Evaluation of the Antifungal Effect of Carvacrol-Rich Essential Oils: In Vitro Study on the Phytopathogenic Fungi Alternaria and Fusarium †
Abstract
1. Introduction
2. Methods
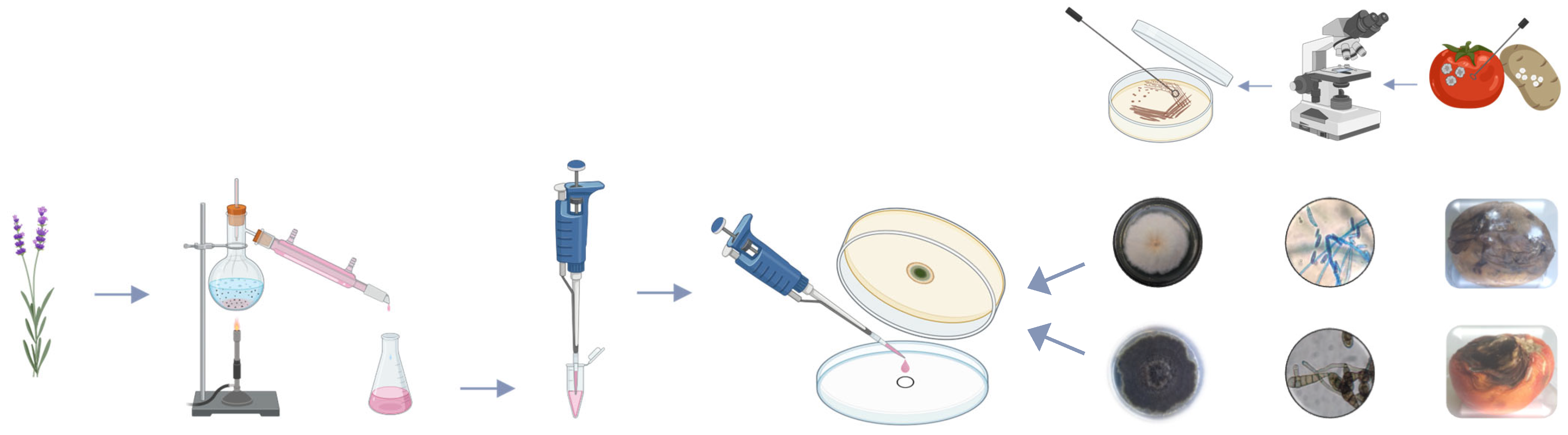
2.1. Experimental Design
2.2. Essential Oils Used in the Experiment
2.3. Preparation of Culture Medium
2.4. Isolation of Phytopathogens
2.5. Fumigant Assay
2.6. Estimation of Fungal Growth
2.7. Statistical Analysis
3. Results and Discussion
3.1. Composition of Essential Oils
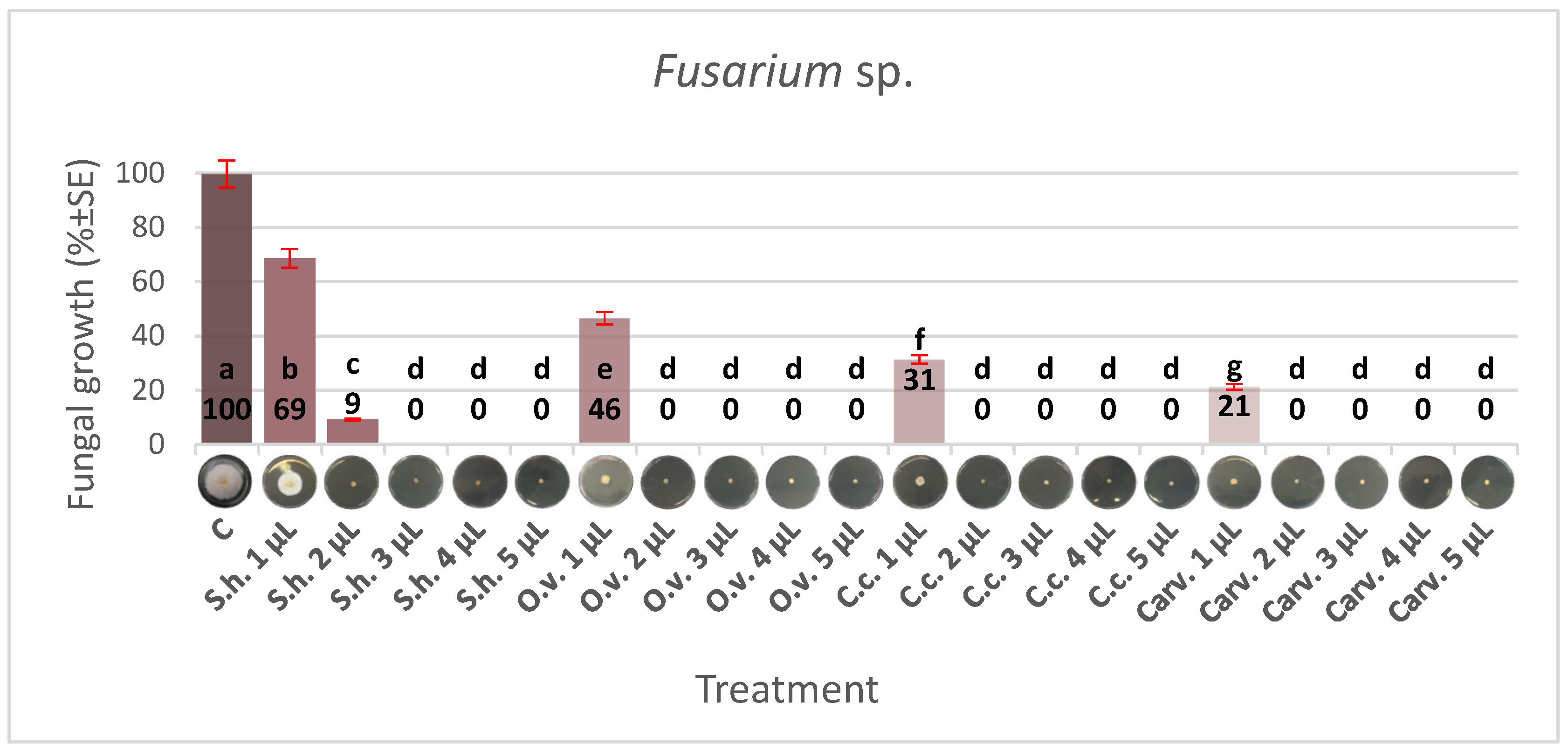
3.2. Effect of EOs on Fusarium sp. Growth
3.3. Effect of EOs on Alternaria sp. Growth
3.4. Mode of EOs’ Effect on Mycelial Growth
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Q.; Chen, T.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Molecular Basis of Pathogenesis of Postharvest Pathogenic Fungi and Control Strategy in Fruits: Progress and Prospect. Mol. Hortic. 2021, 1, 2. [Google Scholar] [CrossRef]
- Shahid, M.; Khan, M.S.; Zaidi, A. Fungicide Toxicity to Legumes and Its Microbial Remediation. In Pesticides in Crop Production: Physiological and Biochemical Action; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 15–33. [Google Scholar] [CrossRef]
- D’Aquino, S.; Palma, A. Reducing or Replacing Conventional Postharvest Fungicides with Low Toxicity Acids and Salts. In Postharvest Pathology of Fresh Horticultural Produce; CRC Press: Boca Raton, FL, USA, 2019; pp. 595–632. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of Fungicides. In Veterinary Toxicology: Basic and Clinical Principles, Third Edition; Academic Press: New York, NY, USA, 2018; pp. 569–580. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS, Plant- and Animal-Derived Compounds as Alternatives to Conventional Fungicides for the Control of Postharvest Diseases of Fresh Horticultural Produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Santamarina, M.P.; Ibáñez, M.D.; Marqués, M.; Roselló, J.; Giménez, S.; Blázquez, M.A. Bioactivity of Essential Oils in Phytopathogenic and Post-Harvest Fungi Control. Nat. Prod. Res. 2017, 31, 2675–2679. [Google Scholar] [CrossRef]
- Lee, S.O.; Choi, G.J.; Jang, K.S.; Lim, H.K.; Cho, K.Y.; Kim, J.C. Antifungal Activity of Five Plant Essential Oils as Fumigant Against Postharvest and Soilborne Plant Pathogenic Fungi. Plant Pathol. J. 2007, 23, 97–102. [Google Scholar] [CrossRef]
