Tyrosinase Inhibition Ability Provided by Hop Tannins: A Mechanistic Investigation †
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Hop Tannins Extract
2.2. GPC
2.3. 13C-NMR Analysis
2.4. Acid-Cleavage Coupled with HPLC-ESI-MS/MS Analysis
2.5. Tyrosinase Inhibition Kinetic Assay
2.6. CD
2.7. MD
2.8. Antioxidant Ability
2.9. ICP-OES
2.10. Cell Culture and Cytotoxicity Assay
2.11. The Effect of Hop Tannins on Intracellular Melanin Production and Tyrosinase Activity of B16F10 Melanoma Cells
2.12. Statistical Analysis
3. Results and Discussion
3.1. Structural Analysis
3.2. Inhibitory Effect, Mechanism, and Type of Hop Tannins on the Tyrosinase
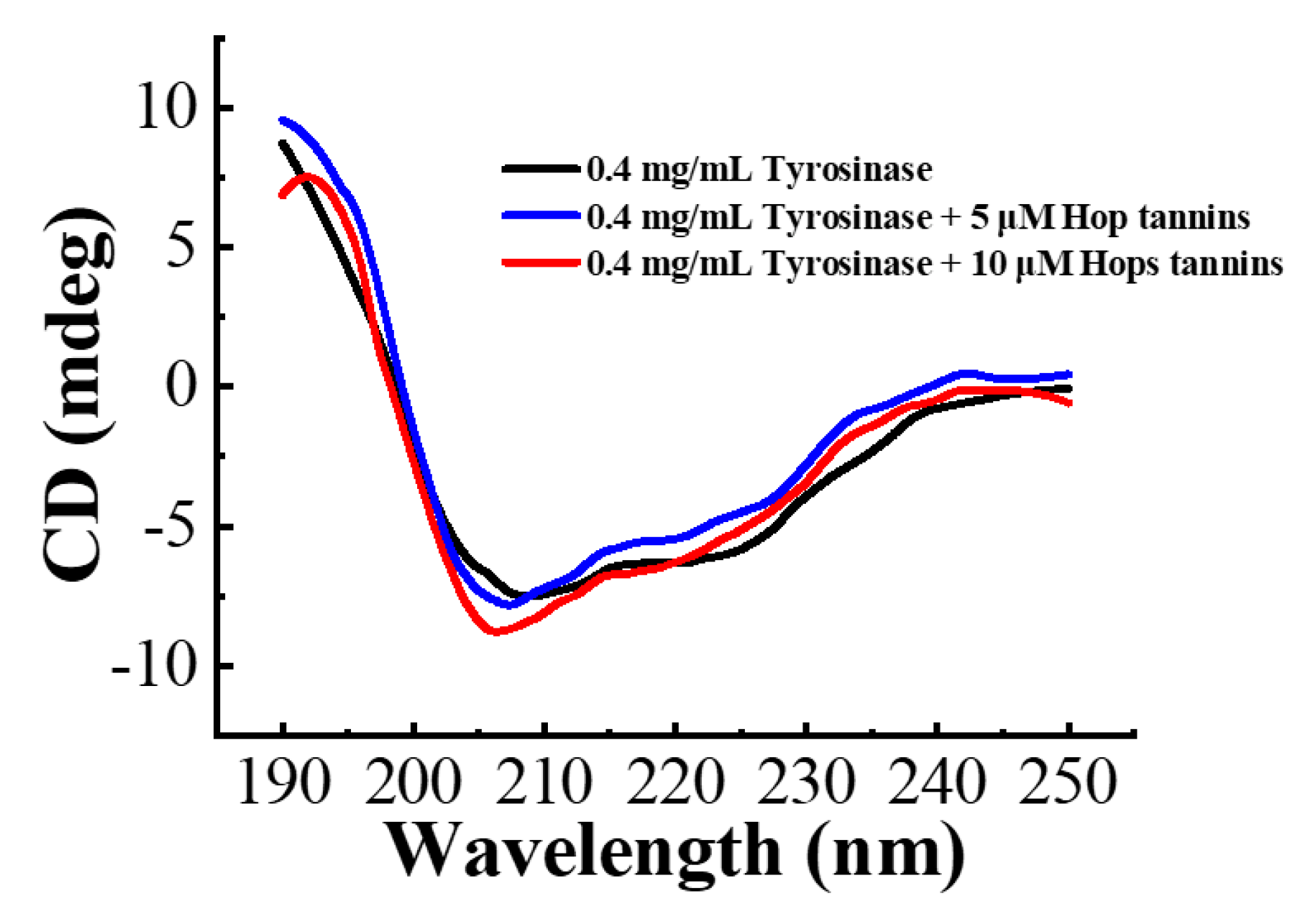
3.3. CD
3.4. Copper Ion Chelating and Antioxidant Abilities
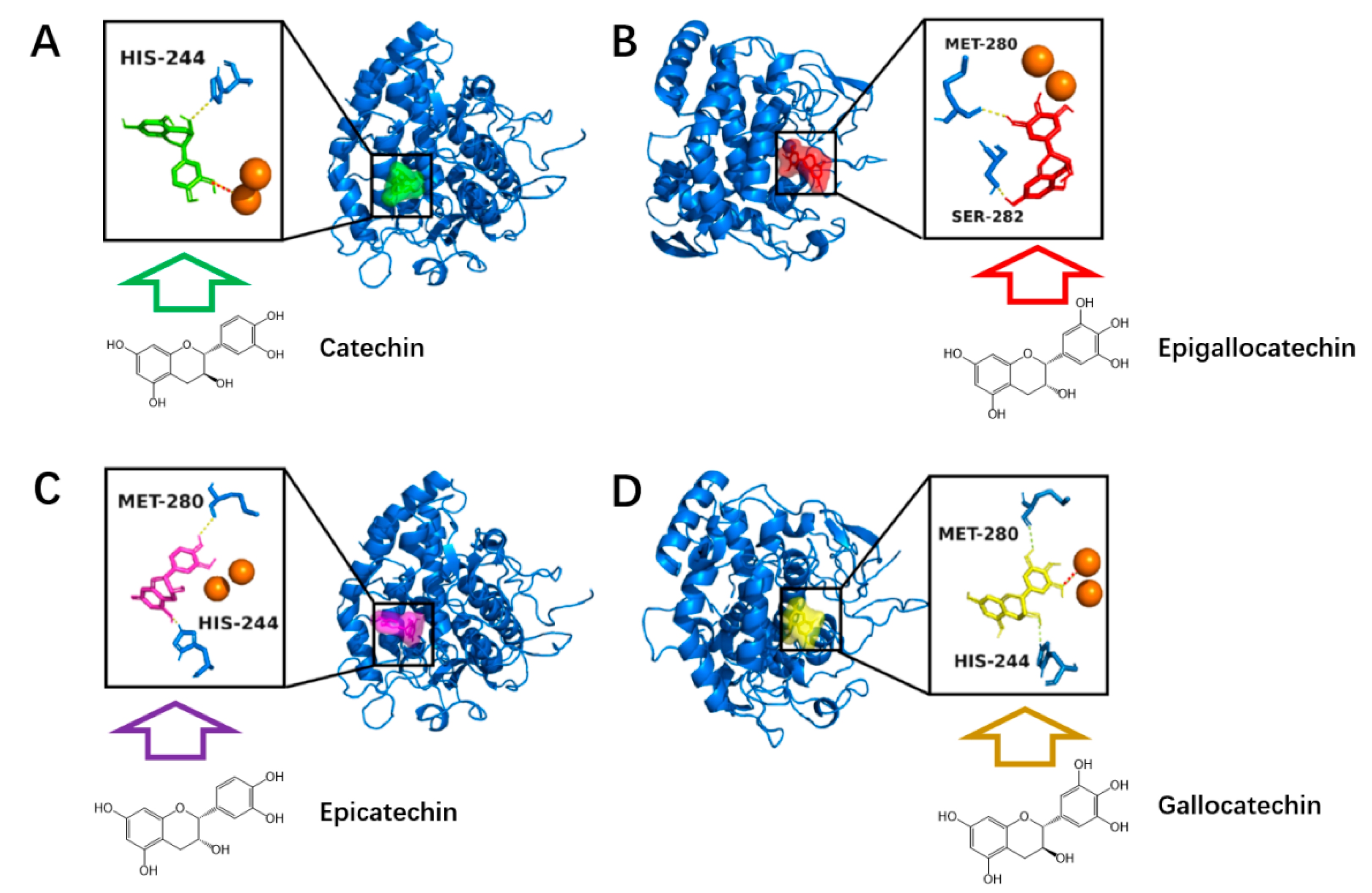
3.5. Molecular Docking Analysis
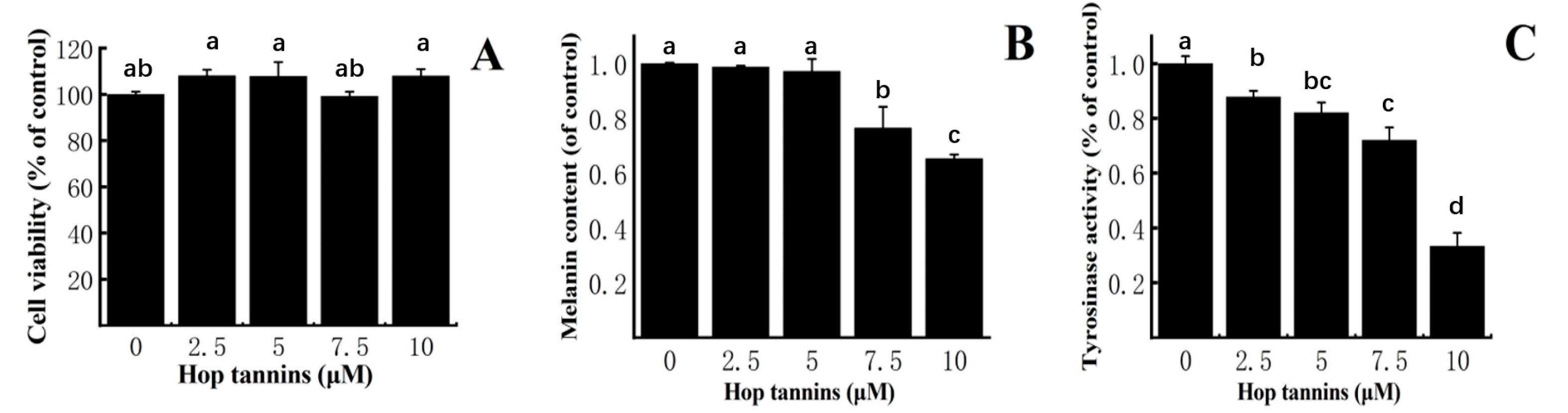
3.6. Effect of Hop Tannin on Cell Viability, Melanin Synthesis, and Tyrosinase Activity in B16F10 Mouse Melanoma Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chai, W.-M.; Wei, Q.-M.; Deng, W.-L.; Zheng, Y.-L.; Chen, X.-Y.; Huang, Q.; Chong, O.-Y.; Peng, Y.-Y. Anti-melanogenesis properties of condensed tannins from Vigna angularis seeds with potent antioxidant and DNA damage protection activities. Food Funct. 