Abstract
An increasing focus in cancer therapy is on the investigation of fungal products with anticancer activity and potential to affect specific targets. Main goal of this study is the analysis of the effects of Phellinus linteus and Lentinus edodes, edible and medicinal mushrooms, on the significant migratory/invasive markers in the first steps of cancer metastasis. Both treatments increased antimigratory marker E-cadherin and decreased promigratory/proinvasive proteins N-cadherin and Vimentin. A lowered concentration of proinvasive protein MMP-9 was also observed in treated HCT-116 and SW-480 cells. PL and LE exerted cell selectivity, whereas PL had better activity on SW-480, while LE had a more prominent effect on HCT-116 cells.
1. Introduction
Cancer presents a prominent health problem worldwide and its treatment is challenging. In previous decades, studies focused on the investigation of fungi and their constitutive compounds with anticancer properties became interesting for research [1]. Alternative approaches to cancer treatment based on the use of fungi are considered attractive worldwide, because of their abundance in bioactive compounds and confirmed therapeutic effects [1,2]. Moreover, cancer fungotherapy, as a promising scientific field, focuses on the investigation of fungal products with prominent anticancer potential and target-specific activity [2]. Phellinus linteus (Berk. et Curt.) Teng, known as meshima, and Lentinus edodes (Berk.) Pegler, also known as shiitake mushroom, are highly valued species with prominent beneficial effects for health and in the treatment of various ailments. Among their confirmed medicinal activities, their antitumor, immunomodulating, antiviral and antioxidant effects are reported [3,4]. Their significant potential is due to the accumulation of a variety of bioactive primary and secondary metabolites in their fruiting bodies. Among these are mineral compounds, vitamins, oils, lipids, organic acids, polysaccharides, proteins, phenols (flavonoids and phenolic acids). It is already known that mushrooms can prevent the genesis of cancer, exert direct antitumor activity and inhibit metastasis [1].
In addition to their cytotoxicity, the effects of mushrooms regarding the regulation of crucial steps in cancer metastasis have been poorly investigated. Our study aimed to analyze the effects of two edible and medicinal mushroom species on markers of migration and invasion as key steps of cancer metastasis.
2. Methods
Methanol extracts of commercially cultivated edible and medicinal mushroom species Phellinus linteus (PL) and Lentinus edodes (LE) were examined in two selected concentrations (10 and 50 µg/mL) for their antimigratory and antiinvasive potential. Two colorectal carcinoma cell lines (HCT-116, SW-480) were used, and treatment effects were evaluated after 24 h. The expression and localization of antimigratory protein E-cadherin, and promigratory markers N-cadherin and Vimentin were performed using the immunofluorescent method [2], and concentration of matrixmetalloproteinase 9 (MMP-9) was determined using a colorimetric ELISA assay [5].
3. Results and Discussion
According to the results, PL and LE induced obvious cell-selective effects, whereas PL exerted a more prominent effect on SW-480 cells, whilst LE had a stronger effect on HCT-116 cells.
PL increased the expression of antimigratory marker E-cadherin and significantly decreased the level of promigratory/proinvasive proteins N-cadherin and Vimentin in SW-480 cells. Meanwhile, LE induced a similar response in HCT-116 cells, increasing E-cadherin and lowering N-cadherin and Vimentin (Figure 1).
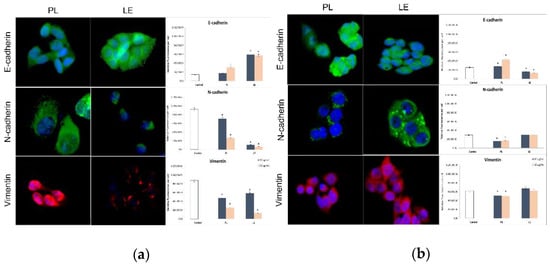
Figure 1.
Effects of PL and LE treatments on HCT-116 (a) and SW-480 cells (b). Figure contains representative micrographs and calculation of relative fluorescence per cell, whereat * p > 0.05 is considered as statistical significance.
Regarding proinvasive protein MMP-9, the applied treatments significantly decreased its concentration in both tested cell lines. Once again, cell selectivity was observed regarding the effects of these treatments. Namely, the best effect on SW-480 cell line exerted PL, which significantly lowered the level of MMP-9, and LE, was more potent in reducing the level of this proinvasive protein in HCT-116 cells (Figure 2).
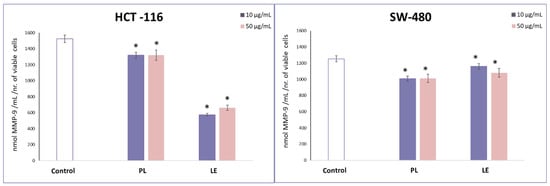
Figure 2.
Effects of PL and LE treatments on MMP-9 concentration in HCT-116 and SW-480 cells. * p > 0.05 is considered as statistical significance.
4. Discussion
Although mushrooms are known for their medicinal properties [1], the molecular mechanisms underlying their biological effects remain to be elucidated. Mushrooms are a rich source of various active substances in high amounts, including polyphenols [2]. Our earlier study confirmed the antimigratory effects of methanolic extracts of two commercially available mushrooms on HCT-116 and SW-480 colorectal cancer cell lines [1]. We also analyzed the chemical profile of our PL methanol extract and showed that it is abundant in flavonoids [1]. The literature data reported that active substances such as flavonoids are able to reduce the expression of Wnt signal pathway regulatory proteins, primarily β-catenin [6].
