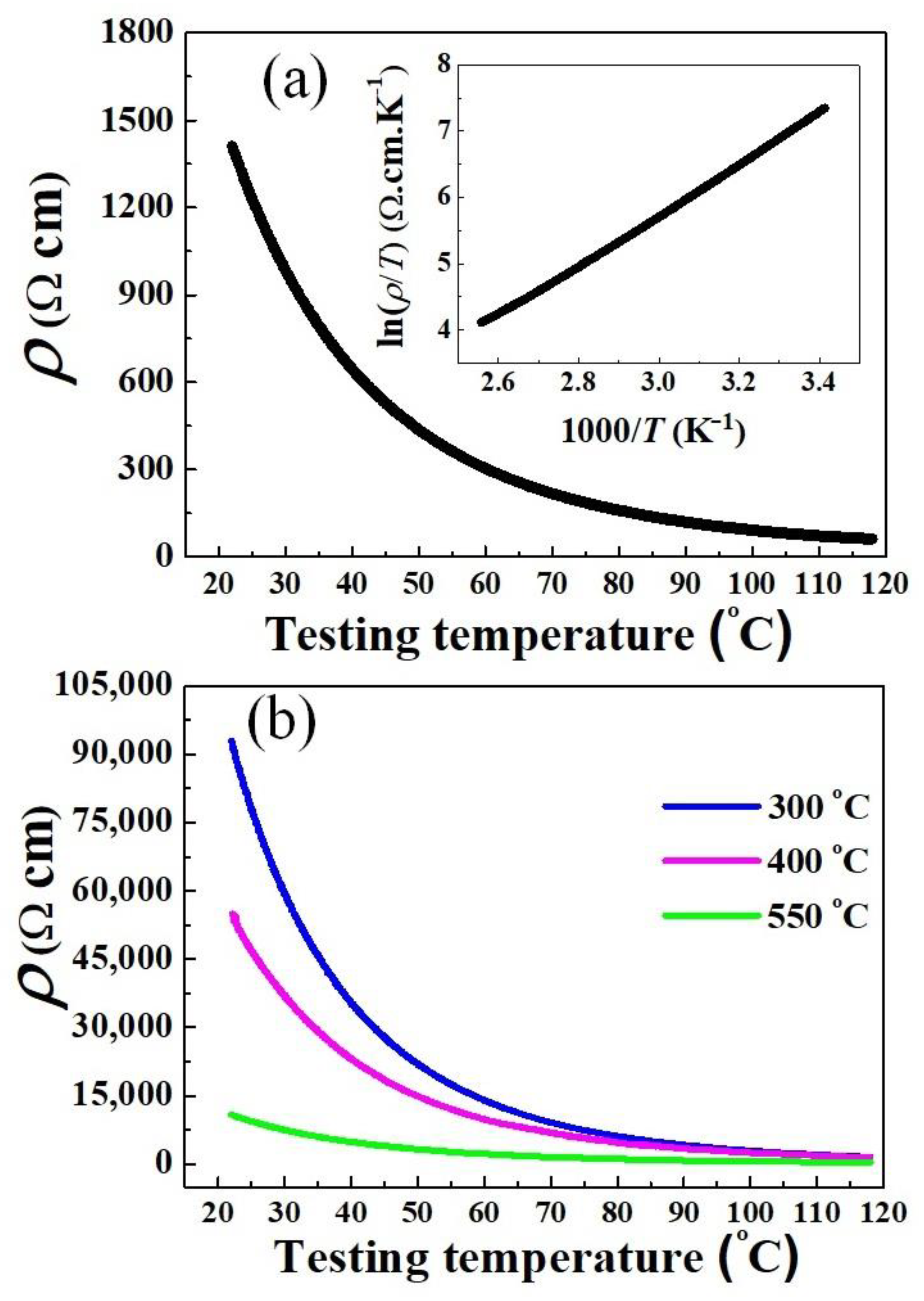
Measurement of the electrical resistivity–temperature (ρ–T) dependence was carried out on pellets sintered at 1100 °C, and the results are presented in Figure 9a. It is found that as the testing temperature increased (295–391 K), the resistivity progressively decreased. Moreover, the inset illustrates a nearly linear relationship between ln(ρ/T) and 1000/T. Obviously, Figure 9a reveals a typical NTCR characteristic of nickel manganites that can be conducted to the hopping mechanism presented by the Arrhenius model [2,3,4,5,36,37]. The thermistor parameters of the sintered samples are listed in Table 3. The ρ25 and B25/85 were calculated to be 1232 Ω cm and 3676 K, respectively, which are suitable for use in low-resistance thermistors. These parameters are comparable to those of conventional Mn–Ni–Co–O ceramics sintered at 1200 °C and above [9,36,37]. Otherwise, to gain more information about the electrical properties, we provide the ρ–T tested results for the samples derived from powders calcined at 300 °C, 400 °C, and 550 °C, as shown in Figure 9b. It is found that under the same sintering conditions (1100°C, 2 h), the NTCR behavior is also revealed for all these ceramics, however, with much higher ρ25 (Table 3). These results confirm that the electrical conductivity is worse when using incomplete crystallization powders.