2019, 10, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Gheida, S.F.; El Maghraby, G.M.; Farag, Z.E. Evaluation of the efficacy and safety of combinations of hydroquinone, glycolic acid, and hyaluronic acid in the treatment of melasma. J. Cosmet. Dermatol. 2015, 14, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Teng, B.; Hayasaka, Y.; Smith, P.A.; Bindon, K.A. Effect of Grape Seed and Skin Tannin Molecular Mass and Composition on the Rate of Reaction with Anthocyanin and Subsequent Formation of Polymeric Pigments in the Presence of Acetaldehyde. J. Agric. Food Chem. 2019, 67, 8938–8949. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, C.G. Enzyme kinetics: Partial and complete uncompetitive inhibition. Biochem. Educ. 2000, 28, 144–147. [Google Scholar] [CrossRef]
- Li, X.; Jiang, H.; Pu, Y.; Cao, J.; Jiang, W. Inhibitory Effect of Condensed Tannins from Banana Pulp on Cholesterol Esterase and Mechanisms of Interaction. J. Agric. Food Chem. 2019, 67, 14066–14073. [Google Scholar] [CrossRef] [PubMed]
- Heitz, M.P.; Rupp, J.W. Determining mushroom tyrosinase inhibition by imidazolium ionic liquids: A spectroscopic and molecular docking study. Int. J. Biol. Macromol. 2018, 107, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zeng, X.; Du, Z.; Ding, X.; Jiang, W. Characterization of the direct interaction between apple condensed tannins and cholesterol in vitro. Food Chem. 2020, 309, 125762. [Google Scholar] [CrossRef] [PubMed]
