1. Introduction
According to the GLOBOCAN estimates, there were 57,043 malignant melanoma deaths (0.6% of the total cancer death) worldwide in 2020 [
1,
2]. Almost 50% of deaths (26,360 cases) occurred in Europe (about 14,500 in men and 11,500 in women). The estimated five-year survival rates for malignant melanoma are relatively low (≤20% for metastatic disease) while rates for localized disease are about 90% [
3,
4].
The world regions with the greatest mortality rates from malignant melanoma are Europe and North America, at ≥1.3 per 100,000 [
2]. In contrast, regions like South-Central Asia, Micronesia, and Northern Africa reported the lowest rates at 0.2 per 100,000. In the world, mortality from malignant melanoma was higher in men than in women (0.7 vs. 0.4 per 100,000). Globally, an overall decreasing trend in malignant melanoma mortality was observed throughout many different populations over the past decades [
1,
2,
3,
4].
For malignant melanoma of skin, one of the most aggressive skin cancers, great differences in mortality across the world have been observed during the last decades. The purpose of this study was to assess the mortality of malignant melanoma of skin in Serbia in 1991–2019.
2. Materials and Methods
For this descriptive epidemiological study, we used the annual underlying cause of death data from Serbia to describe trends in mortality from malignant melanoma for the period 1991–2019. Data on persons who died of malignant melanoma (site code 172 revision 9 and C43 revision 10 of the International Classification of Diseases (ICD) to classify death, injury, and cause of death) were obtained from the Statistical Office of the Republic of Serbia (unpublished data). In Serbia, from 1991 to 1996, data about the main cause of death were classified by the ICD-9th revision. Since 1997, the data processing of mortality statistics is based on the ICD-10th revision. Based on the indicators of mortality statistics, the WHO assessed the registration of death data in Serbia as having medium quality [
5].
The study comprised the entire population of the Republic of Serbia (all ages). The entire population of Serbia comprised about 7 million inhabitants in 2019. Data on the number and composition of the Serbian population by gender and age were presented according to the 1991, 2002, and 2011 censuses.
Three types of death rates were calculated: crude, specific (age- and sex-specific), and age-standardized (expressed per 100,000 persons). The age-standardized rate (ASR) was calculated by a direct method (the world population was used as the standard population, stratified by a five-year age strata).
Mortality trends from suicides were assessed using the joinpoint regression analysis (joinpoint regression software, available through the Surveillance Research Program of the US National Cancer Institute). The results are presented as straight lines connected at change points on a log scale, and these trends in the annual age-standardized mortality rates are characterized with an annual percentage change (APC) between successive change points, with 95% confidence intervals (CI). The goal of the comparability test was to answer whether the two regression mean functions were identical (test of coincidence) or parallel (test of parallelism). A p value of <0.05 was considered statistically significant for all tests.
3. Results
During the observed period, nearly 6500 (about 3600 men and 2700 women) malignant melanoma deaths occurred in Serbia, with the average annual age-standardized mortality rate being 7.0 per 100,000 inhabitants (
Table 1). The average annual ASR of malignant melanoma of skin mortality was greater in men than in women (11.2 per 100,000 and 3.3 per 100,000, respectively).