- Frankova, A.; Smid, J.; Bernardos, A.; Finkousova, A.; Marsik, P.; Novotny, D.; Legarová, V.; Pulkrabek, J.; Kloucek, P. The Antifungal Activity of Essential Oils in Combination with Warm Air Flow against Postharvest Phytopathogenic Fungi in Apples. Food Control 2016, 68, 62–68. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Bashyal, B.M.; Shanmugam, V.; Lal, M.K.; Kumar, R.; Sharma, S.; Vinod; Gaikwad, K.; Singh, B.; Aggarwal, R. Impact of Fusarium Dry Rot on Physicochemical Attributes of Potato Tubers during Postharvest Storage. Postharvest Biol. Technol. 2021, 181, 111638. [Google Scholar] [CrossRef]
- Mondani, L.; Chiusa, G.; Pietri, A.; Battilani, P. Monitoring the Incidence of Dry Rot Caused by Fusarium Proliferatum in Garlic at Harvest and during Storage. Postharvest Biol. Technol. 2021, 173, 111407. [Google Scholar] [CrossRef]
- Suzuki, T.; Kim, Y.K.; Yoshioka, H.; Iwahashi, Y. Regulation of Metabolic Products and Gene Expression in Fusarium Asiaticum by Agmatine Addition. Mycotoxin Res. 2013, 29, 103–111. [Google Scholar] [CrossRef]
- Amsellem, Z.; Kleifeld, Y.; Kerenyi, Z.; Hornok, L.; Goldwasser, Y.; Gressel, J. Isolation, Identification, and Activity of Mycoherbicidal Pathogens from Juvenile Broomrape Plants. Biol. Control 2001, 21, 274–284. [Google Scholar] [CrossRef]
- Ueno, Y.; Sawano, M.; Ishii, K. Production of Trichothecene Mycotoxins by Fusarium Species in Shake Culture. Appl. Microbiol. 1975, 30, 4–9. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, R.; Xue, H.; Bi, Y.; Li, L.; Zhang, Q.; Kouasseu, C.J.; Nan, M.; Prusky, D. Ozone Controls Potato Dry Rot Development and Diacetoxyscirpenol Accumulation by Targeting the Cell Membrane and Affecting the Growth of Fusarium Sulphureus. Physiol. Mol. Plant Pathol. 2022, 118, 101785. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Diseases Caused by Fungi. In Plant Pathology, 5th ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Chaerani, R.; Voorrips, R.E. Tomato Early Blight (Alternaria Solani): The Pathogen, Genetics, and Breeding for Resistance. J. General. Plant Pathol. 2006, 72, 335–347. [Google Scholar] [CrossRef]
- Kumar, V.; Haldar, S.; Pandey, K.K.; Singh, R.P.; Singh, A.K.; Singh, P.C. Cultural, Morphological, Pathogenic and Molecular Variability amongst Tomato Isolates of Alternaria Solani in India. World J. Microbiol. Biotechnol. 2008, 24, 1003–1009. [Google Scholar] [CrossRef]
- Chen, A.; Mao, X.; Sun, Q.; Wei, Z.; Li, J.; You, Y.; Zhao, J.; Jiang, G.; Wu, Y.; Wang, L.; et al. Alternaria Mycotoxins: An Overview of Toxicity, Metabolism, and Analysis in Food. J. Agric. Food Chem. 2021, 69, 7817–7830. [Google Scholar] [CrossRef]
