Natural Deep Eutectic Solvents as the Main Solvents for the Extraction of Total Polyphenols from Orange Peel †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Chemical and Reagents
2.3. Preparation of Natural Deep Eutectic Solvents
2.4. Extraction Procedure
2.5. Determination of the Total Polyphenol Content by UV–Vis Spectroscopy
2.6. Statistical Analyses
3. Results and Discussion
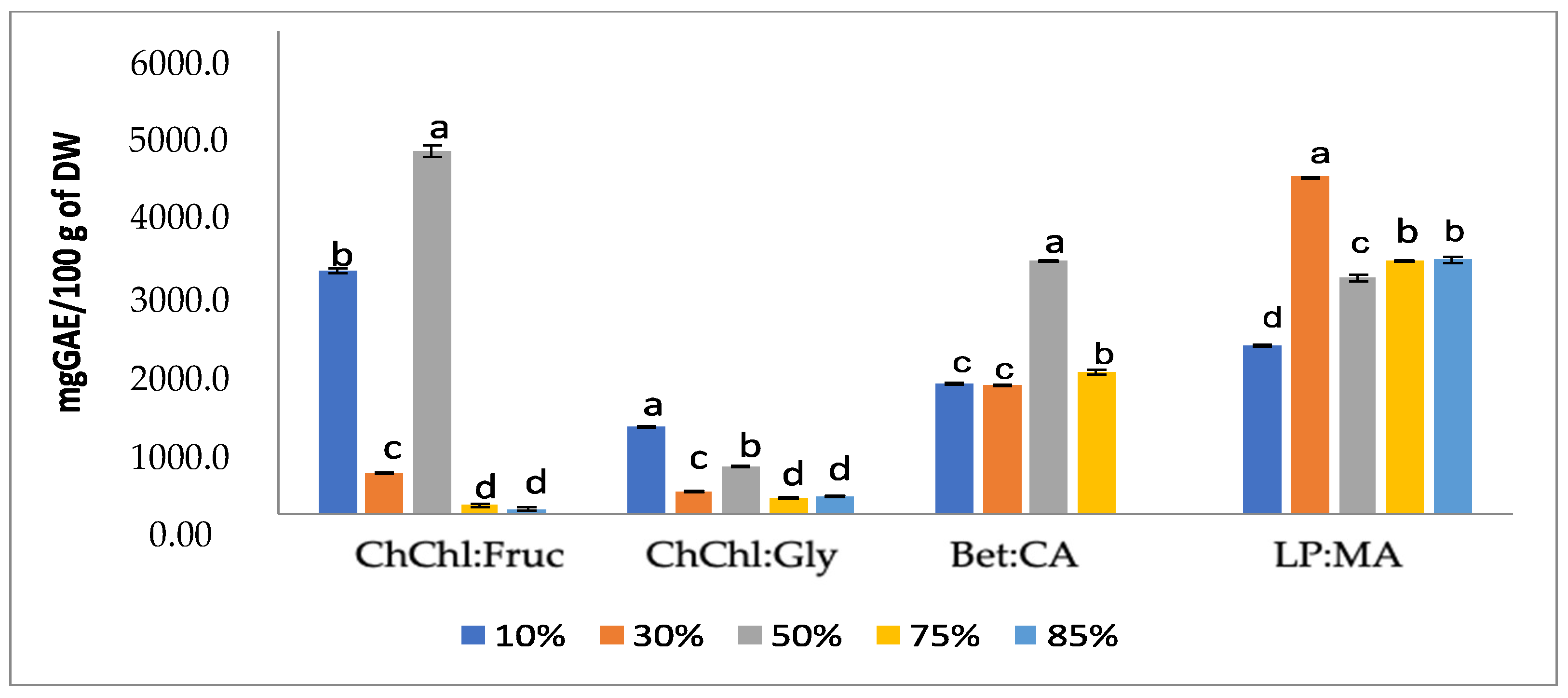
3.1. NADESs Mixed with Water
3.2. Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martínez-Navarrete, N.; del Mar Camacho Vidal, M.; José Martínez Lahuerta, J. Los compuestos bioactivos de las frutas y sus efectos en la salud. Act. Dietética 2008, 12, 64–68. [Google Scholar]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural deep eutectic solvent as extraction media for the main phenolic compounds from olive oil processing wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Teixeira, F.; dos Santos, B.A.; Nunes, G.; Soares, J.M.; do Amaral, L.A.; de Souza, G.H.O.; de Resende, J.T.V.; Menegassi, B.; Rafacho, B.P.M.; Schwarz, K.; et al. Addition of orange peel in orange jam: Evaluation of sensory, physicochemical, and nutritional characteristics. Molecules 2020, 25, 1670. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Ross, T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Microbiol. 1996, 81, 501–508. [Google Scholar] [CrossRef]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valor. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Benvenutti, L.; del Pilar Sanchez-Camargo, A.; Zielinski, A.A.F.; Ferreira, S.R.S. NADES as potential solvents for anthocyanin and pectin extraction from Myrciaria cauliflora fruit by-product: In silico and experimental approaches for solvent selection. J. Mol. Liq. 2020, 315, 113761. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]

| Acronym | HBA | HBD | Molar Ratio |
|---|---|---|---|
| ChChl:Fruc | Choline Chloride | Fructose | 1.9:1 |
| ChChl:Gly | Choline Chloride | Glycerol | 1:2 |
| Bet:CA | Betaine | Citric Acid | 1:1 |
| LP:MA | L-Proline | Malic Acid | 1:1 |
