Acute and Chronic Effects of Medium-Chain Triglyceride Supplementation on Metabolic Parameters and Working Memory in Rats †
Abstract
:1. Introduction
2. Materials and Methods
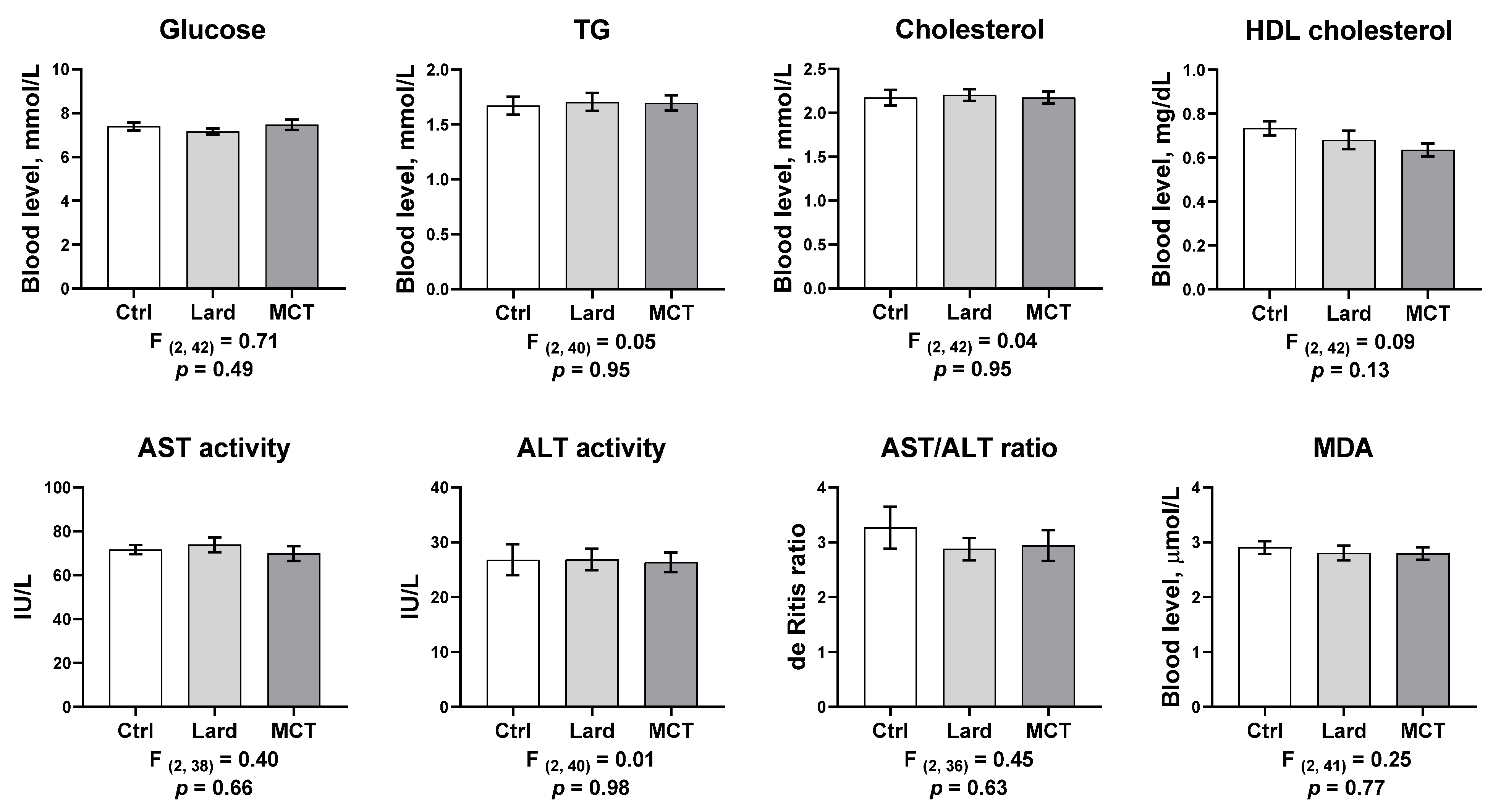
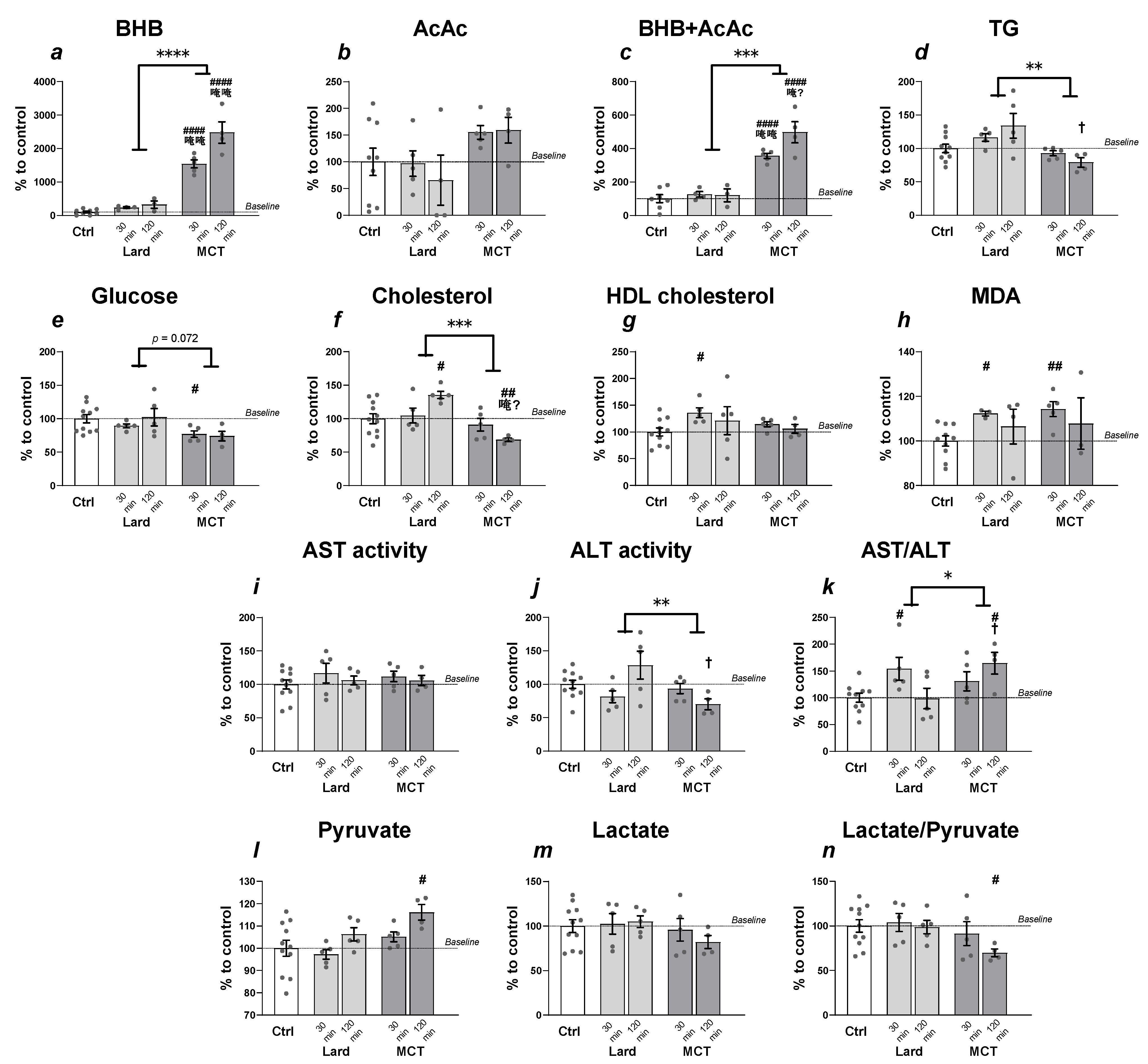
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bach, A.C.; Babayan, V.K. Medium-chain triglycerides: An update. Am. J. Clin. Nutr. 1982, 36, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Leray, V.; Diez, M.; Serisier, S.; Le Bloc’h, J.; Siliart, B.; Dumon, H. Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 2008, 92, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Seaton, T.B.; Welle, S.L.; Warenko, M.K.; Campbell, R.G. Thermic effect of medium-chain and long-chain triglycerides in man. Am. J. Clin. Nutr. 1986, 44, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Courchesne-Loyer, A.; Fortier, M.; Tremblay-Mercier, J.; Chouinard-Watkins, R.; Roy, M.; Nugent, S.; Castellano, C.-A.; Cunnane, S.C. Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: Estimated potential contribution to brain energy metabolism. Nutrition 2013, 29, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, M.; Rho, J.M.; Mattson, M.P. The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res. Rev. 2009, 59, 293–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, Z.; D’Agostino, D.P.; Diamond, D.; Kindy, M.S.; Rogers, C.; Ari, C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front. Psychiatry 2019, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone odies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- Croteau, E.; Castellano, C.A.; Fortier, M.; Bocti, C.; Fulop, T.; Paquet, N.; Cunnane, S.C. A cross-sectional comparison of brain glucose and ketone metabolism in cognitively healthy older adults, mild cognitive impairment and early Alzheimer’s disease. Exp. Gerontol. 2018, 107, 18–26. [Google Scholar] [CrossRef]
- Nugent, S.; Tremblay, S.; Chen, K.W.; Ayutyanont, N.; Roontiva, A.; Castellano, C.-A.; Fortier, M.; Roy, M.; Courchesne-Loyer, A.; Bocti, C.; et al. Brain glucose and acetoacetate metabolism: A comparison of young and older adults. Neurobiol. Aging 2014, 35, 1386–1395. [Google Scholar] [CrossRef]