- Rychlik, M.; Lepper, H.; Weidner, C.; Asam, S. Risk Evaluation of the Alternaria Mycotoxin Tenuazonic Acid in Foods for Adults and Infants and Subsequent Risk Management. Food Control 2016, 68, 181–185. [Google Scholar] [CrossRef]
- Wang, H.; Guo, Y.; Luo, Z.; Gao, L.; Li, R.; Zhang, Y.; Kalaji, H.M.; Qiang, S.; Chen, S. Recent Advances in Alternaria Phytotoxins: A Review of Their Occurrence, Structure, Bioactivity, and Biosynthesis. J. Fungi 2022, 8, 168. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria Host-Specific (HSTs) Toxins: An Overview of Chemical Characterization, Target Sites, Regulation and Their Toxic Effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- El-Alam, I.; Raveau, R.; Fontaine, J.; Verdin, A.; Laruelle, F.; Fourmentin, S.; Chahine, R.; Makhlouf, H.; Lounès-Hadj Sahraoui, A. Antifungal and Phytotoxic Activities of Essential Oils: In Vitro Assays and Their Potential Use in Crop Protection. Agronomy 2020, 10, 825. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Jia, X.; Xin, L.; Zhai, H. Antifungal Effects and Potential Mechanism of Essential Oils on Collelotrichum Gloeosporioides In Vitro and In Vivo. Molecules 2019, 24, 3386. [Google Scholar] [CrossRef]
- Ćosić, J.; Vrandečić, K.; Poštić, J.; Jurković, D.; Ravlić, M. In Vitro Antifungal Activity of Essential Oils on Growth of Phytopathogenic Fungi. Poljoprivreda 2010, 16, 25–28. [Google Scholar]
- El Khetabi, A.; Ezrari, S.; El Ghadraoui, L.; Tahiri, A.; Haddou, L.A.; Belabess, Z.; Merah, O.; Lahlali, R. In Vitro and In Vivo Antifungal Activities of Nine Commercial Essential Oils against Brown Rot in Apples. Horticulturae 2021, 7, 545. [Google Scholar] [CrossRef]
- Tejeswini, M.G.; Sowmya, H.V.; Swarnalatha, S.P.; Negi, P.S. Antifungal Activity of Essential Oils and Their Combinations in in Vitro and in Vivo Conditions. Arch. Phytopathol. Plant Prot. 2014, 47, 564–570. [Google Scholar] [CrossRef]
- Prasad, C.S.; Shukla, R.; Kumar, A.; Dubey, N.K. In Vitro and in Vivo Antifungal Activity of Essential Oils of Cymbopogon Martini and Chenopodium Ambrosioides and Their Synergism against Dermatophytes. Mycoses 2010, 53, 123–129. [Google Scholar] [CrossRef]
- Dardioti, A.; Hanlidou, E.; Lanaras, T.; Kokkini, S. The Essential Oils of the Greek Endemic Satureja Horvatii Ssp. Macrophylla in Relation to Bioclimate. Chem. Biodivers. 2010, 7, 1968–1977. [Google Scholar] [CrossRef]
