Profile Assessment of Bioactive Peptides in the Greek Traditional Cheese “Tsalafouti”
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Biopeptide Separation
2.3. Biopeptide Identification
2.4. Biopeptide Characterization pre and after In Silico Digestion
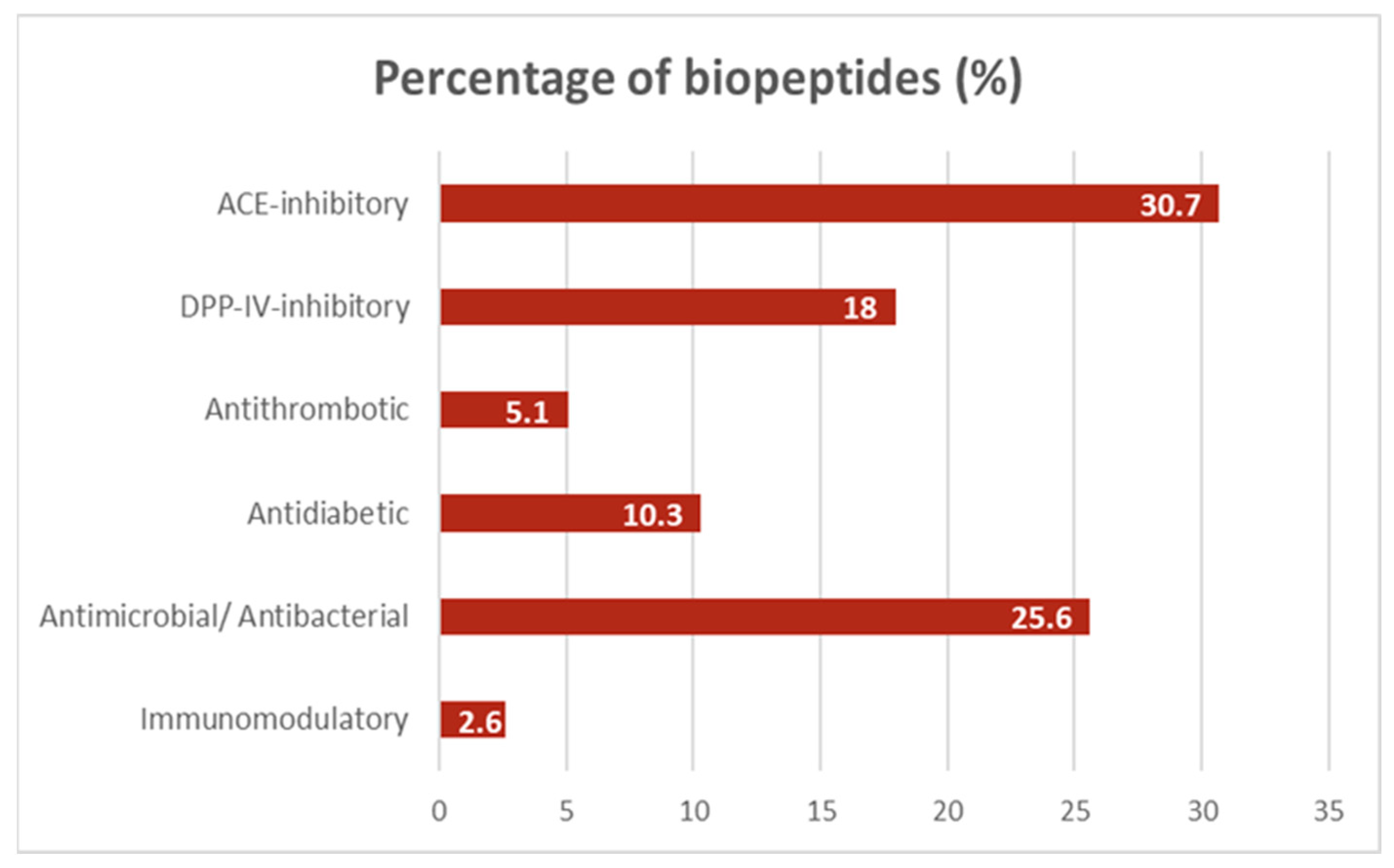
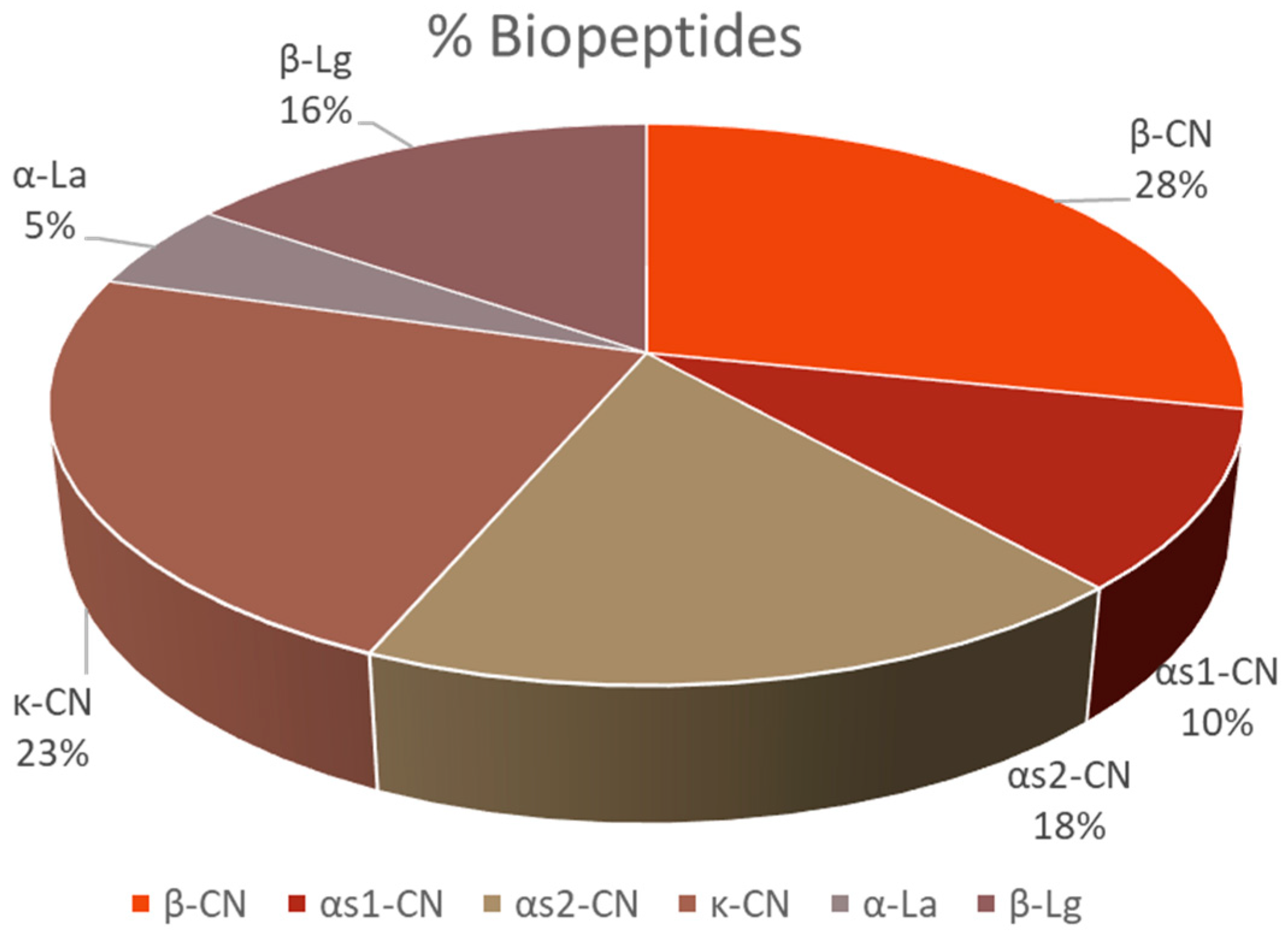
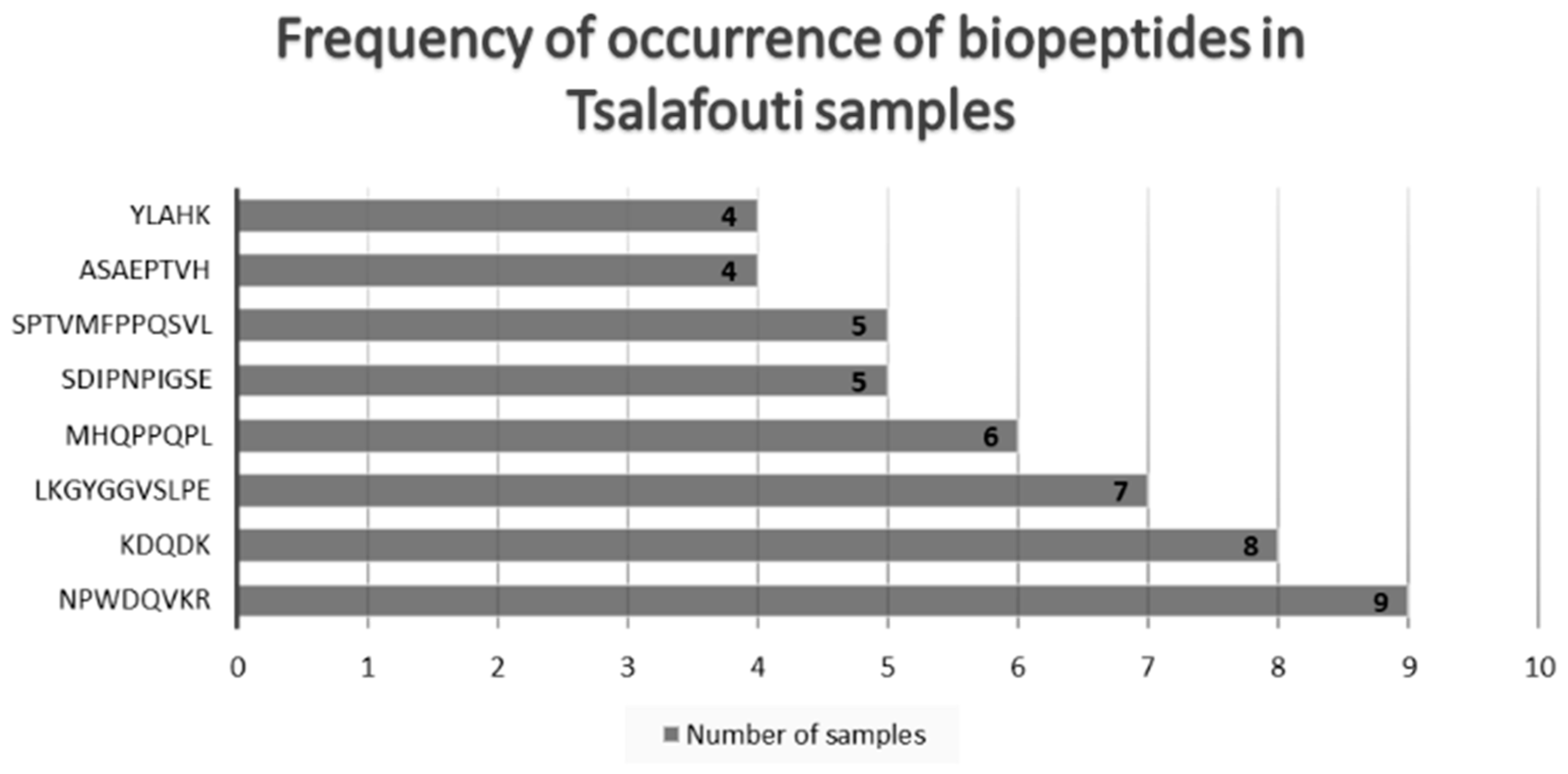
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fontenele, M.A.; do SRBastos, M.; Dos Santos, K.M.; Bemquerer, M.P.; do Egito, A.S. Peptide profile of Coalho cheese: A contribution for Protected Designation of Origin (PDO). Food Chem. 2017, 219, 382–390. [Google Scholar] [CrossRef]
- Roberts, T.A.; Cordier, J.L.; Gram, L.; Tompkin, R.B.; Pitt, J.I.; Gorris, L.G.M.; Swanson, K.M.J. Cereals and cereal products. In Micro-Organisms in Foods 6: Microbial Ecology of Food Commodities; Springer: Boston, MA, USA, 2005; pp. 392–439. [Google Scholar]
- Michailidou, S.; Pavlou, E.; Pasentsis, K.; Rhoades, J.; Likotrafiti, E.; Argiriou, A. Microbial profiles of Greek PDO cheeses assessed with amplicon metabarcoding. Food Microbiol. 2021, 99, 103836. [Google Scholar] [CrossRef]
- Pappa, E.C.; Kondyli, E.; Vlachou, A.M.; Kakouri, A.; Malamou, E.; Samelis, J. Semi industrial production of Tsalafouti dairy product. AIMS Agric. Food 2022, 7, 444–460. [Google Scholar] [CrossRef]
- Malissiova, E.; Meleti, E.; Samara, A.; Alexandraki, M.; Manouras, A. The traditional Greek cheese Tsalafouti: History, technology, nutrition and gastronomy. J. Ethn. Food 2023, 10, 18. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Losito, I.; Gobbetti, M.; Carbonara, T.; De Bari, M.D.; Zambonin, P. Antibacterial activities of peptides from the water-soluble extracts of Italian cheese varieties. J. Dairy Sci. 2005, 88, 2348–2360. [Google Scholar] [CrossRef]
- Kamau, S.M.; Cheison, S.C.; Chen, W.; Liu, X.M.; Lu, R.R. Alpha-lactalbumin: Its production technologies and bioactive peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–212. [Google Scholar] [CrossRef]
- Tidona, F.; Criscione, A.; Guastella, A.M.; Zuccaro, A.; Bordonaro, S.; Marletta, D. Bioactive peptides in dairy products. Ital. J. Anim. Sci. 2009, 8, 315–340. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-Derived Bioactive Peptides in Human Health: Challenges and Opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R.; Kapila, S. Biofunctional properties of bioactive peptides of milk origin. Food Rev. Int. 2009, 25, 28–43. [Google Scholar] [CrossRef]
- Haque, E.; Chand, R. Antihypertensive and antimicrobial bioactive peptides from milk proteins. Eur. Food Res. Technol. 2008, 227, 7–15. [Google Scholar] [CrossRef]
- Atanasova, J.; Ivanova, I. Antibacterial peptides from goat and sheep milk proteins. Biotechnol. Biotechnol. Equip. 2010, 24, 1799–1803. [Google Scholar] [CrossRef]
- Pisanu, S.; Pagnozzi, D.; Pes, M.; Pirisi, A.; Roggio, T.; Uzzau, S.; Addis, M.F. Differences in the peptide profile of raw and pasteurised ovine milk cheese and implications for its bioactive potential. Int. Dairy J. 2015, 42, 26–33. [Google Scholar] [CrossRef]