- Xin, L.; Ipek, Ö.; Beaumont, M.; Shevlyakova, M.; Christinat, N.; Masoodi, M.; Greenberg, N.; Gruetter, R.; Cuenoud, B. Nutritional Ketosis Increases NAD+/NADH Ratio in Healthy Human Brain: An in Vivo Study by 31P-MRS. Front. Nutr. 2018, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Croteau, E.; Castellano, C.-A.; Richard, M.A.; Fortier, M.; Nugent, S.; Lepage, M.; Duchesne, S.; Whittingstall, K.; Turcotte, É.E.; Bocti, C.; et al. Ketogenic Medium Chain Triglycerides Increase Brain Energy Metabolism in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Ota, M.; Matsuo, J.; Ishida, I.; Hattori, K.; Teraishi, T.; Tonouchi, H.; Ashida, K.; Takahashi, T.; Kunugi, H. Effect of a ketogenic meal on cognitive function in elderly adults: Potential for cognitive enhancement. Psychopharmacology 2016, 233, 3797–3802. [Google Scholar] [CrossRef] [PubMed]
- Rebello, C.J.; Keller, J.N.; Liu, A.G.; Johnson, W.D.; Greenway, F.L. Pilot feasibility and safety study examining the effect of medium chain triglyceride supplementation in subjects with mild cognitive impairment: A randomized controlled trial. BBA Clin. 2015, 3, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Fortier, M.; Castellano, C.-A.; Croteau, E.; Langlois, F.; Bocti, C.; St-Pierre, V.; Vandenberghe, C.; Bernier, M.; Roy, M.; Descoteaux, M.; et al. A ketogenic drink improves brain energy and some measures of cognition in mild cognitive impairment. Alzheimers. Dement. 2019, 15, 625–634. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Page, K.A.; Williamson, A.; Yu, N.; McNay, E.C.; Dzuira, J.; McCrimmon, R.J.; Sherwin, R.S. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 2009, 58, 1237–1244. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.N.; Carrasco-Pozo, C.; McDonald, T.S.; Puchowicz, M.; Borges, K. Tridecanoin is anticonvulsant, antioxidant, and improves mitochondrial function. J. Cereb. Blood Flow Metab. 2017, 37, 2035–2048. [Google Scholar] [CrossRef]
- Wang, D.; Mitchell, E.S. Cognition and Synaptic-Plasticity Related Changes in Aged Rats Supplemented with 8- and 10-Carbon Medium Chain Triglycerides. PLoS ONE 2016, 11, e0160159. [Google Scholar] [CrossRef] [Green Version]
- Hollis, F.; Mitchell, E.S.; Canto, C.; Wang, D.; Sandi, C. Medium chain triglyceride diet reduces anxiety-like behaviors and enhances social competitiveness in rats. Neuropharmacology 2018, 138, 245–256. [Google Scholar] [CrossRef]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Wein, S.; Wolffram, S.; Schrezenmeir, J.; Gasperiková, D.; Klimes, I.; Seböková, E. Medium-chain fatty acids ameliorate insulin resistance caused by high-fat diets in rats. Diabetes. Metab. Res. Rev. 2009, 25, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M.; Leo, M.A.; Mak, K.M.; Ponomarenko, A.; Ren, C.; Wang, X. Beneficial effects versus toxicity of medium-chain triacylglycerols in rats with NASH. J. Hepatol. 