- Jafari, A.A.; Tafti, A.F.; Hoseiny, S.M.; Kazemi, A. Antifungal Effect of Zataria Multiflora Essence on Experimentally Contaminated Acryl Resin Plates With Candida Albicans. Iran. Red. Crescent Med. J. 2015, 17, e16552. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Alsanius, B.W. Cassia Oil for Controlling Plant and Human Pathogens on Fresh Strawberries. Food Control 2012, 28, 157–162. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J.E. A Study of the Minimum Inhibitory Concentration and Mode of Action of Oregano Essential Oil, Thymol and Carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary Metabolites in Fungus-Plant Interactions. Front. Plant Sci. 2015, 6, 145754. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal Activity of Thymol and Carvacrol against Postharvest Pathogens Botrytis Cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium Graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef]
- Yfanti, P.; Patakioutas, G.; Douma, D.; Lekka, M.E. In Vitro Antifungal Activity of Satureja Horvatii Ssp. Macrophylla Against 3 Tomato Phytopathogenic Fungi. Nat. Prod. Commun. 2021, 16, 1934578X211025165. [Google Scholar] [CrossRef]
- Feng, W.; Chen, J.; Zheng, X.; Liu, Q. Thyme Oil to Control Alternaria Alternata in Vitro and in Vivo as Fumigant and Contact Treatments. Food Control 2011, 22, 78–81. [Google Scholar] [CrossRef]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Aghdam, M.A.; Kafil, H.S. Carvacrol and Thymol: Strong Antimicrobial Agents against Resistant Isolates. Rev. Res. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal Efficacy of Thymol, Carvacrol, Eugenol and Menthol as Alternative Agents to Control the Growth of Food-Relevant Fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical Composition and Antimicrobial Activity of Satureja, Thymus, and Thymbra Species Grown in Lebanon. Chem. Biodivers. 2017, 14, e1600236. [Google Scholar] [CrossRef]
- Haloc, I.E.; Toska, V.; Baldisserotto, A.; Goci, E.; Vertuani, S.; Manfredini, S. Evaluation of Antifungal Activity of Satureja Montana Essential Oil Before and After Inclusion in Beta-Cyclodextrine. Int. J. Pharm. Pharm. Sci. 2014, 6, 189–191. [Google Scholar]