- Recio, I.; Visser, S. Antibacterial and binding characteristics of bovine, ovine and caprine lactoferrins: A comparative study. Int. Dairy J. 2000, 10, 597–605. [Google Scholar] [CrossRef]
- Ahmed, T.; Sun, X.; Udenigwe, C.C. Role of structural properties of bioactive peptides in their stability during simulated gastrointestinal digestion: A systematic review. Trends Food Sci. Technol. 2022, 120, 265–273. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Sato, H.H. A response surface approach on optimization of hydrolysis parameters for the production of egg white protein hydrolysates with antioxidant activities. Biocatal. Agric. Biotechnol. 2015, 4, 55–62. [Google Scholar] [CrossRef]
- Gomez-Ruiz, J.A.; Ramos, M.; Recio, I. Angiotensin-converting enzyme inhibitory peptides in Manchego cheeses manufactured with different starter cultures. Int. Dairy J. 2002, 12, 697–706. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D.; Hernandez-Escalante, V.M. Bioavailability of Bioactive Peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Ma, H.; Chen, S. Isolation and identification of dipeptidyl peptidase IV-inhibitory peptides from trypsin/chymotrypsin-treated goat milk casein hydrolysates by 2D-TLC and LC–MS/MS. J. Agric. Food Chem. 2015, 63, 8819–8828. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Gao, J.; Wang, Y.; Luo, Q.; Guo, K.; Ren, F.; Mao, X. Identification of novel peptides from goat milk casein that ameliorate high-glucose-induced insulin resistance in HepG2 cells. J. Dairy Sci. 2020, 103, 4907–4918. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-L.; Zhang, S.-S.; Wang, W.; Feng, F.-Q.; Shan, W.-G. A Novel Angiotensin I Converting Enzyme Inhibitory Peptide from the Milk Casein: Virtual Screening and Docking Studies. Agric. Sci. China 2011, 10, 463–467. [Google Scholar] [CrossRef]
- Qian, Z.-Y.; Jollès, P.; Migliore-Samour, D.; Fiat, A.-M. Isolation and characterization of sheep lactoferrin, an inhibitor of platelet aggregation and comparison with human lactoferrin. Biochim. Et Biophys. Acta (BBA)—Gen. Subj. 1995, 1243, 25–32. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.; Ramos, M.; Recio, I. Identification and formation of angiotensin-converting enzyme-inhibitory peptides in Manchego cheese by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2004, 1054, 269–277. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-Converting Enzyme Inhibitory Activity of Peptides Derived from Caprine Kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef]
- Lacroix, I.M.; Meng, G.; Cheung, I.W.; Li-Chan, E.C. Do whey protein derived peptides have dual dipeptidyl-peptidase IV and angiotensin I-converting enzyme inhibitory activities? J. Funct. Foods 2016, 21, 87–96. [Google Scholar] [CrossRef]
- Hayes, M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part II: Bioactive peptide functions. Biotechnol. J. 2007, 2, 435–449. [Google Scholar] [CrossRef]
