1. Introduction
Quinoa (
Chenopodium quinoa Willd.) is recognized as a highly nutritious food due to its elevated seed protein content, ranging from 13.8% to 21.9% [
1], and its balanced profile of essential amino acids [
2,
3]. This crop also stands out for its remarkable adaptability to diverse environments and its tolerance to multiple biotic and abiotic stresses, which has contributed to its global expansion [
4].
Among the factors threatening agricultural productivity, excessive rainfall is an increasingly frequent problem associated with climate change as it can cause crop waterlogging with detrimental effects on yield [
5]. This phenomenon is particularly severe in areas with flat topography, poorly drained soils, high clay content, or compaction [
5,
6]. Soils are considered waterlogged when excess water saturates soil pores, thereby hindering gas exchange between roots and the atmosphere [
6]. Under such conditions, hypoxia drastically reduces oxygen availability in the rhizosphere, limiting root respiration and energy production, which inhibits growth and may ultimately lead to tissue death [
7,
8]. At the shoot level, waterlogging induces stomatal closure [
9], restricting CO
2 uptake and transpiration, decreasing photosynthesis, accelerating senescence, and ultimately causing significant yield losses [
5].
Several studies in grain crops have shown that waterlogging susceptibility depends on the phenological stage. In rapeseed and buckwheat, the vegetative stage is reported as the most vulnerable [
10,
11], whereas in rice, maize, cotton, wheat, soybean, and barley, the reproductive stage results in greater yield losses [
5,
12]. These findings suggest that crop response to waterlogging is modulated by three main factors: crop species, timing of stress occurrence, and duration of the event [
5,
11].
In quinoa, although it is considered tolerant to multiple stresses, knowledge of its response to excess water remains limited. In particular, little is known about the relative sensitivity of different phenological stages to waterlogging. Therefore, the objective of this study was to identify the developmental stage most susceptible to waterlogging stress in quinoa by quantifying its effects on growth and yield.
2. Materials and Methods
The experiment was conducted under greenhouse conditions at the School of Agronomy, Universidad Nacional de Loja (UNL), Loja, Ecuador (4°02′14″ S, 79°12′02″ W). Experimental units consisted of 15 L pots filled with a substrate composed of clay loam soil, sand, and biocompost at a 2:1:1 ratio. One seed of quinoa (var. Tunkahuan) was sown per pot. Weeds were manually controlled, and preventive applications against pests and diseases were performed during the crop cycle.
The experiment was arranged in a completely randomized design with seven treatments and seven replications, using one plant per pot as the experimental unit. Treatments consisted of a non-waterlogged control and six waterlogging applications imposed at different phenological stages, according to the BBCH scale: lateral shoot development (BBCH 25), floral bud initiation (BBCH 50), anthesis onset (BBCH 60), mid-anthesis (BBCH 67), grain filling onset (BBCH 70), and late grain filling (BBCH 85). Each waterlogging event lasted 12 days. For this, pots were placed in 20 L containers filled with water until reaching 1 cm above the soil surface. After each period, pots were removed, kept without irrigation for 10 days to allow free drainage, and then watered normally.
Growth variables were recorded at the end of each waterlogging event. Canopy cover was quantified using the Canopeo mobile application, while relative chlorophyll content was measured with a SPAD-502 chlorophyll meter (Minolta). At physiological maturity, plants were harvested by cutting at soil level. Aboveground biomass was dried in a forced-air oven at 65 ± 5 °C for 72 h until constant weight. Yield components were determined by counting the number of grains per plant and measuring the weight of 1000 grains. Grain yield (R) per plant was calculated as follows:
where
NG = number of grains per plant;
PG = average grain weight.
Data were tested for normality, homogeneity of variances, and independence. Subsequently, an analysis of variance (ANOVA) was performed at a significance level of 0.05, followed by Tukey’s HSD test (p ≤ 0.05).