- Si, C.-L.; Wu, L.; Shen, T.; Huang, X.-F.; Du, Z.-G.; Ren, X.-D.; Luo, X.-G.; Hu, W.-C. Recovery of Low-molecular Weight Galloyltannins from Agricultural Residue of Juglans sigillata Dode Seed Husks and their Tyrosinase Inhibitory Effect. Bioresources 2014, 9, 2226–2236. [Google Scholar] [CrossRef][Green Version]
- Haslam, E. Natural Polyphenols (Vegetable Tannins) as Drugs: Possible Modes of Action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, L.-L.; Ren, Y.-J.; Wei, S.-D.; Yang, H.-B. Inhibitory effects and molecular mechanism on mushroom tyrosinase by condensed tannins isolation from the fruit of Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chow. Int. J. Biol. Macromol. 2020, 165, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Ros, J.R.; Rodríguez-López, J.N.; García-Cánovas, F. Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem. J. 1993, 295 Pt 1, 309–312. [Google Scholar] [CrossRef] [PubMed]
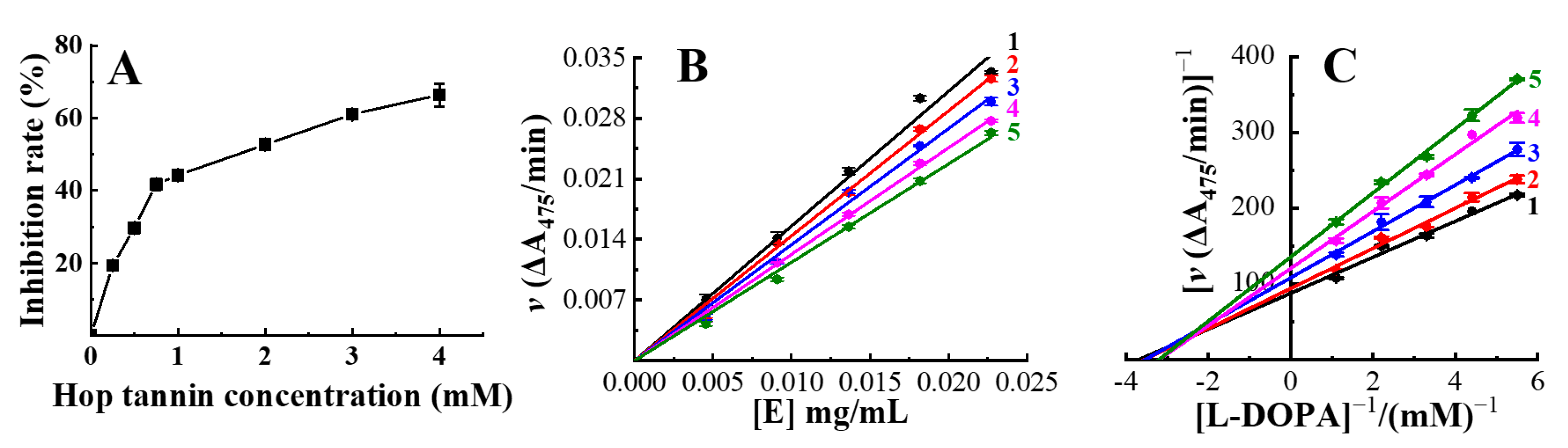
| IC50 (mg/mL) | Inhibition Mechanism | Inhibition Type | Inhibition Constants (mM) | ||
|---|---|---|---|---|---|
| Hop tannin | 76.52 ± 6.56 µM a | reversible | mixed | KIS = 2.61 ± 0.16 | KI = 1.79 ± 0.12 |
| Kojic acid | 49.54 ± 2.08 µM b | -- | -- | -- | -- |