2008, 48, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.; Bracco, E.F.; Hashim, S.A. Enhanced thermogenesis and diminished deposition of fat in response to overfeeding with diet containing medium chain triglyceride. Am. J. Clin. Nutr. 1982, 35, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Tholstrup, T.; Ehnholm, C.; Jauhiainen, M.; Petersen, M.; Høy, C.-E.; Lund, P.; Sandström, B. Effects of medium-chain fatty acids and oleic acid on blood lipids, lipoproteins, glucose, insulin, and lipid transfer protein activities. Am. J. Clin. Nutr. 2004, 79, 564–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakura, L.; Lottenberg, A.M.; Neves, M.Q.; Nunes, V.S.; Rocha, J.C.; Passarelli, M.; Nakandakare, E.R.; Quintão, E.C. Dietary medium-chain triacylglycerol prevents the postprandial rise of plasma triacylglycerols but induces hypercholesterolemia in primary hypertriglyceridemic subjects. Am. J. Clin. Nutr. 2000, 71, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Han, J.R.; Deng, B.; Sun, J.; Chen, C.G.; Corkey, B.E.; Kirkland, J.L.; Ma, J.; Guo, W. Effects of dietary medium-chain triglyceride on weight loss and insulin sensitivity in a group of moderately overweight free-living type 2 diabetic Chinese subjects. Metabolism 2007, 56, 985–991. [Google Scholar] [CrossRef]
- Schwartz, R.M.; Boyes, S.; Aynsley-Green, A. Metabolic effects of three ketogenic diets in the treatment of severe epilepsy. Dev. Med. Child Neurol. 1989, 31, 152–160. [Google Scholar] [CrossRef]
- Reger, M.A.; Henderson, S.T.; Hale, C.; Cholerton, B.; Baker, L.D.; Watson, G.S.; Hyde, K.; Chapman, D.; Craft, S. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol. Aging 2004, 25, 311–314. [Google Scholar] [CrossRef]
