Optical Methods to Determine the Gas Atmosphere in Various Modified Atmosphere Packages: Applications and Correlation in Meat Spoilage †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optical Measurement Systems
2.1.1. Fluorescence Quenching to Detect O2
2.1.2. MIR Spectroscopy to Detect CO2
2.2. Sample Preparation
2.3. Non-Destructive Gas Determination
2.4. Microbiological Analysis
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results
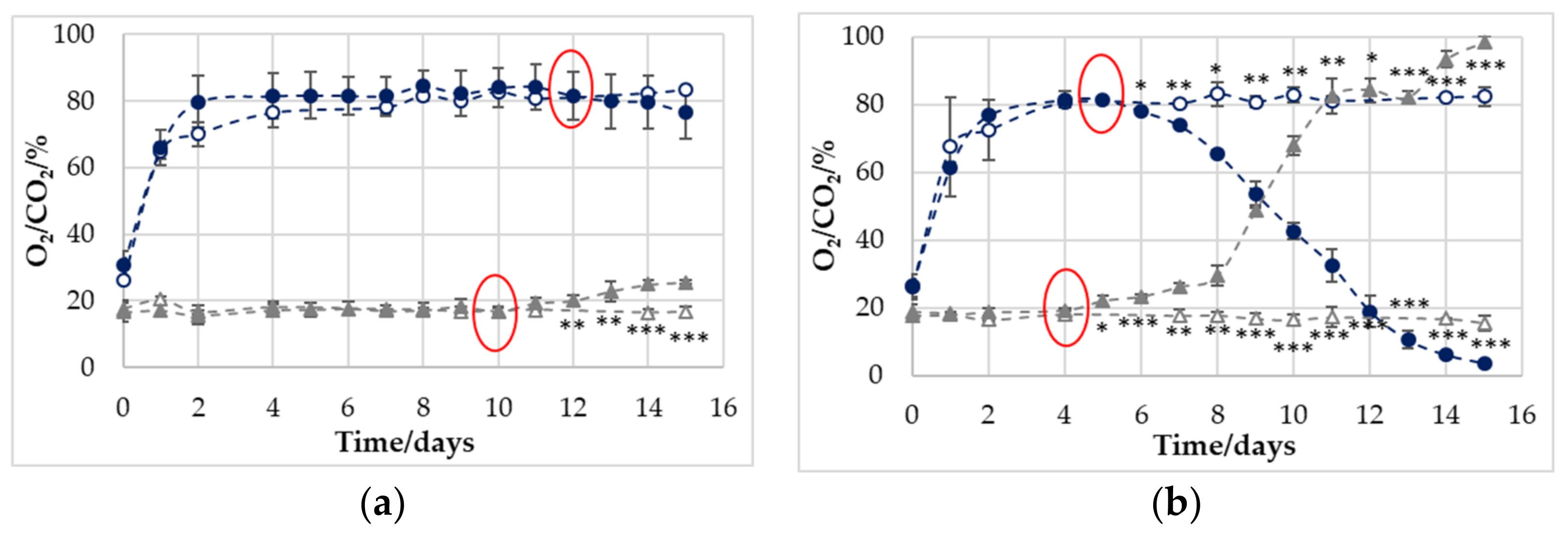
3.1. Development of Gas Concentration in Empty and Filled Trays
3.2. Microbiological Analysis
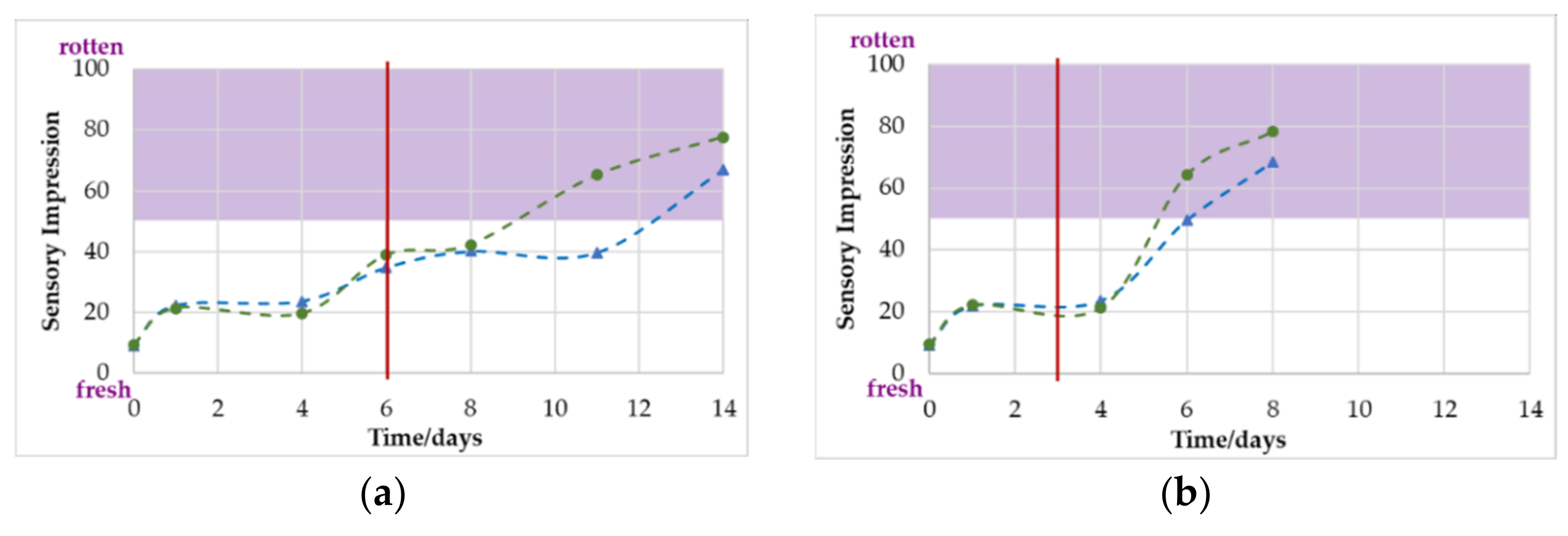
3.3. Sensory Evaluation
4. Discussion
4.1. Correlation of Results
4.1.1. Correlations between O2 and CO2 Concentrations and Microbial Spoilage
4.1.2. Correlation of O2/CO2 with Sensory Evaluation
4.2. Influence of the Microbiome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament. Food Waste: The Problem in the EU in Numbers. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20170505STO73528/food-waste-the-problem-in-the-eu-in-numbers-infographic (accessed on 2 August 2021).