- Lopez-Exposito, I.; Gomez-Ruiz, J.A.; Amigo, L.; Recio, I. Identification of antibacterial peptides from ovine alpha s2-casein. Int. Dairy J. 2006, 16, 1072–1080. [Google Scholar] [CrossRef]
- Miguel, M.; Gómez-Ruiz, J.Á.; Recio, I.; Aleixandre, A. Changes in arterial blood pressure after single oral administration of milk-casein-derived peptides in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2010, 54, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. In silico approaches to predict the potential of milk protein-derived peptides as dipeptidyl peptidase IV (DPP-IV) inhibitors. Peptides 2014, 57, 43–51. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mazzocchi, C.; Paolella, S.; FitzGerald, R.J. Release of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides from milk protein isolate (MPI) during enzymatic hydrolysis. Food Res. Int. 2017, 94, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Almaas, H.; Eriksen, E.; Sekse, C.; Comi, I.; Flengsrud, R.; Holm, H.; Jensen, E.; Jacobsen, M.; Langsrud, T.; Vegarud, G.E. Antibacterial peptides derived from caprine whey proteins, by digestion with human gastrointestinal juice. Br. J. Nutr. 2011, 106, 896–905. [Google Scholar] [CrossRef]
- Geerlings, A.; Villar, I.C.; Zarco, F.H.; Sánchez, M.; Vera, R.; Gomez, A.Z.; Boza, J.; Duarte, J. Identification and characterization of novel angiotensin-converting enzyme inhibitors obtained from goat milk. J. Dairy Sci. 2006, 89, 3326–3335. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. Inhibition of dipeptidyl peptidase IV and xanthine oxidase by amino acids and dipeptides. Food Chem. 2013, 141, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Recio, I.; Ramos, M.; Amigo, L. Preparation of ovine and caprine β-lactoglobulin hydrolysates with ACE-inhibitory activity. Identification of active peptides from caprine β-lactoglobulin hydrolysed with thermolysin. Int. Dairy J. 2002, 12, 805–812. [Google Scholar] [CrossRef]
- Chobert, J.M.; El-Zahar, K.; Sitohy, M.; Dalgalarrondo, M.; Métro, F.; Choiset, Y.; Haertlé, T. Angiotensin I-converting-enzyme (ACE)-inhibitory activity of tryptic peptides of ovine β-lactoglobulin and of milk yoghurts obtained by using different starters. Le Lait 2005, 85, 141–152. [Google Scholar] [CrossRef]
- Silveira, S.T.; Martínez-Maqueda, D.; Recio, I.; Hernández-Ledesma, B. Dipeptidyl peptidase-IV inhibitory peptides generated by tryptic hydrolysis of a whey protein concentrate rich in β-lactoglobulin. Food Chem. 2013, 141, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Tavares, T.; Contreras, M.d.M.; Amorim, M.; Pintado, M.; Recio, I.; Malcata, F.X. Novel whey-derived peptides with inhibitory effect against angiotensin-converting enzyme: In vitro effect and stability to gastrointestinal enzymes. Peptides 2011, 32, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Ohmori, T.; Nakagami, T. Prolylendopeptidase Inhibitory Activity of a Glial Fibrillary Acidic Protein Fragment and Other Proline-rich Peptides. Biosci. Biotechnol. Biochem. 