According to our previous results [1], the treatments with mushroom extracts that exerted the highest antimigratory activity were able to reduce β-catenin, or relocate it to the intercellular connections in the tested colorectal carcinoma cell lines (LE in HCT-116 and PL in SW-480 cells). The lentinan polysaccharides detected in LE are responsible for the increased localization of β-catenin to the cell membrane in colon cancer cells [1]. It is known that the expression of N-cadherin and Vimentin, as promigratory markers, is regulated by the Wnt/β-catenin signaling pathway; thus, the reduction in β-catenin expression in these lines and its relocation to intercellular connections results in a reduction in the expression of the examined promigratory markers [7].
The differences between these two cell lines, considering that HCT-116 cells have wild-type APC gene and mutant β-catenin, while SW-480 cells bear mutant APC and wildtype β-catenin, should be noted [8]. However, different cell mechanisms decreased the level of β-catenin in the tested cells, yet an increased level of β-catenin in intercellular connections was observed in both tested cell lines, which resulted in suppressed migratory/invasive activity [9]. LE extract obviously increased cytoplasmic β-catenin in HCT-116 cells, which most probably led to increased E-cadherin expression. This correlates with the reduction of cell motility/invasion, also presented through a reduction in the concentration of MMP-9 [9].
Considering the increase in cytoplasmic β-catenin, which was mostly located in intercellular connections, a reduction in nuclear β-catenin pool is logical, and, via this protein, the suppression of Wnt/β-catenin signaling occurred. Furthermore, extracts affected the expression of promigratory proteins N-cadherin and Vimetin, most probably because of this reduction in nuclear β-catenin, which, when located in the nucleus, acts as a transcriptional factor whose target genes are N-cadherin and Vimentin [10]. Therefore, it is clear why the expression of these proteins was detected at a lower level after treatment with LE in HCT-116 cells and PL in the SW-480 cell line.
5. Conclusions
The investigated PL and LE extracts show significant effects on the suppression of promigratory markers N-cadherin and Vimentin as a consequence of the increased level of E-cadherin protein and reduction in nuclear β-catenin in HCT-116 and SW-480 cells. PL and LE extracts induced the suppression of cell invasion by reducing the level of MMP-9. Further studies should be conducted regarding these mushrooms, which possess obvious and important antimigratory/antiinvasive potential, especially their application as food supplements.
Author Contributions
Conceptualization, D.S.Š. and S.D.M.; methodology, D.S.Š.; software, D.S.Š.; validation, S.D.M.; formal analysis, M.Ž.; investigation, M.M.J.; resources, S.D.M.; data curation, D.S.Š.; writing—original draft preparation, M.M.J. and D.S.Š.; writing—review and editing, D.S.Š. and M.M.J.; visualization, K.V. and J.G.; supervision, S.D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Education, Science and Technological Development of the Republic of Serbia (grant number 451-03-9/2021-14/200378 and 451-03-9/2021-14/200122).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Šeklić, D.S.; Stanković, M.; Milutinović, M.; Topuzović, M.D.; Štajn, A.; Marković, S.D. Cytotoxic, antimigratory, pro- and antioxidative activities of extracts from medicinal mushrooms on colon cancer cell lines. Arch. Biol. Sci. 2016, 68, 93–105. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A Review: The Bioactivities and Pharmacological Applications of Phellinus linteus. Molecules 2019, 24, 1888. [Google Scholar] [CrossRef] [Green Version]
- Bisen, P.; Baghel, R.; Sanodiya, B.; Thakur, G.; Prasad, G. Lentinus edodes: A Macrofungus with Pharmacological Activities. Curr. Med. Chem. 2010, 17, 2419–2430. [Google Scholar] [CrossRef]
- Koepke, J.; Dresel, M.; Schmid, S.; Greulich, T.; Beutel, B.; Schmeck, B.; Vogelmeier, C.F.; Janciauskiene, S.; Koczulla, A.R. Therapy with Plasma Purified Alpha1-Antitrypsin (Prolastin®) Induces Time-Dependent Changes in Plasma Levels of MMP-9 and MPO. PLoS ONE 2015, 10, e0117497. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Li, G.; Kim, J.S.; Jing, K.; Kim, T.D.; Kim, J.P.; Seo, S.B.; Yoo, J.K.; Park, H.D.; Hwang, B.D.; et al. Protein-bound polysaccharide from Phellinus linteus inhibits tumor growth, invasion, and angiogenesis and alters Wnt/β-catenin in SW480 human colon cancer cells. BMC Cancer 2011, 11, 307–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shi, J.; Chai, K.; Ying, X.; Zhou, B.P. The Role of Snail in EMT and Tumorigenesis. Curr. Cancer Drug Targets 2013, 13, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, M.; Tomlinson, I.P.M.; Rowan, A.; Pignatelli, M.; Bodmer, W. β-Catenin mutations in cell lines established from human colorectal cancers. Proc. Natl. Acad. Sci. USA 1997, 94, 10330–10334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albring, K.F.; Weidemüller, J.; Mittag, S.; Weiske, J.; Friedrich, K.; Geroni, M.C.; Lombardi, P.; Huber, O. Berberine acts as a natural inhibitor of Wnt/β-catenin signaling-Identification of more active 13-arylalkyl derivatives. BioFactors 2013, 39, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kwon, Y.; Jang, M.; Park, M.; Kim, J.; Cho, S.; Jang, D.G.; Lee, W.-B.; Jung, S.H.; Choi, H.J.; et al. β-catenin activation down-regulates cell-cell junction-related genes and induces epithelial-to-mesenchymal transition in colorectal cancers. Sci. Rep. 2019, 9, 18440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).