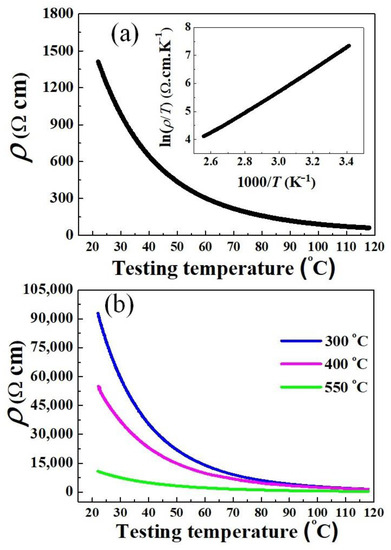
Figure 9.
Resistivity–temperature (ρ–T) dependence of sintered ceramics (1100 °C, 2 h), produced from powders calcined at (a) 650 °C, and (b) lower than 650 °C. The insert in (a) shows the plot of ln(ρ/T) versus (1000/T).

Table 3.
The electrical properties of ceramics sintered at 1100 °C.
The drift in resistance (ΔR/R) due to aging and/or long time operation, the so-called aging coefficient, is also vital for practical applications. The ΔR/R describes the electrical stability, and it should be as low as possible to maintain external responses [38]. Table 3 also shows an average ΔR/R value of 1.43% after the ceramics were aged for 500 h, demonstrating extremely high stability. It has been reported that aging behavior is primarily caused by the oxidation reactions during metallization [39,40]. Therefore, the little change in resistance here can be supported by the dense and fine-grained microstructure shown in Figure 7 (AGS~1.82 μm), which suppresses oxygen adsorption, leading to more stable spinel lattices [41].
It should be again mentioned that the oxidation of Mn2+ to Mn3+ ions was noted as the key factor, which induces the formation of cubic spinel structure at low temperatures. For better understanding, the formation mechanism of present Mn–Ni–Co–O powders was proposed as follows:
NH4Cl → NH4+ + Cl−
NH4+ + OH− ↔ NH3·H2O
The metal amine complexes (M(NH3)n2+) are formed as follows:
M2+ + nNH3 ↔ Mn(NH3)n2+
M−Mn, Ni, and Co
The metal amine complexes then combine with OH− groups:
M(NH3)n2+ + OH− → [M(NH3)n](OH)2
Once the oxidizing agent (H2O2) is added, some M2+ ions are oxidized to form M3+:
3[Mn(NH3)n](OH)2 + H2O2 → [Mn2+,Mn3+]3O4 + 4H2O+ 3nNH3
Finally, under thermal energy of calcination, the spinel phase is formed:
[Mn2+,Mn3+]3O4 → [(Mn2+)(Mn3+)2]O4
4. Conclusions
In this work, the cubic spinel nano-sized powder was successful produced via a simple solution method, from a mixed chloride aqueous solution. The addition of H2O2 induced the partial oxidation of Mn2+ to Mn3+ ions in solution, which was noted as the key factor that enables the crystallization to begin at 300 °C. Notably, powder calcined at 650 °C possessed quite uniform particles with a size smaller than 150 nm, which promoted high densification in the resulting ceramics. As a result, a relative density (RD) of 97.1% was obtained for pellets sintered at 1100 °C, while maintaining a pure cubic spinel phase. Furthermore, these ceramics exhibited a typical NTCR behavior, with average ρ25, B25/85, and ΔR/R of 1232 Ω cm, 3676 K, and 1.43%, respectively. The results demonstrate a feasible approach for the fabrication of spinel nano-sized thermistor powders.