1996, 60, 358–359. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Amigo, L.; Martínez-Maqueda, D.; Hernández-Ledesma, B. In Silico and In Vitro Analysis of Multifunctionality of Animal Food-Derived Peptides. Foods 2020, 9, 991. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A.; Koskinen, P.; Piilola, K.; Tupasela, T.; Korhonen, H. Angiotensin I-converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of active peptides. J. Dairy Res. 2000, 67, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.; Henriksson, A.; Singh, T.; Vasiljevic, T.; Shah, N. ACE-inhibitory activity of probiotic yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Mullally, M.M.; Meisel, H.; FitzGerald, R.J. Identification of a novel angiotensin-I-converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine β-lactoglobulin. FEBS Lett. 1997, 402, 99–101. [Google Scholar] [CrossRef] [PubMed]
- van Platerink, C.J.; Janssen, H.-G.M.; Haverkamp, J. Application of at-line two-dimensional liquid chromatography–mass spectrometry for identification of small hydrophilic angiotensin I-inhibiting peptides in milk hydrolysates. Anal. Bioanal. Chem. 2008, 391, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Meisel, H. Overview on Milk Protein-derived Peptides. Int. Dairy J. 1998, 8, 363–373. [Google Scholar] [CrossRef]
- Tomazou, M.; Oulas, A.; Anagnostopoulos, A.K.; Tsangaris, G.T.; Spyrou, G.M. In Silico Identification of Antimicrobial Peptides in the Proteomes of Goat and Sheep Milk and Feta Cheese. Proteomes 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, W.; Xu, Y. Comprehensive Identification of Short and Medium-Sized Peptides from Pixian Broad Bean Paste Protein Hydrolysates Using UPLC-Q–TOF–MS and UHPLC-Q Exactive HF-X. J. Agric. Food Chem. 2022, 70, 8288–8299. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Aita, S.E.; Capriotti, A.L.; Cavaliere, C.; Montone, A.M.I.; Montone, C.M.; Laganà, A. Investigating the Short Peptidome Profile of Italian Dry-Cured Ham at Different Processing Times by High-Resolution Mass Spectrometry and Chemometrics. Int. J. Mol. Sci. 2022, 23, 3193. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Du, A.; Jia, W. New insights into the bioaccessibility and metabolic fates of short-chain bioactive peptides in goat milk using the INFOGEST static digestion model and an improved data acquisition strategy. Food Res. Int. 2023, 169, 112948. [Google Scholar] [CrossRef] [PubMed]
- Pappa, E.; Robertson, J.; Rigby, N.; Mellon, F.; Kandarakis, I.; Mills, E. Application of proteomic techniques to protein and peptide profiling of Teleme cheese made from different types of milk. Int. Dairy J. 2008, 18, 605–614. [Google Scholar] [CrossRef]
| Peptide Sequence | Protein | Fragment | Peptide/Fragment Mass (Da) | Type of Milk | Bioactivity | Reference |
|---|---|---|---|---|---|---|
| SAMPLE 1 | ||||||
