Chenopodium quinoa to Modulate Innate Myeloid Cells in the Induction of Obesity †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metabolic Mouse Models
2.2. Hemogram
2.3. Markers of Selective Functional Differentiation Intrahepatic Macrophages
2.4. Statistical Analyses
3. Results
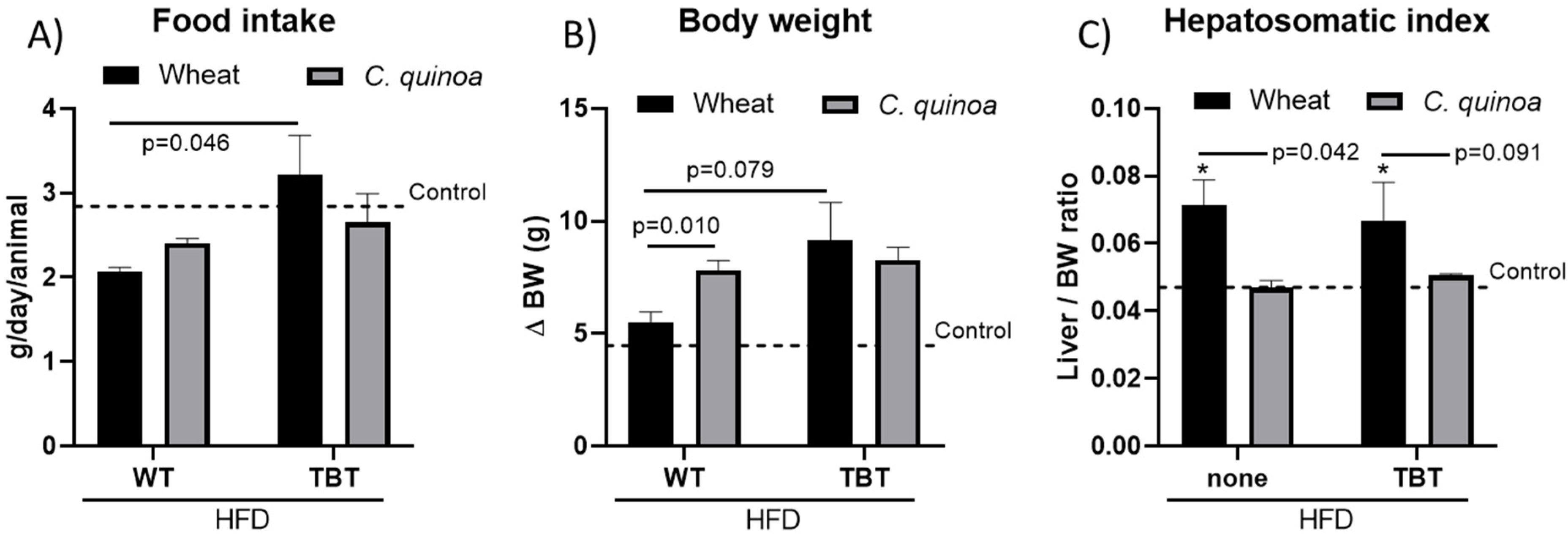
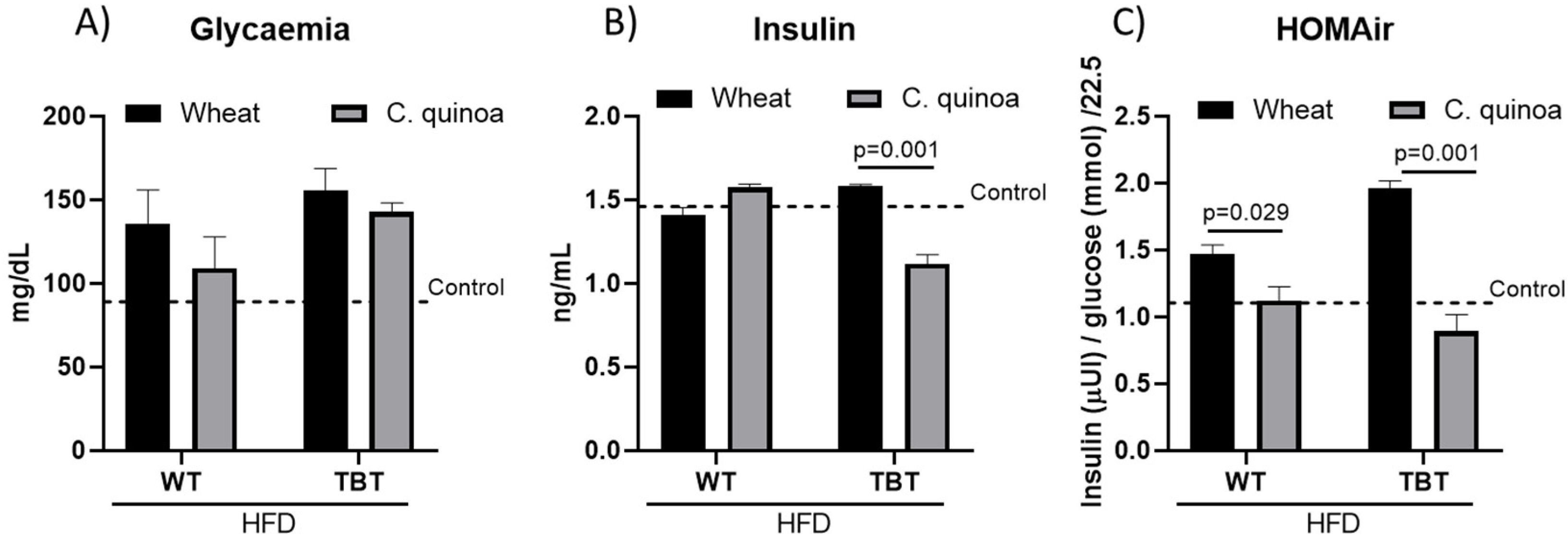
3.1. Food Intake and Morphometric Measurements
3.2. Variations on Innate Immune Myeloid Cell Population
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krenkel, O.; Hundertmark, J.; Abdallah, A.T.; Kohlhepp, M.; Puengel, T.; Roth, T.; Branco, D.P.P.; Mossanen, J.C.; Luedde, T.; Trautwein, C.; et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut 2020, 69, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Eter, L.; Lanzetta, N.; Abrishami, S.; Varghese, M.; McKernan, K.; Muir, L.; Lane, J.; Lumeng, C.N.; Singer, K. TLR4, TRIF, and MyD88 are essential for myelopoiesis and CD11c+ adipose tissue macrophage production in obese mice. J. Biol. Chem. 2018, 293, 8775–8786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selma-Gracia, R.; Haros, C.M.; Laparra Llopis, J.M. Inclusion of Salvia hispanica L. and Chenopodium quinoa into bread formulations improves metabolic imbalances derived from a high-fat intake in hyperglycaemic mice. Food Funct. 2020, 11, 7994–8002. [Google Scholar] [CrossRef]
- Srdić, M.; Ovčina, I.; Fotschki, B.; Haros, C.M.; Laparra, J.M. C. quinoa and S. hispanica L. seeds provide immunonutritional agonists to selectively polarize macrophages. Cells 2020, 9, 593. [Google Scholar] [CrossRef] [Green Version]