3. Results
Waterlogging significantly reduced quinoa growth and physiological performance across all phenological stages tested (
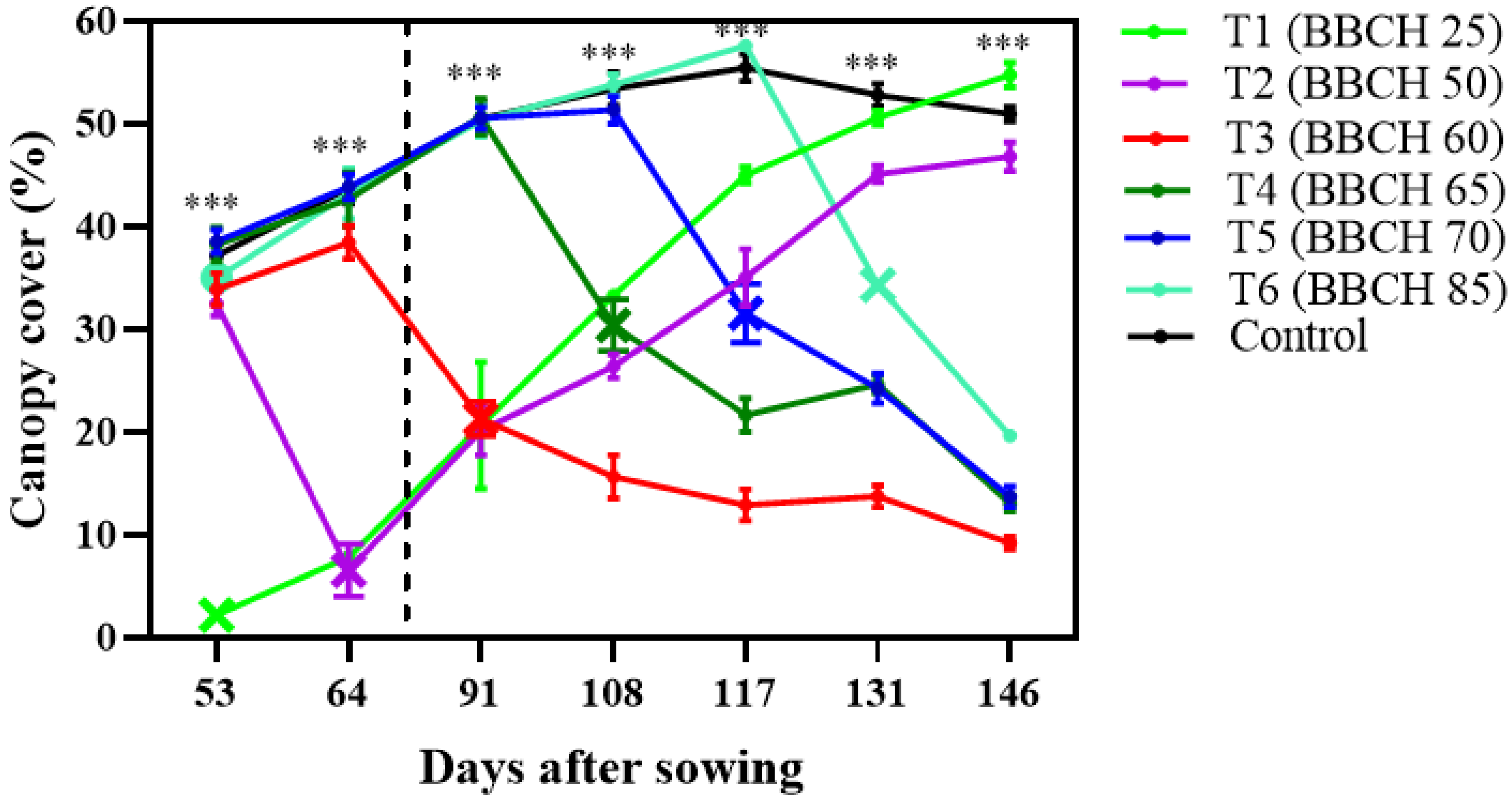
p ≤ 0.05). Canopy cover decreased markedly under stress at all stages (
Figure 1). Plants subjected to waterlogging during the five lateral shoots (BBCH 25) and floral bud initiation (BBCH 50) stages exhibited the strongest reductions immediately after stress; however, these plants partially recovered once waterlogging ended. In contrast, waterlogging imposed during reproductive stages resulted in poor recovery capacity, and canopy cover remained low until the end of the crop cycle.