| MHQPPQPL | β-CN | 159–166 | 947.106/341.300 (QPP) | Sheep | DPP-IV inhibitory | [23] |
| SPTVMFPPQSVL | β-CN | 165–178 | 1302.536/430.450 (PQSV) | Sheep | DPP-IV inhibitory | [23] |
| QEPVLGPVRGPFP | β-CN | 207–219 | 1392.601/475.500 (VRGPF) | Sheep | Antidiabetic | [24] |
| KVLILA | β-CN | 2–7 | 656.700 | Sheep | ACE inhibitory | [25] |
| TAQVTSTEV | κ-CN | 184–192 | 934.981/707.700 (TAQVTST) | Sheep | Antithrombotic | [26] |
| SDIPNPIGSE | αs1-CN | 195–204 | 1028.066/502.500 (PIGSE) | Sheep/Goat | Antidiabetic | [23] |
| VPSERY | αs1-CN | 101–106 | 750.900 | Sheep | ACE inhibitory | [27] |
| VRYL | αs2-CN | 220–223 | 550.800 | Sheep | ACE-inhibitory | [27] |
| KFAWPQ | αs2-CN | 189–194 | 776.900 | Sheep | ACE-inhibitory | [28] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/232.200 (GGV) | Sheep/Goat | DPP-IV inhibitory | [29] |
| SAMPLE 2 | ||||||
| QTPVVVPPF | β-CN | 94–102 | 983.200 | Sheep | Immunomodulatory | [30] |
| NAGPFTPT | αs2-CN | 131–138 | 803.800 | Sheep | Antibacterial | [31] |
| YAKPVA | κ-CN | 82–87 | 647.600 | Sheep | ACE-inhibitory | [32] |
| LKKISQ | αs2-CN | 180–185 | 716.600 | Sheep/Goat | Antimicrobial | [31] |
| NPWDQVKR | αs2-CN | 123–130 | 1042.144/659.350 (NPWDQ) | Goat | Antidiabetic | [24] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/658.100 (GGVSLPE) | Sheep/Goat | DPP-IV inhibitory | [29] |
| IPIQY | κ-CN | 47–51 | 633.600 | Goat | DPP-IV inhibitory | [33] |
| GPFPILV | β-CN | 216–222 | 741.911/342.400 (PIL) | Goat | DPP-IV inhibitory | [23] |
| SAMPLE 3 | ||||||
| TGPIPN | β-CN | 78–83 | 597.652/383.200 (GPIP) | Sheep | ACE-inhibitory | [15] |
| GPFPILV | β-CN | 216–222 | 741.911/342.400 (PIL) | Sheep | DPP-IV inhibitory | [23] |
| SPTVMFPPQSVL | β-CN | 167–178 | 1302.536/430.400 (PQSV) | Sheep | DPP-IV inhibitory | [23] |
| MHQPPQPL | β-CN | 159–166 | 947.106/454.350 (PQPL) | Sheep | DPP-IV inhibitory | [23] |
| VPSERY | αs1-CN | 101–106 | 750.750 | Sheep | ACE inhibitory | [27] |
| VDQHQKAMKPWTQ-PKTNAIPYVRYL | αs2-CN | 184–208 | 3013.491/628.500 (PWTQP) | Sheep | Antibacterial | [31] |
| VLVLDTDYK | B-Lg | 110–118 | 1065.213/659.600 (VLVLDT) | Sheep | DPP-IV inhibitory/ACE inhibitory | [34] |
| SAMPLE 4 | ||||||
| PPKKDQDKTEVPA | κ-CN | 130–142 | 1452.608/341.300 (PPK) | Goat | Antimicrobial | [35] |
| ASAEPTVH | κ-CN | 147–154 | 810.844/474.250 (ASAEP) | Goat | Antimicrobial | [35] |
| PTVHSTPTTE | κ-CN | 151–160 | 1069.118/ 635.400 (STPTTE) | Goat | Antimicrobial | [35] |
| ENLLRF | αs1-CN | 33–38 | 790.900/662.600 (NLLRF) | Goat | ACE-inhibitory | [28] |
| NPWDQVKR | αs2-CN | 123–130 | 1042.144/830.800 (WDQVKR) | Goat | Antidiabetic | [24] |
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| SDIPNPIGSENSEK | αs1-CN | 195–208 | 1486.536/668.600 (DIPNPI) | Goat | Antibacterial | [30] |
| SAMPLE 5 | ||||||
| SPTVMFPPQSVL | β-CN | 167–178 | 1302.536/304.200 (STP) | Sheep | DPP-IV inhibitory | [23] |
| YQEPVLGP | β-CN | 206–213 | 1302.536/739.600 (QEPVLGP) | Goat | Antioxidant/Antidiabetic | [24] |