- Salameh, T.S.; Rhea, E.M.; Banks, W.A.; Hanson, A.J. Insulin resistance, dyslipidemia, and apolipoprotein E interactions as mechanisms in cognitive impairment and Alzheimer’s disease. Exp. Biol. Med. (Maywood) 2016, 241, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Henderson, S.T.; Vogel, J.L.; Barr, L.J.; Garvin, F.; Jones, J.J.; Costantini, L.C. Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: A randomized, double-blind, placebo-controlled, multicenter trial. Nutr. Metab. 2009, 6, 31. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Ogura, Y.; Furuta, M.; Kakehashi, C.; Funabashi, T.; Akema, T. Ketogenic diet does not impair spatial ability controlled by the hippocampus in male rats. Brain Res. 2015, 1622, 36–42. [Google Scholar] [CrossRef]
- Rahim, N.S.; Lim, S.M.; Mani, V.; Abdul Majeed, A.B.; Ramasamy, K. Enhanced memory in Wistar rats by virgin coconut oil is associated with increased antioxidative, cholinergic activities and reduced oxidative stress. Pharm. Biol. 2017, 55, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Brownlow, M.L.; Benner, L.; D’Agostino, D.; Gordon, M.N.; Morgan, D. Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology. PLoS ONE 2013, 8, e75713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães, J.; Bargut, T.C.L.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Medium-chain triglyceride reinforce the hepatic damage caused by fructose intake in mice. Prostaglandins. Leukot. Essent. Fatty Acids 2019, 140, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kesl, S.L.; Poff, A.M.; Ward, N.P.; Fiorelli, T.N.; Ari, C.; Van Putten, A.J.; Sherwood, J.W.; Arnold, P.; D’Agostino, D.P. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr. Metab. 2016, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Bach, A.; Weryha, A.; Schirardin, H. Influence of oral MCT or LCT load on glycemia in Wistar and Zucker rats and in guinea pigs. Ann. Biol. Anim. Biochim. Biophys. 1979, 19, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.M.; Williamson, D.H. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Physiol. Rev. 1980, 60, 143–187. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chen, S.; Wang, S.; Tu, Z.; Zhang, G.; Zhu, H.; Li, X.; Xiong, J.; Liu, Y. Medium-Chain Triglycerides Attenuate Liver Injury in Lipopolysaccharide-Challenged Pigs by Inhibiting Necroptotic and Inflammatory Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 3697. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Li, X.; Gao, Q.; Fan, L.; Jin, H.; Guo, Y. Differential effects of olive oil, soybean oil, corn oil and lard oil on carbon tetrachloride-induced liver fibrosis in mice. Biosci. Rep. 2019, 39, BSR20191913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, C.; Myer, S.; Munson, S.; Turet, P.; Birdsall, T.C. A cross-over study of the effect of a single oral feeding of medium chain triglyceride oil vs. canola oil on post-ingestion plasma triglyceride levels in healthy men. Altern. Med. Rev. 1999, 4, 23–28. [Google Scholar]