- Schmutzler, M.; Beganovic, A.; Böhler, G.; Huck, C.W. Methods for detection of pork adulteration in veal product based on FT-NIR spectroscopy for laboratory, industrial and on-site analysis. Food Control 2015, 57, 258–267. [Google Scholar] [CrossRef]
- Banerjee, S.; Kelly, C.; Kerry, J.P.; Papkovsky, D.B. High throughput non-destructive assessment of quality and safety of packaged food products using phosphorescent oxygen sensors. Trends Food Sci. Technol. 2016, 50, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Kolbeck, S.; Reetz, L.; Hilgarth, M.; Vogel, R.F. Quantitative Oxygen Consumption and Respiratory Activity of Meat Spoiling Bacteria Upon High Oxygen Modified Atmosphere. Front. Microbiol. 2019, 10, 2398. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, S.; Hilgarth, M.; Vogel, R.F. Proof of concept: Predicting the onset of meat spoilage by an integrated oxygen sensor spot in MAP packages. Lett. Appl. Microbiol. 2021, 73, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Höll, L.; Behr, J.; Vogel, R.F. Identification and growth dynamics of meat spoilage microorganisms in modified atmosphere packaged poultry meat by MALDI-TOF MS. Food Microbiol. 2016, 60, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, S.; Klausmann, S.; Kreyenschmidt, J. Effect of high-oxygen and oxygen-free modified atmosphere packaging on the spoilage process of poultry breast fillets. Poult. Sci. 2015, 94, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, J.; Becker, B.; Stephan, R. (Eds.) Mikrobiologische Untersuchung von Lebensmitteln: Ein Leitfaden Für das Studium, 6th ed.; B.Behr’s Verlag GmbH & Co., KG: Hamburg, Germany, 2016; ISBN 978-3-95468-333-8. [Google Scholar]
- Franke, C.; Höll, L.; Langowski, H.-C.; Petermeier, H.; Vogel, R.F. Sensory evaluation of chicken breast packed in two different modified atmospheres. Food Packag. Shelf Life 2017, 13, 66–75. [Google Scholar] [CrossRef]
| Day 0 | Day 15 | Shelf Life Expired | |
|---|---|---|---|
| 80/20 4 °C | 1.36 × 104 CFUg−1 | 4.00 × 109 CFUg−1 | Day 6 |
| 70/30 4 °C | 1.27 × 104 CFUg−1 | 4.29 × 109 CFUg−1 | Day 7 |
| 80/20 10 °C | 1.36 × 104 CFUg−1 | 2.19 × 1010 CFUg−1 | Day 3 |
| 70/30 10 °C | 1.27 × 104 CFUg−1 | 4.47 × 109 CFUg−1 | Day 4 |
| 4 °C | 10 °C | |||
|---|---|---|---|---|
| 80/20 | 70/30 | 80/20 | 70/30 | |
| Cross-over O2 | 12 | 13 | 5 | 5 |
| p ≤ 0.05 O2 | - | - | 6 | 7 |
| Cross-over CO2 | 10 | - | 4 | 4 |
| p ≤ 0.05 CO2 | 12 | - | 5 | 6 |
| Microbiologically spoiled | 6 | 7 | 3 | 4 |
| Olfactory spoiled | 11 | 14 | 6 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dold, J.; Hollmann, C.; Kehr, C.; Langowski, H.-C. Optical Methods to Determine the Gas Atmosphere in Various Modified Atmosphere Packages: Applications and Correlation in Meat Spoilage. Biol. Life Sci. Forum 2021, 6, 36. https://doi.org/10.3390/Foods2021-11098
Dold J, Hollmann C, Kehr C, Langowski H-C. Optical Methods to Determine the Gas Atmosphere in Various Modified Atmosphere Packages: Applications and Correlation in Meat Spoilage. Biology and Life Sciences Forum. 2021; 6(1):36. https://doi.org/10.3390/Foods2021-11098
Chicago/Turabian StyleDold, Jasmin, Clarissa Hollmann, Caroline Kehr, and Horst-Christian Langowski. 2021. "Optical Methods to Determine the Gas Atmosphere in Various Modified Atmosphere Packages: Applications and Correlation in Meat Spoilage" Biology and Life Sciences Forum 6, no. 1: 36. https://doi.org/10.3390/Foods2021-11098
APA StyleDold, J., Hollmann, C., Kehr, C., & Langowski, H.-C. (2021). Optical Methods to Determine the Gas Atmosphere in Various Modified Atmosphere Packages: Applications and Correlation in Meat Spoilage. Biology and Life Sciences Forum, 6(1), 36. https://doi.org/10.3390/Foods2021-11098