| SDIPNPIGSE | αs1-CN | 195–204 | 1028.066/668.700 (DIPNP) | Goat | Antidiabetic | [24] |
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| DAQSAPLR | B-Lg | 51–58 | 858.000 | Goat | Antimicrobial | [35] |
| TGPIPN | β-CN | 78–83 | 597.652/383.200 (GPIP) | Sheep | ACE-inhibitory | [36] |
| NPWDQVKR | αs2-CN | 123–130 | 1042.144/659.600 (NPWDQ) | Goat | Antidiabetic | [24] |
| YPYY | κ-CN | 79–82 | 605.500 | Sheep | DPP-IV inhibitory/ACE-inhibitory/Opioid | [33,37] |
| YLAHK | a-La | 104–108 | 630.600 | Goat | ACE-inhibitory | [15] |
| LKPTPEGD | B-Lg | 46–53 | 969.085/314.350 (PTP) | Goat | ACE-inhibitory | [38] |
| SAMPLE 6 | ||||||
| MHQPPQPL | β-CN | 159–166 | 947.106/341.300 (QPP) | Sheep | DPP-IV inhibitory | [23] |
| ASAEPTVH | κ-CN | 147–154 | 810.844/473.400 (ASAEP) | Goat | Antimicrobial | [35] |
| SDIPNPIGSENSEK | αs1-CN | 195–208 | 1028.066/502.450 (PIGSE) | Goat | Antibacterial | [30] |
| SPTVMFPPQSVL | β-CN | 167–178 | 1302.536/778.400 (SPTVMFP) | Sheep | DPP-IV inhibitory | [23] |
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| NPWDQVKR | αs2-CN | 123–130 | 659.500/ (NPWDQ) 402.400 (VKR) | Goat | Antidiabetic | [24] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/1.046.300 (GGVSLPEWVC) | Sheep/Goat | DPP-IV inhibitory | [29] |
| YLAHK | a-La | 104–108 | 630.600 | Goat | ACE-inhibitory | [15] |
| LKPTPEGD | B-Lg | 46–53 | 855.926/628.600 (KPTPEG) | Goat | ACE-inhibitory | [38] |
| ALPMHIR | B-Lg | 142–148 | 837.037/360.350 (LPM) | Sheep | ACE-inhibitory | [39] |
| SAMPLE 7 | ||||||
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| VPSERY | αs1-CN | 101–106 | 749.804/863.000 (VPSERYL) | Sheep | ACE inhibitory | [27] |
| SPTVMFPPQSVL | β-CN | 167–178 | 1302.536/341.300 (PPQ) 360.350 (FPP) | Sheep | DPP-IV inhibitory | [23] |
| NPWDQVKR | αs2-CN | 123–127 | 1042.144/659.550 (NPWDQ) | Sheep | Antidiabetic | [24] |
| IPAVF | B-Lg | 96–100 | 545.662/286.350 (PAV) | Sheep | DPP-IV inhibitory | [40] |
| TGPIPN | β-CN | 78–83 | 597.652/286.350 (GPI) | Sheep | ACE-inhibitory | [36] |
| SAMPLE 8 | ||||||
| SLPQ | β-CN | 84–87 | 443.484/558.600 (NSLPQ) | Sheep/Goat | ACE-inhibitory | [36] |
| MHQPPQPL | β-CN | 159–166 | 947.106/284.350 (HQ) | Sheep/Goat | DPP-IV inhibitory | [23] |
| SPTVMFPPQSVL | β-CN | 167–178 | 1302.536/360.400 (FPP) | Sheep/Goat | DPP-IV inhibitory | [23] |
| NPWDQVKR | αs2-CN | 123–130 | 1302.536/230.300 (NP) | Goat | Antidiabetic | [24] |
| INNQFLPYPY | κ-CN | 72–81 | 1268.415/360.400 (INN) | Goat | DPP-IV inhibitory | [23] |
| ALNEINQF | αs2-CN | 96–104 | 948.026/878.000 (LNEINQF) | Goat | Antimicrobial | [15] |
| TAQVTSTEV | κ-CN | 184–192 | 934.981/418.500 (TAQV) | Sheep/Goat | Antithrombotic | [26] |
| LKPTPEGD | B-Lg | 64–72 | 855.926/314.400 (PTP) | Sheep/Goat | DPP-IV inhibitory | [29] |
| SAMPLE 9 | ||||||
| KFAWPQ | αs2-CN | 174–179 | 775.887/550.500 (KFAW) | Goat | ACE-inhibitory | [28] |
| YPFTGPIPN | β-CN | 60–68 | 1005.12/286.400 (GPI) | Goat | Antimicrobial | [16] |
