Development of Photothermal Membrane for Treatment of Infected Wound: A Proof-of-Concept †
Abstract
:1. Introduction
2. Methods
2.1. Preparation of CS/PVA/Pd Dressing
2.2. Evaluation of the Antibacterial Properties of CS/PVA/Pd Membrane
3. Results and Discussion
3.1. Characterization of CS/PVA/Pd Dressing
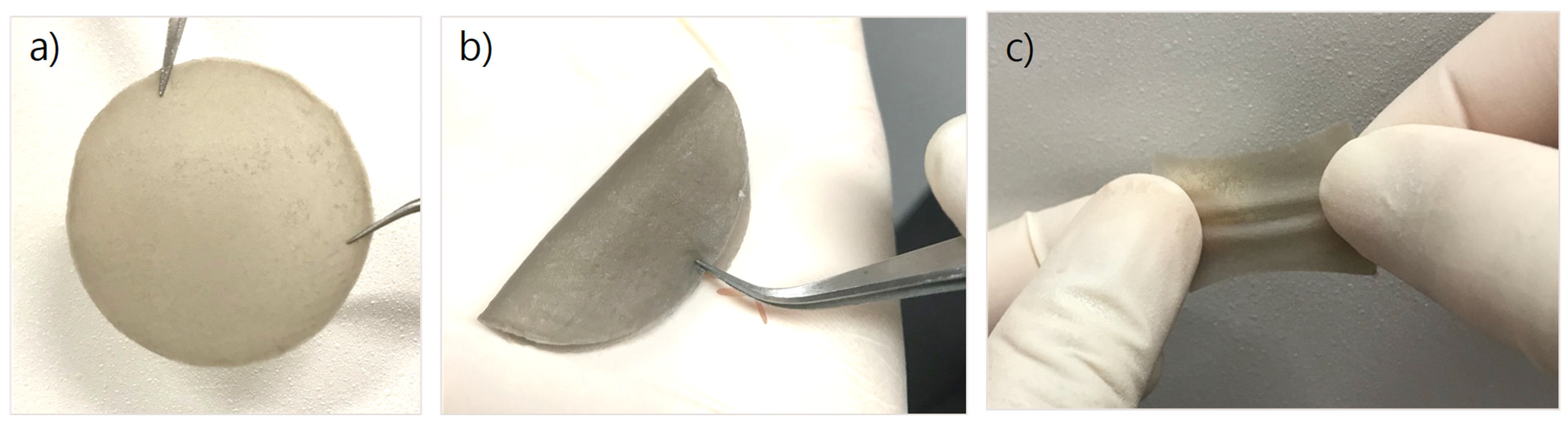
3.1.1. Physical Properties of CS/PVA/Pd Dressing
3.1.2. Surface and Morphology of CS/PVA/Pd Dressing
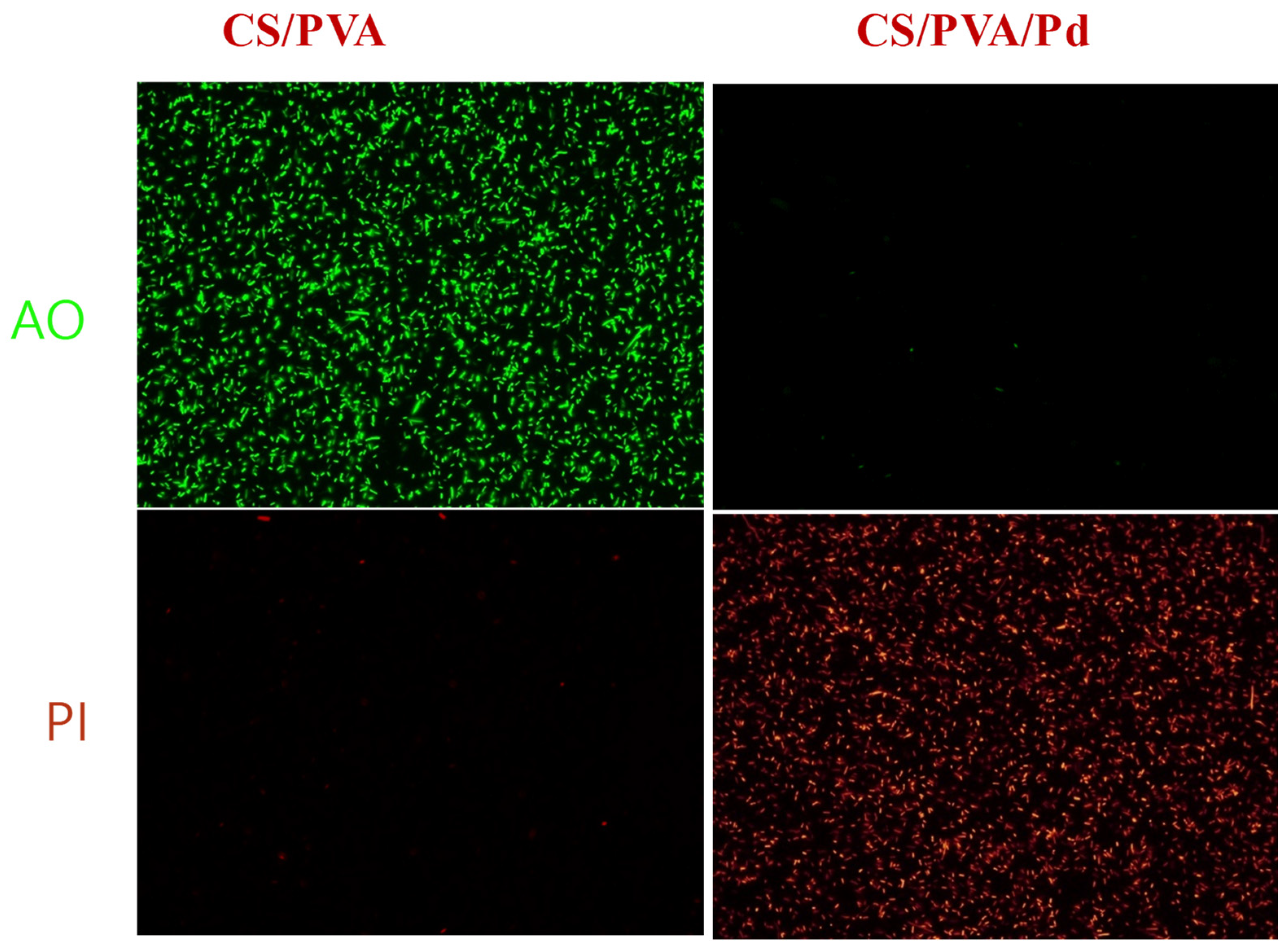
3.2. Anti-Bacterial Performance of CS/PVA/Pd Dressing
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, K.-L.; Hosseinkhani, H. Development of 3D In Vitro Technology for Medical Applications. Int. J. Mol. Sci. 2014, 15, 17938–17962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, T.T.V.; Hoang, G.; Nguyen, V.T.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.; Matthew, H. Porous chitosan scafolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, T.T.V. Development of Photothermal Membrane for Treatment of Infected Wound: A Proof-of-Concept. Biol. Life Sci. Forum 2021, 7, 9. https://doi.org/10.3390/ECB2021-10279
Phan TTV. Development of Photothermal Membrane for Treatment of Infected Wound: A Proof-of-Concept. Biology and Life Sciences Forum. 2021; 7(1):9. https://doi.org/10.3390/ECB2021-10279
Chicago/Turabian StylePhan, Thi Tuong Vy. 2021. "Development of Photothermal Membrane for Treatment of Infected Wound: A Proof-of-Concept" Biology and Life Sciences Forum 7, no. 1: 9. https://doi.org/10.3390/ECB2021-10279
APA StylePhan, T. T. V. (2021). Development of Photothermal Membrane for Treatment of Infected Wound: A Proof-of-Concept. Biology and Life Sciences Forum, 7(1), 9. https://doi.org/10.3390/ECB2021-10279