Author Contributions
Conceptualization, D.T.L. and J.H.C.; methodology, D.T.L.; formal analysis, D.T.L. and J.H.C.; investigation, D.T.L. and J.H.C.; resources, J.H.C.; data curation, D.T.L.; writing—original draft preparation, D.T.L.; writing—review and editing, J.H.C.; supervision, J.H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by a grant from the R&D program for Material/Parts Technology Development funded by the Ministry of Trade, Industry and Energy, Republic of Korea, grant number, 20016729.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Price, B.Y.; Hardal, G. Electrical properties of Ni0.5Co0.8Mn1.7O4 and Ni0.5Co1.1Mn1.4O4 negative temperature coefficientceramics doped with B2O3. J. Mater. Sci. Mater. Electron. 2021, 32, 8983–8990. [Google Scholar] [CrossRef]
- Feteira, A. Negative Temperature Coefficient Resistance (NTCR) Ceramic Thermistors: An Industrial Perspective. J. Am. Ceram. Soc. 2009, 92, 967–983. [Google Scholar] [CrossRef]
- Schulze, H.; Li, J.; Dickey, E.C.; Trolier–McKinstry, S. Synthesis, Phase Characterization, and Properties of Chemical Solution–Deposited Nickel Manganite Thermistor Thin Films. J. Am. Ceram. Soc. 2009, 92, 738–744. [Google Scholar] [CrossRef]
- Sachse, H.F. Semiconducting Temperature Sensors and Their Applications; Wiley: New York, NY, USA, 1975. [Google Scholar]
- Metz, R. Electrical properties of N.T.C. thermistors made of manganite ceramics of general spinel structure: Mn3−x−x′ MxNx′O4 (0 ≤ x + x’ ≤ 1; M and N being Ni, Co or Cu). Aging phenomenon study. J. Mater. Sci. 2000, 35, 4705–4711. [Google Scholar] [CrossRef]
- Luo, W.; Yao, H.M.; Yang, P.H.; Chen, C.S. Negative temperature coefficient material with low thermal constant and high resistivity for low–temperature thermistor applications. J. Am. Ceram. Soc. 2009, 92, 2682–2686. [Google Scholar] [CrossRef]
- Jadhav, R.N.; Mathad, S.N.; Puri, V. Studies on the properties of Ni0.6Cu0.4Mn2O4 NTC ceramic due to Fe doping. Ceram. Int. 2012, 38, 5181–5188. [Google Scholar] [CrossRef]
- Vidales, J.L.M.; Garcia-Chain, P.; Rojas, R.M.; Vila, E.; Garcia Martinez, O. Preparation and characterization of spinel type Mn–Ni–Co–O negative temperature coefficient ceramic thermistors. J. Mater. Sci. 1998, 33, 1491–1496. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Y.; Yao, J.; Tang, X.; Wang, J.; Chang, A. NTC thermo–sensitive ceramics with low B value and high resistance at low temperature in Li–doped Mn0.6Ni0.9Co1.5O4 system. J. Mater. Sci. Mater. Electron. 2020, 31, 1403–1410. [Google Scholar] [CrossRef]
- Park, K.; Lee, J.K. Mn–Ni–Co–Cu–Zn–O NTC thermistors with high thermal stability for low resistance applications. Scr. Mater. 2007, 57, 329–332. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Hu, Z.; Yao, J.; Chang, A. Effect of Fe addition on microstructure and electrical properties of Co1.5Mn1.5–xFexO4 (0.2 ≤ x ≤ 1.0) NTC thermistors. J. Mater. Sci. Mater. Electron. 2017, 28, 7243–7247. [Google Scholar] [CrossRef]
- Kocjan, A.; Logar, M.; Shen, Z. The agglomeration, coalescence and sliding of nanoparticles, leading to the rapid sintering of zirconia nanoceramics. Sci. Rep. 2017, 7, 2541. [Google Scholar] [CrossRef]