| SLSSSEESITH | β-CN | 30–40 | 1176.184/477.300 (EESI) | Goat | Antidiabetic | [24] |
| SDIPNPIGSE | αs1-CN | 195–204 | 1028.066/286.400 (PIG) | Goat | Antidiabetic | [24] |
| NPWDQVKR | αs2-CN | 123–130 | 1042.144/402.400 (VKR) | Goat | Antidiabetic | [24] |
| MHQPPQPL | β-CN | 159–166 | 947.106/454.400 (PQPL) | Sheep | DPP-IV inhibitory | [23] |
| ALPMHIR | B-Lg | 142–148 | 837.037/384.200 (LPM) | Sheep | ACE-inhibitory | [39] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/536.600 (LKGYG) | Sheep/Goat | DPP-IV inhibitory | [29] |
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| SAMPLE 10 | ||||||
| MHQPPQPL | β-CN | 159–166 | 947.106/ 381.200 (HQP) | Sheep | DPP-IV inhibitory | [23] |
| SDIPNPIGSE | αs1-CN | 195–204 | 1028.066/1544.500 (APSFSDIPNPIGSEN) | Goat | Antidiabetic | [24] |
| NPWDQVKR | αs2-CN | 123–130 | 1042.144/1854.200 (IVLNPWDQVKRNAGPF) | Goat | Antidiabetic | [24] |
| GPFPILV | β-CN | 216–222 | 741.911/342.400 (PIL) | Goat | DPP-IV inhibitory | [23] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/431.300 (GGVSL) | Sheep/Goat | DPP-IV inhibitory | [29] |
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| YQEPVLGP | β-CN | 206–213 | 901.997/649.600 (LYQEP) | Goat | Antioxidant/Antidiabetic | [24] |
| SAMPLE 11 | ||||||
| KDQDK | κ-CN | 133–137 | 633.600 | Sheep | Antithrombotic | [26] |
| NPWDQVKR | αs2-CN | 123–130 | 1042.144/545.400 (PWDQ) 402.400 (VKR) | Goat | Antidiabetic | [24] |
| ASAEPTVH | κ-CN | 147–154 | 810.844/376.200 (ASAE) | Goat | Antimicrobial | [35] |
| PTVHSTPTTE | κ-CN | 151–160 | 1069.118/541.600 (HSTPT) | Goat | Antimicrobial | [35] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/231.300 (GGV) | Sheep/Goat | DPP-IV inhibitory | [29] |
| ENLLRF | αs1-CN | 33–38 | 791.00 | Goat | ACE-inhibitory | [28] |
| YAKPVA | κ-CN | 82–87 | 647.600 | Sheep | ACE-inhibitory | [32] |
| LKKISQ | αs2-CN | 180–185 | 715.874/ 446.500 (KIS) | Sheep/Goat | Antimicrobial | [31] |
| YLAHK | a-La | 104–108 | 630.727/468.450 (LAHK) | Goat | ACE-inhibitory | [15] |
| SAMPLE 12 | ||||||
| SDIPNPIGSE | αs1-CN | 195–204 | 1028.066/555.700 (DIPNP) | Goat | Antidiabetic | [24] |
| KFAWPQ | αs2-CN | 189–194 | 775.887/429.550 (WPQ) | Sheep | ACE-inhibitory | [28] |
| KDQDK | κ-CN | 133–137 | 633.600/261.800 (KD) | Sheep | Antithrombotic | [26] |
| LKGYGGVSLPE | a-La | 34–44 | 1119.265/261.800 (GVS) | Sheep/Goat | DPP-IV inhibitory | [29] |
| YLAHK | a-La | 104–108 | 630.700 | Goat | ACE-inhibitory | [15] |
| LLF | B-Lg | 103–105 | 391.494/555.700 (YLLF) | Goat | ACE-inhibitory | [38] |
| YAKPVA | κ-CN | 82–87 | 647.600 | Sheep | ACE-inhibitory | [32] |
| ASAEPTVH | κ-CN | 147–154 | 810.844/218.100 (AE) | Goat | Antimicrobial | [35] |
| Biopeptides after Digestion | |||||
|---|---|---|---|---|---|
| Original Peptide | Peptide after Digestion | Protein | Type of Milk | Bioactivity | Reference |
| LKGYGGVSLPE | KGYGGVSL | a-La | Sheep/Goat | ACE inhibitor | [40] |
| IPIQY | IPI | κ-CN | Goat | DPP IV inhibitor | [41] |