| Cu2+ Chelating (%) | DPPH Free Radical Scavenging Activity (IC50 μM) | ABTS Free Radical Scavenging Activity (IC50 μM) | |
|---|---|---|---|
| Hop tannins | 44.77 ± 0.45 | 1.17 ± 0.08 b | 1.52 ± 0.02 b |
| Ascorbic acid | - | 22.04 ± 0.28 a | 17.83 ± 0.30 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Chen, Y.; Zhang, X.; Zheng, J.; Wang, J.; Hu, W.; Teng, B. Tyrosinase Inhibition Ability Provided by Hop Tannins: A Mechanistic Investigation. Biol. Life Sci. Forum 2021, 6, 66. https://doi.org/10.3390/Foods2021-11096
Liu J, Chen Y, Zhang X, Zheng J, Wang J, Hu W, Teng B. Tyrosinase Inhibition Ability Provided by Hop Tannins: A Mechanistic Investigation. Biology and Life Sciences Forum. 2021; 6(1):66. https://doi.org/10.3390/Foods2021-11096
Chicago/Turabian StyleLiu, Jiaman, Yanbiao Chen, Xinxin Zhang, Jie Zheng, Jiaying Wang, Weiying Hu, and Bo Teng. 2021. "Tyrosinase Inhibition Ability Provided by Hop Tannins: A Mechanistic Investigation" Biology and Life Sciences Forum 6, no. 1: 66. https://doi.org/10.3390/Foods2021-11096
APA StyleLiu, J., Chen, Y., Zhang, X., Zheng, J., Wang, J., Hu, W., & Teng, B. (2021). Tyrosinase Inhibition Ability Provided by Hop Tannins: A Mechanistic Investigation. Biology and Life Sciences Forum, 6(1), 66. https://doi.org/10.3390/Foods2021-11096