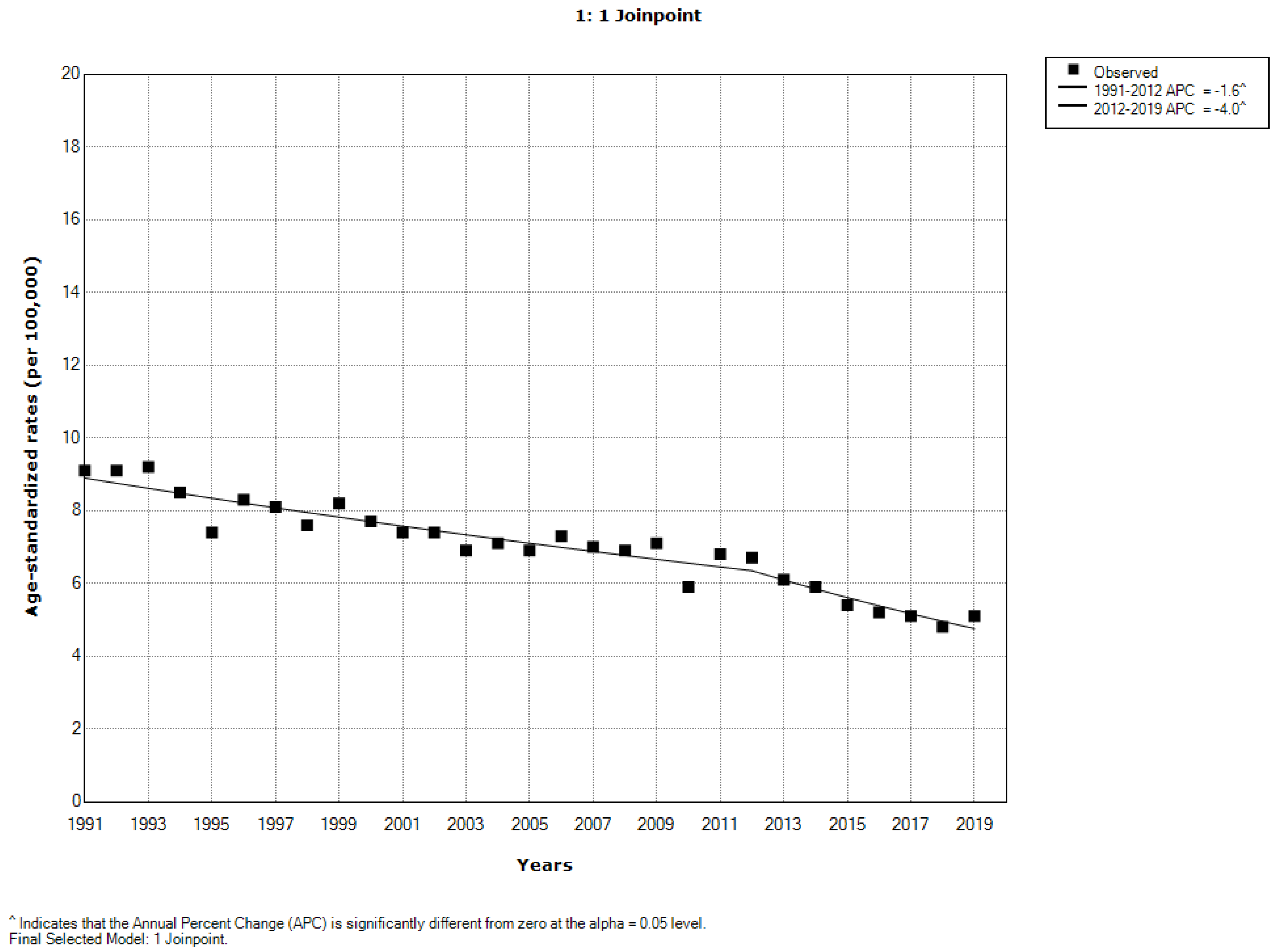
Overall, a significantly decreasing trend for malignant melanoma of skin mortality was observed (AAPC = −2.0% per year, 95% CI = −2.3 to −1.7). However, one joinpoint was observed: a significant decrease of malignant melanoma mortality from 1991 to 2012 (by −1.6% per year, 95% CI = −2.0 to −1.2) was followed by a significant decrease onwards (by −4.0% per year, 95% CI = −5.9 to −2.2) (
Figure 1).
Trends from malignant melanoma of skin mortality rates significantly decreased both in men (AAPC = −1.6% per year; 95% CI = −1.9 to −1.4) and women (AAPC = −3.5% per year; 95% CI = −3.9 to −3.0) (
Figure 2). However, one joinpoint was observed: a significant decrease of malignant melanoma mortality from 1991 to 2012 (by −1.2% per year, 95% CI = −1.6 to −0.9) was followed by a significant decrease onwards (by −3.6% per year, 95% CI = −5.4 to −1.8). Mortality trends in males and females were not parallel (the final selected model rejected parallelism,
p = 0.0002) and not coincident (the final selected model rejected coincidence,
p = 0.0002).
Malignant melanoma mortality rates in both sexes increased with age and were greater in males than in females in all age groups (
Figure 3).