| Independent Variable | Level | |||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| Solid/liquid ratio | X1 | 5 | 15 | 25 |
| NADES (%, v/v) | X2 | 10 | 50 | 85 |
| Extraction time (min) | X3 | 5 | 15 | 30 |
| Runs | Extraction | Total Polyphenol Content (mg GAE/100 g DW) | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | LP:MA | ChChl:Fruc | ChChl:Gly | Bet:CA | |
| 1 | 15 | 50 | 10 | 2804.7 ± 146.6 | 1045.4 ± 73.6 | 296.3 ± 22.6 | 1767.4 ± 221.5 |
| 2 | 25 | 10 | 15 | 2341.5 ± 149.3 | 1688.0 ± 107.0 | 512.3 ± 3.3 | 1711.7 ± 117.5 |
| 3 | 5 | 50 | 5 | 2029.4 ± 57.5 | 4128.3 ± 109.0 | 535.2 ± 32.5 | 1445.9 ± 47.8 |
| 4 | 15 | 30 | 15 | 3582.8 ± 506.3 | 1503.2 ± 32.2 | 611.4 ± 13.8 | 1889.4 ± 181.9 |
| 5 | 5 | 20 | 20 | ND | 3337.5 ± 339.4 | 1706.1 ± 125.7 | ND |
| 6 | 25 | 30 | 5 | 2569.1 ± 59.4 | 913.8 ± 76.6 | 177.4 ± 15.7 | 1254.7 ± 136.6 |
| 7 | 10 | 50 | 30 | 3712.2 ± 1222.6 | 7577.3 ± 385.1 | 596.1 ± 16.3 | 3004.4 ± 238.1 |
| 8 | 15 | 30 | 15 | 3079.6 ± 221.3 | 1482.8 ± 80.0 | 609.7 ± 23.7 | 1852.6 ± 87.2 |
| 9 | 15 | 30 | 15 | 4334.1 ± 441.5 | 1360.8 ± 37.6 | 649.9 ± 53.1 | 1698.9 ± 52.9 |
| 10 | 15 | 10 | 30 | 2130–6 ± 43.3 | 3220.8 ± 245.0 | 1088.2 ± 20.2 | 1630.3 ± 47.9 |
| 11 | 25 | 30 | 5 | 2645.8 ± 50.3 | 778.9 ± 24.1 | 190.2 ± 6.6 | 1194.1 ± 183.9 |
| 12 | 25 | 10 | 30 | 2598.8 ± 133.6 | 3241.5 ± 40.3 | 631.4 ± 15.1 | 1713.4 ± 243.7 |
| 13 | 5 | 20 | 20 | ND | 3637.5 ± 118.2 | 1717.0 ± 56.4 | ND |
| 14 | 10 | 10 | 5 | 2187.8 ± 92.8 | 2744.4 ± 768.2 | 927.2 ± 9.5 | 1317.2 ± 76.7 |
| 15 | 5 | 40 | 20 | ND | 1480.6 ± 72.4 | 266.1 ± 13.0 | ND |
| 16 | 25 | 30 | 30 | 4121.5 ± 231.1 | 515.7 ± 10.3 | 285.2 ± 16.9 | 1657.4 ± 93.0 |
| 17 | 25 | 50 | 15 | 1161.1 ± 11.2 | 354.9 ± 7.8 | 214.4 ± 10.0 | 694.1 ± 70.4 |
| 18 | 20 | 10 | 5 | 2134.5 ± 186.4 | 729.9 ± 11.1 | 438.8 ± 14.3 | 1318.4 ± 9.7 |
| 19 | 15 | 30 | 15 | 4706.2 ± 592.9 | 1773.3 ± 32.1 | 594.4 ± 15.3 | 1742.2 ± 142.3 |
| 20 | 5 | 30 | 5 | 486.7 ± 14.7 | 2563.9 ± 10.8 | 944.7 ± 36.6 | 1455.4 ± 63.0 |
| 21 | 15 | 75 | 10 | 3208.8 ± 573.9 | 55.7 ± 1.2 | 27.9 ± 1.2 | 1458.3 ± 194.0 |