| IPIQY | QY | κ-CN | Goat | DPP IV inhibitor | [42] |
| GPFPILV | LV | β-CN | Goat | DPP IV inhibitor | [43] |
| TGPIPN | TGPIPN | β-CN | Sheep | ACE inhibitor | [15] |
| SPTVMFPPQSVL | VL | β-CN | Sheep | DPP IV inhibitor | [43] |
| VPSERY | RY | αs1-CN | Sheep | Antioxidative | [44] |
| VDQHQKAMKPWTQPKTNAIPYVRYL | RYL | αs2-CN | Sheep | Antioxidative | [44] |
| VDQHQKAMKPWTQPKTNAIPYVRYL | PYVRYL | αs2-CN | Sheep | Antioxidative | [31] |
| VLVLDTDYK | VLDTDYK | B-Lg | Sheep | ACE inhibitor | [45] |
| PPKKDQDKTEVPA | PPK | κ-CN | Goat | Antithrombotic | [30] |
| ASAEPTVH | ASAEPTVH | κ-CN | Goat | Antimicrobial | [35] |
| SDIPNPIGSENSEK | SDIPNPIGSENSEK | αs1-CN | Goat | Antibacterial | [30] |
| YQEPVLGP | YQEPVL | β-CN | Goat | ACE inhibitor | [46] |
| YPYY | YPY | κ-CN | Sheep | DPP IV inhibitor | [33] |
| YPYY | YY | κ-CN | Sheep | DPP IV inhibitor | [43] |
| ALPMHIR | ALPMH | B-Lg | Sheep | ACE inhibitor | [47] |
| SLPQ | PQ | β-CN | Sheep/Goat | ACE inhibitor | [48] |
| LLF | LF | B-Lg | Goat | ACE inhibitor | [49] |
| Short Chain Peptides | Confirmed Bioactivity | Peptide Ranker c |
|---|---|---|
| VRYL | ACE-inhibitory b | 0.3127 |
| IPIQY | DPP-IV Inhibitory b | 0.3607 |
| KDQDK | Antithrombotic a | 0.0764 |
| YPYY | ACE-inhibitory b, DPP-IV Inhibitory a,b, Opioid b | 0.7586 |
| YLAHK | ACE-inhibitory a | 0.1832 |
| IPAVF | ACE-inhibitory b, DPP-IV Inhibitory a,b | 0.6821 |
| SLPQ | ACE-inhibitory b | 0.3658 |
| LLF | ACE-inhibitory b | 0.9389 |
| Short chainPeptides after simulated digestion | Confirmed Bioactivity | Peptide Ranker c |
| PPK | Antithrombotic a | 0.6062 |
| YPY | DPP-IV Inhibitory a,b | 0.7358 |
| LF | ACE-inhibitory b, Immunomodulatory b, Anti-inflammatory b | 0.9869 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meleti, E.; Alexandraki, M.; Samara, A.; Loffi, C.; Tedeshi, T.; Galaverna, G.; Manouras, A.; Koureas, M.; Malissiova, E. Profile Assessment of Bioactive Peptides in the Greek Traditional Cheese “Tsalafouti”. Dietetics 2024, 3, 16-29. https://doi.org/10.3390/dietetics3010002
Meleti E, Alexandraki M, Samara A, Loffi C, Tedeshi T, Galaverna G, Manouras A, Koureas M, Malissiova E. Profile Assessment of Bioactive Peptides in the Greek Traditional Cheese “Tsalafouti”. Dietetics. 2024; 3(1):16-29. https://doi.org/10.3390/dietetics3010002
Chicago/Turabian StyleMeleti, Ermioni, Maria Alexandraki, Antonia Samara, Cecilia Loffi, Tullia Tedeshi, Gianni Galaverna, Athanasios Manouras, Michalis Koureas, and Eleni Malissiova. 2024. "Profile Assessment of Bioactive Peptides in the Greek Traditional Cheese “Tsalafouti”" Dietetics 3, no. 1: 16-29. https://doi.org/10.3390/dietetics3010002
APA StyleMeleti, E., Alexandraki, M., Samara, A., Loffi, C., Tedeshi, T., Galaverna, G., Manouras, A., Koureas, M., & Malissiova, E. (2024). Profile Assessment of Bioactive Peptides in the Greek Traditional Cheese “Tsalafouti”. Dietetics, 3(1), 16-29. https://doi.org/10.3390/dietetics3010002