- Sarrou, E.; Tsivelika, N.; Chatzopoulou, P.; Tsakalidis, G.; Menexes, G.; Mavromatis, A. Conventional Breeding of Greek Oregano (Origanum Vulgare Ssp. Hirtum) and Development of Improved Cultivars for Yield Potential and Essential Oil Quality. Euphytica 2017, 213, 104. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Mol. A J. Synth. Chem. Nat. Prod. Chem. 2017, 22, 989. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, Phytotoxic and Insecticidal Properties of Essential Oil Isolated from Turkish Origanum Acutidens and Its Three Components, Carvacrol, Thymol and p-Cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.D.; Dimić, G.R.; Tanackov, I.J.; Pejin, D.J.; Mojović, L.V.; Pejin, J.D. Antifungal Activity of Oregano (Origanum Vulgare L.) Extract on the Growth of Fusarium and Penicillium Species Isolated from Food. Hem. Ind. 2012, 66, 33–41. [Google Scholar] [CrossRef]
- Hedhili, L.; Romdhane, M.; Planche, H.; Abderrabba, M. Towards Gas Chromatography–Mass Spectrometry Coupling Protocols for Both Identifying and Quantification Essential Oils of Thymus Capitatus Hoff et Link. J. Chromatogr. A 2005, 1064, 129–134. [Google Scholar] [CrossRef]
- Marin, M.; Novaković, M.; Tešević, V.; Vučković, I.; Milojević, N.; Vuković-Gačić, B.; Marin, P.D. Antioxidative, Antibacterial and Antifungal Activity of the Essential Oil of Wild-Growing Satureja Montana L. from Dalmatia, Croatia. Flavour. Fragr. J. 2012, 27, 216–223. [Google Scholar] [CrossRef]
- Alam, S.B.; Benyelles, N.G.; El, M.; Dib, A.; Djabou, N.; Tabti, L.; Paolini, J.; Muselli, A.; Costa, J. Antifungal Activity of Essential Oils of Three Aromatic Plants from Western Algeria against Five Fungal Pathogens of Tomato (Lycopersicon Esculentum Mill). J. Appl. Bot. Food Qual. 2014, 87, 56–61. [Google Scholar]
- Karpouhtsis, I.; Pardali, E.; Feggou, E.; Kokkini, S.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Oregano Essential Oils. J. Agric. Food. Chem. 1998, 46, 1111–1115. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Hanrahan, J. Biologically Active Compounds from Lamiaceae Family: Central Nervous System Effects. Stud. Nat. Prod. Chem. 2021, 68, 255–315. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, Z.; Li, M.; Xing, M.; Xian, T.; Tu, K. Carvacrol and Eugenol Effectively Inhibit Rhizopus Stolonifer and Control Postharvest Soft Rot Decay in Peaches. J. Appl. Microbiol. 2018, 124, 166–178. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Zhang, H.; Wang, P.; Wang, C.; Zhou, Y.; Li, H.; Yu, S.; Wu, R. Inhibitory Effect of Carvacrol against Alternaria Alternata Causing Goji Fruit Rot by Disrupting the Integrity and Composition of Cell Wall. Front. Microbiol. 2023, 14, 1139749. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Wang, P.; Zhao, L.; Zhang, H.; Qu, H.; Xu, F. Inhibitory Effect and Mechanism of Carvacrol against Black Mold Disease Agent Alternaria Alternata in Goji Berries. J. Fungi 2024, 10, 402. [Google Scholar] [CrossRef]