- Lee, A.; Yoo, H.J.; Kim, M.; Kim, M.; Choi, J.-H.; Lee, C.; Lee, J.H. Effects of equivalent medium-chain diacylglycerol or long-chain triacylglycerol oil intake via muffins on postprandial triglycerides and plasma fatty acids levels. J. Funct. Foods 2019, 53, 299–305. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.H.; Bloomer, R.J. Exacerbated postprandial oxidative stress induced by the acute intake of a lipid meal compared to isoenergetically administered carbohydrate, protein, and mixed meals in young, healthy men. J. Am. Coll. Nutr. 2010, 29, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Baseer, A. Increased malondialdehyde levels in coronary heart disease. J. Pak. Med. Assoc. 2000, 50, 261–264. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shcherbakova, K.; Schwarz, A.; Ivleva, I.; Nikitina, V.; Krytskaya, D.; Apryatin, S.; Karpenko, M.; Trofimov, A. Acute and Chronic Effects of Medium-Chain Triglyceride Supplementation on Metabolic Parameters and Working Memory in Rats. Biol. Life Sci. Forum 2021, 7, 22. https://doi.org/10.3390/ECB2021-10282
Shcherbakova K, Schwarz A, Ivleva I, Nikitina V, Krytskaya D, Apryatin S, Karpenko M, Trofimov A. Acute and Chronic Effects of Medium-Chain Triglyceride Supplementation on Metabolic Parameters and Working Memory in Rats. Biology and Life Sciences Forum. 2021; 7(1):22. https://doi.org/10.3390/ECB2021-10282
Chicago/Turabian StyleShcherbakova, Ksenia, Alexander Schwarz, Irina Ivleva, Veronika Nikitina, Darya Krytskaya, Sergey Apryatin, Marina Karpenko, and Alexander Trofimov. 2021. "Acute and Chronic Effects of Medium-Chain Triglyceride Supplementation on Metabolic Parameters and Working Memory in Rats" Biology and Life Sciences Forum 7, no. 1: 22. https://doi.org/10.3390/ECB2021-10282
APA StyleShcherbakova, K., Schwarz, A., Ivleva, I., Nikitina, V., Krytskaya, D., Apryatin, S., Karpenko, M., & Trofimov, A. (2021). Acute and Chronic Effects of Medium-Chain Triglyceride Supplementation on Metabolic Parameters and Working Memory in Rats. Biology and Life Sciences Forum, 7(1), 22. https://doi.org/10.3390/ECB2021-10282