- Aleksic, O.S.; Nikolic, M.V.; Lukovic, M.D.; Nikolic, N.; Radojcic, B.M.; Radovanovic, M.; Djuric, Z.; Mitric, M.; Nikolic, P.M. Preparation and characterization of Cu and Zn modified nickel manganite NTC powders and thick film thermistors. Mater. Sci. Eng. B 2013, 178, 202–210. [Google Scholar] [CrossRef]
- Fang, D.L.; Wang, Z.B.; Yang, P.H.; Liu, W.; Chen, C.S. Preparation of Ultra–Fine Nickel Manganite Powders and Ceramics by a Solid–State Coordination Reaction. J. Am. Ceram. Soc. 2006, 89, 230–235. [Google Scholar] [CrossRef]
- Teichman, C.; Töpfer, J. Sintering and electrical properties of Cu–substituted Zn–Co–Ni–Mn spinel ceramics for NTC thermistors thick films. J. Eur. Ceram. Soc. 2022, 42, 2261–2267. [Google Scholar] [CrossRef]
- Uppuluri, K.; Szwagierczak, D. Fabrication and characterization of screen printed NiMn2O4 spinel based thermistors. Sens. Rev. 2022, 42, 177–186. [Google Scholar] [CrossRef]
- Drouet, C.; Laberty, C.; Fierro, J.L.G.; Alphonse, P.; Rousset, A. X–ray photoelectron spectroscopic study of non–stoichiometric nickel and nickel–copper spinel manganites. Int. J. Inorg. Mater. 2000, 2, 419–426. [Google Scholar] [CrossRef]
- Chanel, C.; Fritsch, S.; Legros, R.; Rousset, A. Controlled Morphology of Nickel Manganite Powders. Key Eng. Mater. 1997, 132, 109–112. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Zhang, H.; Tuokedaerhan, K.; Chang, A. Synthesis of pilot–scale Co2Mn1.5Fe2.1Zn0.4O8 fabricated by hydrothermal method for NTC thermistor. J. Alloys Compd. 2019, 797, 1295–1298. [Google Scholar] [CrossRef]
- Savić, S.M.; Mančić, L.; Vojisavljević, K.; Stojanović, G.; Branković, Z.; Aleksić, O.S.; Branković, G. Microstructural and electrical changes in nickel manganite powder induced by mechanical activation. Mater. Res. Bull. 2011, 46, 1065–1071. [Google Scholar] [CrossRef]
- Zheng, C.H.; Fang, D.L. Preparation of ultra–fine cobalt–nickel manganite powders and ceramics derived from mixed oxalate. Mater. Res. Bull. 2008, 43, 1877–1882. [Google Scholar] [CrossRef]
- Fang, D.L.; Lee, C.G.; Koo, B.H. Preparation of Ultra–Fine FeNiMnO4 Powders and Ceramics by a Solid–State Coordination Reaction. Met. Mater. Int. 2007, 13, 165–170. [Google Scholar] [CrossRef]
- Le, D.T.; Ju, H. Solution Synthesis of Cubic Spinel Mn–Ni–Cu–O Thermistor Powder. Materials 2021, 14, 1389. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Mhin, S.; Han, H.; Kim, K.M.; Shim, K.B.; Lee, J.I.; Ryu, J.H. Electrical properties of Fe doped Ni–Mn–Co–O cubic spinel nanopowders for temperature sensors. J. Ceram. Process. Res. 2017, 18, 247–251. [Google Scholar]
- de Gyoryfalva, G.D.C.C.; Reaney, I.M. Decomposition of NiMn2O4 spinels. J. Mater. Res. 2003, 18, 1301–1308. [Google Scholar] [CrossRef]
- Khirade, P.P.; Birajdar, S.D.; Raut, A.; Jadhav, K. Multiferroic iron doped BaTiO3 nanoceramics synthesized by sol–gel auto combustion: Influence of iron on physical properties. Ceram. Int. 2016, 42, 12441–12451. [Google Scholar] [CrossRef]
- Ahsan, M.; Irshad, M.; Fu, P.F.; Siraj, K.; Raza, R.; Javed, F. The effect of calcination temperature on the properties of Ni–SDC cermet anode. Ceram. Int. 2019, 46, 2780–2785. [Google Scholar] [CrossRef]