- Yfanti, P.; Batistatou, A.; Manos, G.; Lekka, M.E.; Yfanti, P.; Batistatou, A.; Manos, G.; Lekka, M.E. The Aromatic Plant Satureja Horvatii Ssp. Macrophylla Induces Apoptosis and Cell Death to the A549 Cancer Cell Line. Am. J. Plant Sci. 2015, 6, 2092–2103. [Google Scholar] [CrossRef]
- Vicuña, G.C.; Stashenko, E.E.; Fuentes, J.L. Chemical Composition of the Lippia Origanoides Essential Oils and Their Antigenotoxicity against Bleomycin-Induced DNA Damage. Fitoterapia 2010, 81, 343–349. [Google Scholar] [CrossRef]
- Burt, S.A.; Van Der Zee, R.; Koets, A.P.; De Graaff, A.M.; Van Knapen, F.; Gaastra, W.; Haagsman, H.P.; Veldhuizen, E.J.A. Carvacrol Induces Heat Shock Protein 60 and Inhibits Synthesis of Flagellin in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007, 73, 4484. [Google Scholar] [CrossRef]
- Ultee, A.; Kets, E.P.W.; Alberda, M.; Hoekstra, F.A.; Smid, E.J. Adaptation of the Food-Borne Pathogen Bacillus Cereus to Carvacrol. Arch. Microbiol. 2000, 174, 233–238. [Google Scholar] [CrossRef]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors That Interact with the Antibacterial Action of Thyme Essential Oil and Its Active Constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Müller-Riebau, F.; Berger, B.; Yegen, O. Chemical Composition and Fungitoxic Properties to Phytopathogenic Fungi of Essential Oils of Selected Aromatic Plants Growing Wild in Turkey. J. Agric. Food Chem. 1995, 43, 2262–2266. [Google Scholar] [CrossRef]
- Schiro, G.; Verch, G.; Grimm, V.; Müller, M.E.H. Alternaria and Fusarium Fungi: Differences in Distribution and Spore Deposition in a Topographically Heterogeneous Wheat Field. J. Fungi 2018, 4, 63. [Google Scholar] [CrossRef]

| No | RT | RI | Compounds | Area (%) | ||
|---|---|---|---|---|---|---|
| O. vulgare ssp. hirtum | C. capitatus | S. horvatii ssp. macrophylla | ||||
| 1 | 6.09 | 928 | α-Thujene | 2.3 | 0.9 | 1.3 |
| 2 | 6.36 | 937 | α-Pinene | 1.1 | 0.7 | 1.4 |
| 3 | 6.89 | 955 | Camphene | 1.8 | ||
| 4 | 7.76 | 986 | 1-Octen-3-ol | 1.2 | 0.8 | 1.7 |
| 5 | 7.98 | 993 | α-Myrcene | 3.0 | 1.9 | 1.1 |
| 6 | 8.68 | 1012 | α-Phellandrene | 0.6 | 0.4 | 0.2 |
| 7 | 9.03 | 1023 | α-Terpinene | 2.9 | 2.4 | 1.6 |
| 8 | 9.40 | 1033 | p-Cymene | 10.2 | 6.2 | 12.9 |