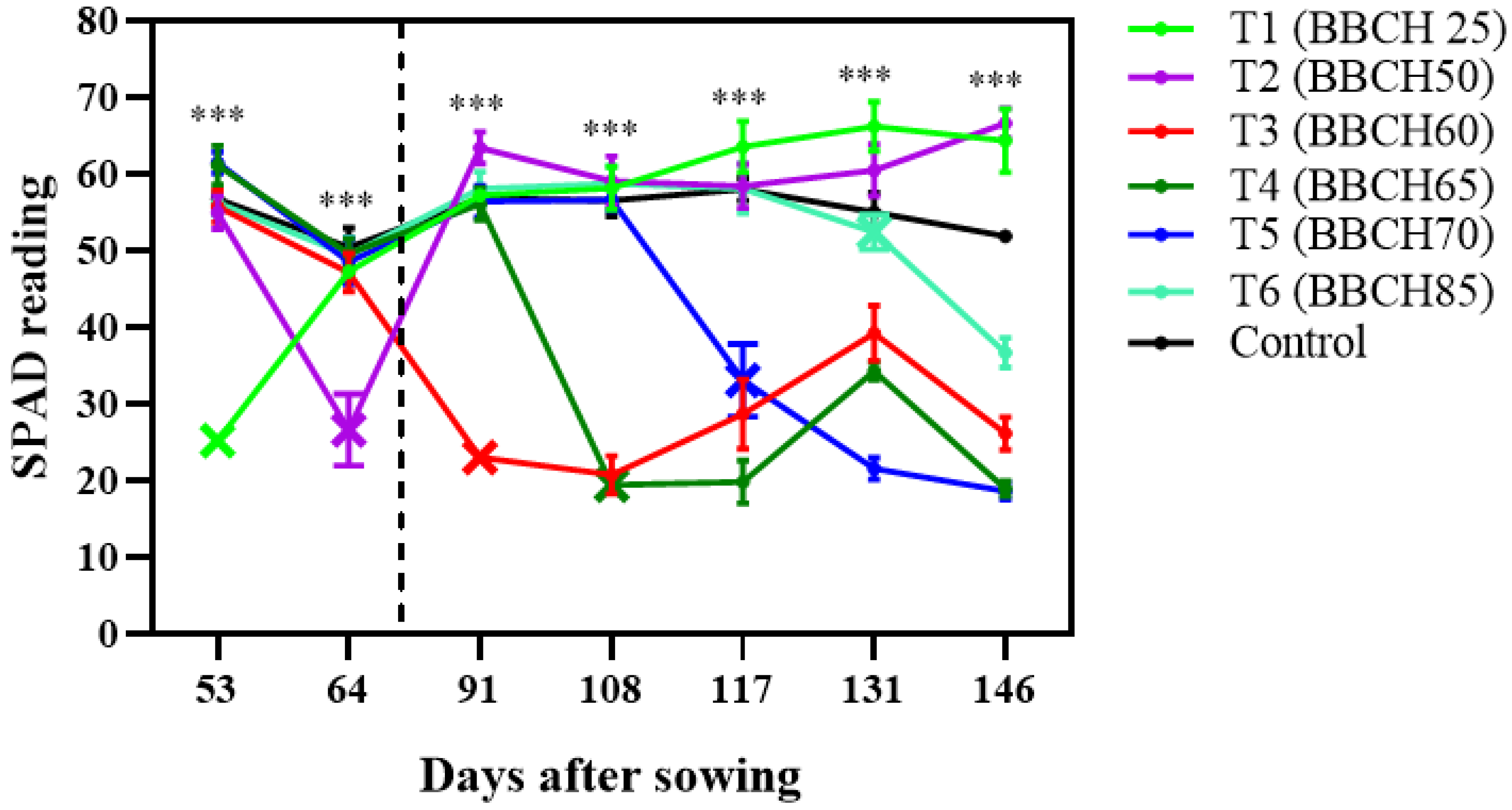
Relative chlorophyll content (SPAD values) was also negatively affected by waterlogging (
Figure 2). During vegetative stages, SPAD readings decreased sharply but gradually recovered as growth progressed. In contrast, waterlogging after anthesis caused sustained reductions in SPAD values, indicating a high sensitivity of post-anthesis physiology and a limited recovery capacity.
Waterlogging markedly reduced grain yield in all treatments compared to the well-drained control (
p ≤ 0.05). The severity of yield reduction depended on the stage of stress application. Yield losses were most pronounced at anthesis onset (BBCH 60) and mid-anthesis (BBCH 67), where yields dropped to 11.83 and 9.63 g plant
−1, corresponding to 71% and 77% reductions relative to the control (41.09 g plant
−1). In contrast, stress imposed during grain filling caused moderate yield reductions, ranging between 22.91 and 27.87 g plant
−1 (
Table 1). High plant mortality was also observed when stress occurred at early stages: 73% at BBCH 25 and 33% at BBCH 50.