- Lee, B.W. Preparation and characterization of spinel LiCoxMn2–xO4 by oxalate precipitation. J. Power Sources 2002, 109, 220–226. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M. XPS Studies of Solvated Metal Atom Dispersed (SMAD) Catalysts. Evidence for Layered Cobalt–Manganese Particles on Alumina and Silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Gao, H.; Ma, C.; Sun, B. Preparation and characterization of NiMn2O4 negative temperature coefficient ceramics by solid–state coordination reaction. J. Mater. Sci. Mater. Electron. 2014, 25, 3990–3995. [Google Scholar] [CrossRef]
- Arinicheva, Y.; Clavier, N.; Neumeier, S.; Podor, R.; Bukaemskiy, A.; Klinkenberg, M.; Roth, G.; Dacheux, N.; Bosbach, D. Effect of powder morphology on sintering kinetics, microstructure and mechanical properties of monazite ceramics. J. Eur. Ceram. Soc. 2018, 38, 227–234. [Google Scholar] [CrossRef]
- Hardal, G.; Price, B.Y. Influence of nano–sized cobalt oxide additions on the structural and electrical properties of nickel–manganite–based NTC thermistors. Mater. Technol. 2016, 50, 923–928. [Google Scholar] [CrossRef]
- Park, K.; Kim, S.J.; Kim, J.G.; Nahm, S. Structural and electrical properties of MgO–doped Mn1.4Ni1.2Co0.4−xMgxO4 (0 ≤ x ≤ 0.25) NTC thermistors. J. Eur. Ceram. Soc. 2007, 27, 2009–2016. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Rafique, M.; Usman, U.; Ain, Q.; Ghaffar, A. Evaluation of densification effects on the properties of 8 mol % yttria stabilized zirconia electrolyte synthesized by cost effective coprecipitation route. Ceram. Int. 2021, 47, 2857–2863. [Google Scholar] [CrossRef]
- Rong, J.; Zhang, H.; Zhao, P.; Qin, Q.; He, D.; Xie, J.; Ding, Y.; Jiang, H.; Wu, B.; Chang, A. Effect of Zn/Fe co–doping on the microstructure, electrical properties and aging behavior of Co–Mn–Ni–O NTC ceramics. Appl. Phys. A 2022, 128, 444. [Google Scholar] [CrossRef]
- Price, B.Y.; Hardal, G. Influence of B2O3 addition on the electrical and microstructure properties of Ni0.5Co0.5CuxMn2−xO4 (0 ≤ x ≤ 0.3) NTC thermistors without calcination. J. Mater. Sci. Mater. Electron. 2016, 27, 9226–9232. [Google Scholar] [CrossRef]
- Muralidharan, M.N.; Rohini, P.R.; Sunny, E.K.; Dayas, K.R.; Seema, A. Effect of Cu and Fe addition on electrical properties of Ni–Mn–Co–O NTC thermistor compositions. Ceram. Int. 2012, 38, 6481–6486. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, W.; Wu, W.; Zhang, M.; Li, Z. Aging characteristic of Cu-doped nickel manganite NTC ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 11784–11790. [Google Scholar] [CrossRef]
- Fritsch, S.; Sarrias, J.; Brieu, M.; Couderc, J.J.; Baudour, J.L.; Snoeck, E.; Rousset, A. Correlation between the structure, the microstructure and the electrical properties of nickel manganite negative temperature coefficient (NTC) thermistors. Solid State Ion. 1998, 109, 229–237. [Google Scholar] [CrossRef]
- Le, D.T.; Cho, J.H.; Ju, H. Electrical properties and stability of low temperature annealed (Zn,Cu) co–doped (Ni,Mn)3O4 spinel thin films. J. Asian Ceram. Soc. 2021, 9, 838–850. [Google Scholar] [CrossRef]
- Cui, M.-M.; Zhang, X.; Liu, K.-G.; Li, H.-B.; Gao, M.-M.; Liang, S. Fabrication of nano–grained negative temperature coefficient thermistors with high electrical stability. Rare Met. 2021, 40, 1014–1019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).