| 9 | 9.53 | 1035 | Limonene | 0.4 | 0.4 | 0.6 |
| 10 | 6.19 | 1038 | b-Phellandrene | 0.6 | 0.5 | |
| 11 | 9.76 | 1039 | 1.8-cineole | 1.4 | ||
| 12 | 10.59 | 1065 | γ-Terpinene | 9.2 | 5.7 | 5.5 |
| 13 | 11.13 | 1180 | cis sabinene hydrate | 1.4 | 1.1 | |
| 14 | 12.25 | 1107 | Linalool | 1.4 | 1.6 | |
| 15 | 15.55 | 1185 | Borneol | 0.8 | 1.8 | 5.0 |
| 16 | 15.84 | 1192 | Terpinene-4-ol | 0.8 | 1.1 | 1.3 |
| 17 | 16.24 | 1201 | p-Cymen-8-ol | 0.3 | ||
| 18 | 16.55 | 1207 | α-Terpineol | 0.4 | ||
| 19 | 18.41 | 1242 | Thymol methyl ether | 3.2 | ||
| 20 | 21.86 | 1304 | Thymol | 18.9 | 6.2 | |
| 21 | 22.68 | 1313 | Carvacrol | 42.5 | 70.0 | 41.4 |
| 22 | 31.417 | 1424 | Caryophyllene | 2.7 | 4.9 | 4.5 |
| 23 | 32.72 | 1444 | Aromadendrene | 0.6 | ||
| 24 | 33.85 | 1461 | α-Humulene | 0.3 | ||
| 25 | 36.06 | 1494 | Viridiflorene | 0.3 | ||
| 26 | 36.347 | 1498 | γ-Elemene | 1.5 | ||
| 27 | 37.10 | 1514 | β-Bisabolene | 1.0 | 0.3 | |
| 28 | 40.63 | 1591 | Spathulenol | 0.9 | ||
| 29 | 40.75 | 1594 | Caryophyllene oxide | 0.4 | 0.8 | 2.0 |
| Monoterpene hydrocarbons | 30.3 | 19.0 | 26.5 | |||
| Oxygenated monoterpenes | 62.9 | 74.2 | 57.6 | |||
| Sesquiterpene hydrocarbons | 3.8 | 5.2 | 7.0 | |||
| Oxygenated sesquiterpenes | 0.4 | 0.8 | 2.9 | |||
| Others | 2.6 | 0.8 | 5.9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papantzikos, V.; Patakioutas, G.; Yfanti, P. Evaluation of the Antifungal Effect of Carvacrol-Rich Essential Oils: In Vitro Study on the Phytopathogenic Fungi Alternaria and Fusarium. Biol. Life Sci. Forum 2025, 54, 1. https://doi.org/10.3390/blsf2025054001
Papantzikos V, Patakioutas G, Yfanti P. Evaluation of the Antifungal Effect of Carvacrol-Rich Essential Oils: In Vitro Study on the Phytopathogenic Fungi Alternaria and Fusarium. Biology and Life Sciences Forum. 2025; 54(1):1. https://doi.org/10.3390/blsf2025054001
Chicago/Turabian StylePapantzikos, Vasileios, Georgios Patakioutas, and Paraskevi Yfanti. 2025. "Evaluation of the Antifungal Effect of Carvacrol-Rich Essential Oils: In Vitro Study on the Phytopathogenic Fungi Alternaria and Fusarium" Biology and Life Sciences Forum 54, no. 1: 1. https://doi.org/10.3390/blsf2025054001
APA StylePapantzikos, V., Patakioutas, G., & Yfanti, P. (2025). Evaluation of the Antifungal Effect of Carvacrol-Rich Essential Oils: In Vitro Study on the Phytopathogenic Fungi Alternaria and Fusarium. Biology and Life Sciences Forum, 54(1), 1. https://doi.org/10.3390/blsf2025054001