| 22 | 5 | 75 | 5 | 1754.8 ± 79.0 | 28.2 ± 1.8 | 16.5 ± 1.4 | 1090.2 ± 53.2 |
| 23 | 10 | 75 | 15 | 3104.5 ± 68.7 | 114.4 ± 14.4 | 87.2 ± 109.1 | 1707.0 ± 99.4 |
| 24 | 25 | 75 | 15 | 2924.7 ± 214.1 | 158.9 ± 7.9 | 35.1 ± 3.0 | 1199.4 ± 73.9 |
| 25 | 15 | 85 | 10 | 3643.2 ± 279.9 | 103.8 ± 12.8 | 37.8 ± 10.0 | ND |
| 26 | 5 | 85 | 5 | 1581.4 ± 87.1 | 41.3 ± 4.9 | 216.1 ± 14.3 | ND |
| 27 | 10 | 85 | 30 | 3232.1 ± 308.2 | 68.8 ± 13.3 | 212.2 ± 18.5 | ND |
| 28 | 25 | 85 | 15 | 3895.8 ± 213.0 | 188.6 ± 34.8 | 115.8 ± 3.3 | ND |
| Acronym | Ratio (solid/liquid) | Extraction Time (min) | Max (mg GAE/100 g DW) | Desirability |
|---|---|---|---|---|
| ChChl:Fruc | 5.0 | 30.0 | 6530.8 | 0.8 |
| ChChl:Gly | 5.2 | 23.3 | 1833.5 | 1.0 |
| Bet:CA | 6.0 | 28.5 | 3218.8 | 1.0 |
| LP:MA | 16.7 | 29.0 | 5389.1 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Urios, C.; Viñas-Ospino, A.; Penadés-Soler, A.; López-Malo, D.; Frígola, A.; Esteve, M.J.; Blesa, J. Natural Deep Eutectic Solvents as the Main Solvents for the Extraction of Total Polyphenols from Orange Peel. Biol. Life Sci. Forum 2021, 6, 110. https://doi.org/10.3390/Foods2021-11043
Gómez-Urios C, Viñas-Ospino A, Penadés-Soler A, López-Malo D, Frígola A, Esteve MJ, Blesa J. Natural Deep Eutectic Solvents as the Main Solvents for the Extraction of Total Polyphenols from Orange Peel. Biology and Life Sciences Forum. 2021; 6(1):110. https://doi.org/10.3390/Foods2021-11043
Chicago/Turabian StyleGómez-Urios, Clara, Adriana Viñas-Ospino, Anna Penadés-Soler, Daniel López-Malo, Ana Frígola, María José Esteve, and Jesús Blesa. 2021. "Natural Deep Eutectic Solvents as the Main Solvents for the Extraction of Total Polyphenols from Orange Peel" Biology and Life Sciences Forum 6, no. 1: 110. https://doi.org/10.3390/Foods2021-11043
APA StyleGómez-Urios, C., Viñas-Ospino, A., Penadés-Soler, A., López-Malo, D., Frígola, A., Esteve, M. J., & Blesa, J. (2021). Natural Deep Eutectic Solvents as the Main Solvents for the Extraction of Total Polyphenols from Orange Peel. Biology and Life Sciences Forum, 6(1), 110. https://doi.org/10.3390/Foods2021-11043