4. Discussion
The trend in malignant melanoma of skin mortality has been decreasing in Serbia in the last decades, both in men and women. Despite decreasing trends, mortality rates for malignant melanoma in Serbia remain among the highest in the world.
Mortality rates for malignant melanoma among men in the world in 2020 vary between 6.7 per 100,000 in New Zealand, 4.0 in Montenegro, 3.8 in Norway and Slovakia, followed by 3.4 in Slovenia, 3.3 in Croatia, 3.2 in Australia, and 3.1 in Serbia [
2]. Contrary, the lowest rates (≤0.2 per 100,000) are found in China, Ethiopia, Nepal, Nigeria, Viet Nam, Barbados, Jordan, and Saudi Arabia. Mortality rates among women in the world in 2020 vary between 2.8 per 100,000 in New Zealand, 2.6 in Norway, followed by 2.3 in Montenegro, 2.2 in Slovakia, 2.1 in Denmark, 1.9 in Slovenia, and 1.7 in Serbia, Croatia, Australia, and Ireland equally [
2].
According to Yang et al. [
6], continuously increasing malignant melanoma mortality for both sexes between 1985 and 2015 was reported in Belgium, Croatia, Germany, Ireland, Italy, Norway, the Republic of Korea, Serbia, and the United Kingdom. Furthermore, continuously decreasing malignant melanoma mortality for both sexes between 1985 and 2015 was reported in the Czech Republic only. Besides, continuously decreasing malignant melanoma mortality for females between 1985 and 2015 was reported in Australia only. Denmark, Estonia, Finland, Hungary, Lithuania, Netherlands, Moldova, Slovakia, Slovenia, and Sweden reported continuously decreasing malignant melanoma mortality for males only between 1985 and 2015.
The large geographic variations in malignant melanoma mortality could be attributed to differences in practice of diagnosis and treatment as well as lifestyle habits [
6,
7,
8]. Some differences by sexes could be due to the fact that men protect themselves less from the sun [
9]. On the other hand, it is more likely for men to engage in outdoor occupations and, therefore, experience more exposure to ultraviolet radiation. Additionally, although women are more likely to practice sun protection, they are also more likely to undergo intentional sun tanning. Some other factors that may be factors behind the sex differences in malignant melanoma mortality across different countries include differences in alcohol intake and diet as well as biological factors.
5. Conclusions
The trend in malignant melanoma of skin mortality has been decreasing in Serbia in the last decades, both in men and women. Despite decreasing trends, mortality rates for malignant melanoma in Serbia remain among the highest in the world. Future epidemiological studies are necessary in order to elucidate the reasons of the malignant melanoma burden in Serbia.
Author Contributions
Conceptualization, I.I. and M.I.; methodology, I.I. and M.I.; software, I.I. and M.I.; validation, I.I. and M.I.; formal analysis, I.I. and M.I.; investigation, I.I. and M.I.; resources, I.I. and M.I.; data curation, I.I. and M.I.; writing—original draft preparation, I.I.; writing—review and editing, I.I. and M.I.; visualization, I.I. and M.I.; supervision, M.I.; project administration, M.I.; funding acquisition, M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the Faculty of Medical Sciences, University of Kragujevac (Ref. No.: 01-4806, 12 May 2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
This study is conducted as the part of project No 175042 supported by the Ministry of Education, Science and Technological Development, Republic of Serbia, 2011–2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020; Available online: https://gco.iarc.fr/today (accessed on 2 June 2021).
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2018; Based on November 2020 SEER Data Submission, Posted to the SEER Web Site; National Cancer Institute: Bethesda, MD, USA, 2021. Available online: https://seer.cancer.gov/csr/1975_2018 (accessed on 22 June 2021).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Mathers, C.D.; Fat, D.M.; Inoue, M.; Rao, C.; Lopez, A.D. Counting the dead and what they died from: An assessment of the global status of cause of death data. Bull. World Health Organ. 2005, 83, 171–177. [Google Scholar]
- Yang, D.D.; Salciccioli, J.D.; Marshall, D.C.; Sheri, A.; Shalhoub, J. Trends in malignant melanoma mortality in 31 countries from 1985 to 2015. Br. J. Dermatol. 2020, 183, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Centers for Disease Control and Prevention (CDC). Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).