Aboveground biomass followed a similar pattern to yield (
p < 0.001). The greatest reduction occurred when waterlogging was applied at anthesis onset (38.79 g plant
−1), representing a 59% decrease compared to the control (94.03 g plant
−1). Waterlogging also reduced the harvest index. The lowest harvest index (0.18) was recorded in plants stressed at mid-anthesis, a 58% reduction relative to the control (0.43). Stress during grain filling stages produced smaller decreases in harvest index (0.36–0.40) (
Table 1).
Yield components were strongly affected (
p < 0.001). The number of grains per plant was highest in the control (13,144 grains plant
−1) and lowest under mid-anthesis stress (3746 grains plant
−1), representing a 72% reduction. Early waterlogging (BBCH 25 and BBCH 50) also reduced yield components, mainly due to high plant mortality. Thousand-grain weight was significantly reduced under stress (
p < 0.05). The control treatment recorded the highest value (3.13 g), while the lowest (2.43 g; 22% reduction) occurred at anthesis onset (
Table 1). Overall, results indicate that quinoa yield is sensitive to waterlogging at all stages, but the reproductive phase, particularly around anthesis, represents the most critical period for irreversible yield losses.
4. Discussion
Our findings demonstrate that quinoa is highly sensitive to waterlogging, with effects varying according to the phenological stage at which stress occurs. The reductions in canopy cover, chlorophyll content, biomass, and yield observed in this study are consistent with previous reports in other grain crops, where hypoxia and impaired root aeration lead to limited photosynthetic activity and reduced assimilate availability for reproductive development [
5,
7,
9].
Early vegetative stress (BBCH 25 and BBCH 50) caused severe initial reductions in canopy cover and SPAD values; however, plants showed partial recovery after the stress was relieved. This pattern reflects the greater plasticity of quinoa during early growth stages, allowing compensation of leaf area and biomass production when favorable conditions return. Nevertheless, high plant mortality (up to 73% at BBCH 25) indicates that waterlogging tolerance in early stages is highly variable and dependent on plant establishment success.
In contrast, reproductive stages were characterized by both a sharp decline in growth and an inability to recover, particularly at anthesis (BBCH 60–67). Yield reductions exceeding 70% were associated with severe decreases in grain number and harvest index, suggesting that assimilate partitioning to reproductive organs is highly compromised under hypoxic conditions. Similar results have been reported in maize, wheat, and soybean, where anthesis is considered the most sensitive phase to flooding due to pollen sterility, ovary abortion, and reduced fertilization success [
5,
11,
12].
Stress imposed during grain filling (BBCH 70–85) reduced yield to a lesser extent. Although biomass accumulation and harvest index were negatively affected, yield losses were mitigated by the fact that fertilization had already occurred, and a significant proportion of grains were retained. This suggests that the critical window for irreversible yield damage in quinoa occurs between floral initiation and anthesis, in agreement with observations in other dicotyledonous grain crops such as buckwheat and rapeseed [
10].
Overall, these results indicate that quinoa exhibits a stage-dependent sensitivity to waterlogging, with anthesis representing the most vulnerable phase. While vegetative stress may be partially reversible, reproductive stress leads to permanent reductions in grain set and harvest index. This highlights the importance of identifying tolerant genotypes and implementing management practices that mitigate waterlogging risk, particularly around the reproductive period.
5. Conclusions
Waterlogging significantly reduced quinoa growth, chlorophyll content, biomass, and yield across all phenological stages. The most critical stage of sensitivity was anthesis (BBCH 60–67), where yield was reduced by up to 77%, mainly due to reductions in grain number and harvest index. Vegetative stages showed partial recovery after stress removal; however, early stress also caused high plant mortality (up to 73%). These findings provide valuable insights for breeding and agronomic management aimed at improving quinoa resilience to excessive rainfall and soil flooding under climate change scenarios.
Author Contributions
Conceptualization, S.C.V.; methodology, S.C.V. and M.M.-M.; formal analysis, S.C.V.; investigation, S.C.V., M.M.-M., L.M., K.L., F.G. and M.C.-M.; resources, S.C.V.; data curation, S.C.V. and W.O.; writing—original draft preparation, S.C.V.; writing—review and editing, S.C.V., M.M.-M., F.G. and M.C.-M.; visualization, S.C.V., W.O. and M.M.-M.; supervision, S.C.V.; project administration, S.C.V.; funding acquisition, S.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Project 06-DI-FARNR-2023, funded by the Research Directorate of the National University of Loja, Ecuador.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data is available from the corresponding author (Santiago C. Vásquez) upon reasonable request.