- Chamorro-García, R.; Sahu, M.; Abbey, R.J.; Laude, J.; Pham, N.; Blumberg, B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ. Health Perspect. 2013, 121, 359–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.S.; Mayes, W.H.; Bracy, D.P.; Bookbinder, L.H.; Hasty, A.H.; Thompson, C.B.; Wasserman, D.H. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Guo, J.; Xi, Z.; Xu, J.; Zuo, Z.; Wang, C. Tributyltin in male mice disrupts glucose homeostasis as well as recovery after exposure: Mechanism analysis. Arch. Toxicol. 2017, 91, 3261–3269. [Google Scholar] [CrossRef] [PubMed]
- Chakarov, S.; Lim, H.Y.; Tan, L.; Lim, S.Y.; See, P.; Lum, J.; Zhang, X.-M.; Foo, S.; Nakamizo, S.; Duan, D.; et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019, 363, eaau0964. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laparra, J.M.; Aguilar-Aguilar, E.; Haros, C.M. Chenopodium quinoa to Modulate Innate Myeloid Cells in the Induction of Obesity. Biol. Life Sci. Forum 2021, 8, 13. https://doi.org/10.3390/blsf2021008013
Laparra JM, Aguilar-Aguilar E, Haros CM. Chenopodium quinoa to Modulate Innate Myeloid Cells in the Induction of Obesity. Biology and Life Sciences Forum. 2021; 8(1):13. https://doi.org/10.3390/blsf2021008013
Chicago/Turabian StyleLaparra, José Moisés, Elena Aguilar-Aguilar, and Claudia Monika Haros. 2021. "Chenopodium quinoa to Modulate Innate Myeloid Cells in the Induction of Obesity" Biology and Life Sciences Forum 8, no. 1: 13. https://doi.org/10.3390/blsf2021008013
APA StyleLaparra, J. M., Aguilar-Aguilar, E., & Haros, C. M. (2021). Chenopodium quinoa to Modulate Innate Myeloid Cells in the Induction of Obesity. Biology and Life Sciences Forum, 8(1), 13. https://doi.org/10.3390/blsf2021008013