Acknowledgments
The authors express their gratitude to María José Uchuari and Magdalena Gaona for their valuable technical assistance during the field experiments. They also thank Beatriz Guerrero León, from the Bromatology Laboratory, and Lucía Quichimbo, from the Plant Physiology Laboratory at the National University of Loja (UNL), for their collaboration in processing the plant material. Finally, the authors acknowledge the Agronomy Department and the Research Directorate of UNL for providing the facilities and support necessary for the completion of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vásquez, S.C.; del Pozo, A.; Castillo, D.; Matus, I.; Gómez-Pando, L.; Zamudio-Ayala, D.; Mignone, C.M.; Bertero, H.D.; Calderini, D.F. The Critical Period for Yield and Grain Protein Determination in Quinoa (Chenopodium quinoa Willd.). Field Crops Res. 2024, 306, 109207. [Google Scholar] [CrossRef]
- Mestanza, C.; Riegel, R.; Silva, H.; Vásquez, S.C. Characterization of the Acetohydroxyacid Synthase Multigene Family in the Tetraploid Plant Chenopodium quinoa. Electron. J. Biotechnol. 2015, 18, 393–398. [Google Scholar] [CrossRef]
- Mestanza, C.; Riegel, R.; Vásquez, S.C.; Veliz, D.; Cruz-Rosero, N.; Canchignia, H.; Silva, H. Discovery of Mutations in Chenopodium quinoa Willd through EMS Mutagenesis and Mutation Screening Using Pre-Selection Phenotypic Data and Next-Generation Sequencing. J. Agric. Sci. 2018, 156, 1196–1204. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global Expansion of Quinoa and Challenges for the Andean Region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Tian, L.; Zhang, Y.; Chen, P.; Zhang, F.; Li, J.; Yan, F.; Dong, Y.; Feng, B. How Does the Waterlogging Regime Affect Crop Yield? A Global Meta-Analysis. Front. Plant Sci. 2021, 12, 634898. [Google Scholar] [CrossRef] [PubMed]
- de San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the Critical Period for Waterlogging on Yield and Its Components in Wheat and Barley. Plant Soil 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Ding, J.; Liang, P.; Wu, P.; Zhu, M.; Li, C.; Zhu, X.; Guo, W. Identifying the Critical Stage Near Anthesis for Waterlogging on Wheat Yield and Its Components in the Yangtze River Basin, China. Agronomy 2020, 10, 130. [Google Scholar] [CrossRef]
- Ghobadi, M.E.; Ghobadi, M.; Zebarjadi, A. Effect of Waterlogging at Different Growth Stages on Some Morphological Traits of Wheat Varieties. Int. J. Biometeorol. 2017, 61, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.D.; Zhou, Y.F.; Yue, Z.X.; Chen, X.F.; Cao, X.; Xu, X.X.; Xing, Y.F.; Jiang, B.; Ai, X.Y.; Huang, R.D. Changes in Photosynthesis, Chloroplast Ultrastructure, and Antioxidant Metabolism in Leaves of Sorghum under Waterlogging Stress. Photosynthetica 2019, 57, 1076–1083. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Cho, S.-W.; Chun, J.-B.; Kwon, S.J.; Roy, S.K.; Sung, J.-K.; Woo, S.-H.; Sakagami, J.-I. Morpho-Physiological Response of Common Buckwheat (Fagopyrum esculentum) to Flooding Stress at Different Growth Stages. J. Crop Sci. Biotechnol. 2021, 24, 41–49. [Google Scholar] [CrossRef]
- Wollmer, A.-C.; Pitann, B.; Mühling, K.H. Waterlogging Events during Stem Elongation or Flowering Affect Yield of Oilseed Rape (Brassica napus L.) but Not Seed Quality. J. Agron. Crop Sci. 2018, 204, 165–174. [Google Scholar] [CrossRef]
- Ploschuk, R.A.; Miralles, D.J.; Colmer, T.D.; Ploschuk, E.L.; Striker, G.G. Waterlogging of Winter Crops at Early and Late Stages: Impacts on Leaf Physiology, Growth and Yield. Front. Plant Sci. 2018, 9, 1863. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).