Revalorization of the Residual Cake from Moringa Seeds as an Alternative Source of Plant-Based Proteins †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Proximal Composition
2.2.2. Nutritional Value Evaluation
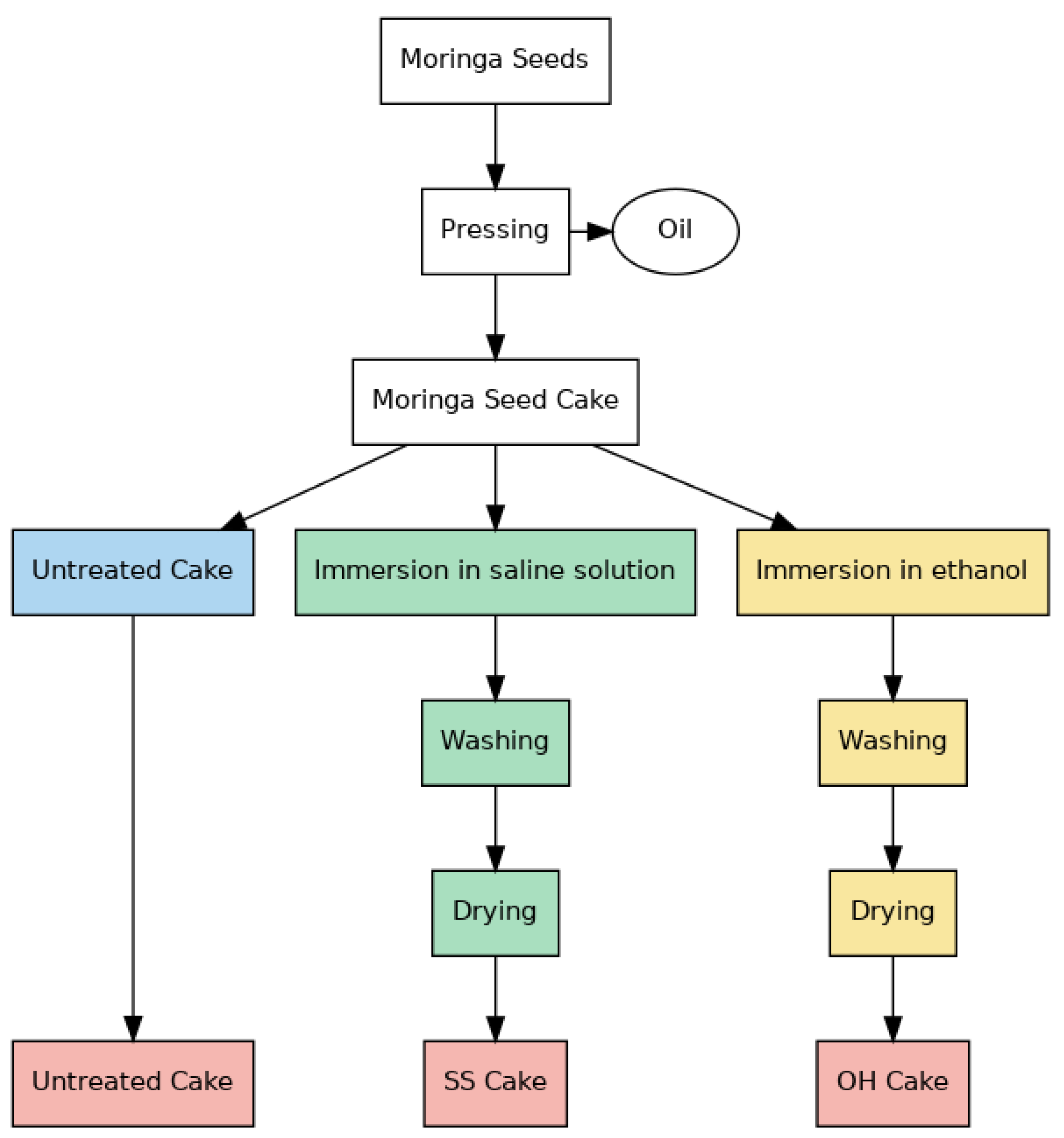
2.2.3. Physicochemical Treatments of Moringa Residual Cake After Oil Extraction
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition of Raw Materials
3.2. Nutritional Value of Raw Materials
3.3. Preliminary Trials
3.4. Proximal and Nutritional Composition of Defatted Moringa Residual Cake Treated with Saline and Ethanol Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cattan, Y.; Patil, D.; Vaknin, Y.; Rytwo, G.; Lakemond, C.; Benjamin, O. Characterization of Moringa oleifera leaf and seed protein extract functionality in emulsion model system. Innov. Food Sci. Emerg. Technol. 2022, 75, 102903. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera seeds and oil: Characteristics and uses for human health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed]
- Amad, A.A.; Zentek, J. The use of Moringa oleifera in ruminant feeding and its contribution to climate change mitigation. Front. Anim. Sci. 2023, 4, 1137562. [Google Scholar] [CrossRef]
- Abiodun, O.A.; Adegbite, J.A.; Omolola, A.O. Chemical and Physicochemical Properties of Moringa Flours and Oil. Glob. J. Sci. Front. Res. Biol. Sci. 2012, 12, 12–17. [Google Scholar]
- Silva Jaimes, M.I.; Cibej López, F.E.; SalváRuíz, B.; Guevara Perez, A.; Pascual Chagman, G. Effect of the debittered moringa seed cake (Moringa oleifera) on its proximal composition and its nutritional and toxicological profile. Sci. Agropecu. 2018, 9, 247–257. [Google Scholar] [CrossRef]
- Sanz-Penella, J.M.; Tamayo-Ramos, J.A.; Sanz, Y.; Haros, M. Phytate reduction in bran-enriched bread by phytase-producing bifidobacteria. J. Agric. Food Chem. 2009, 57, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Ballester-Sánchez, J.; Millán-Linares, M.C.; Fernández-Espinar, M.T.; Haros, C.M. Development of healthy, nutritious bakery products by incorporation of Quinoa. Foods. 2019, 8, 379. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Jin, Y.; Piao, J.; Kok, F.; Guusje, B.; Jacobsen, E. Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. J. Agric. Food Chem. 2005, 53, 10285–10290. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, R.F. Phytic acid degradation as a means of improving iron absorption. Int. J. Vitam. Nutr. Res. 2004, 74, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Dietary reference values for nutrients summary report. EFSA Support. Public. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Pérez-Heras, A.; Ros, E. Dietary fibre, nuts and cardiovascular diseases. Br. J. Nutr. 2007, 96, S45–S51. [Google Scholar] [CrossRef] [PubMed]
- FAO. Ingesta de Referencia de Población para Proteínas: Recomendaciones para Adultos; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Rome, Italy, 2017. [Google Scholar]
- FAO/WHO/UNU. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; WHO Technical Report Series No. 935; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- NAS. National Academy of Sciences. Dietary Reference Intakes. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 1 September 2025).
- NAS. National Academy of Sciences. Dietary Reference Intakes: Dietary Reference Intakes for Calcium and Vitamin D. Available online: https://www.ncbi.nlm.nih.gov/books/NBK56070/ (accessed on 1 September 2025).
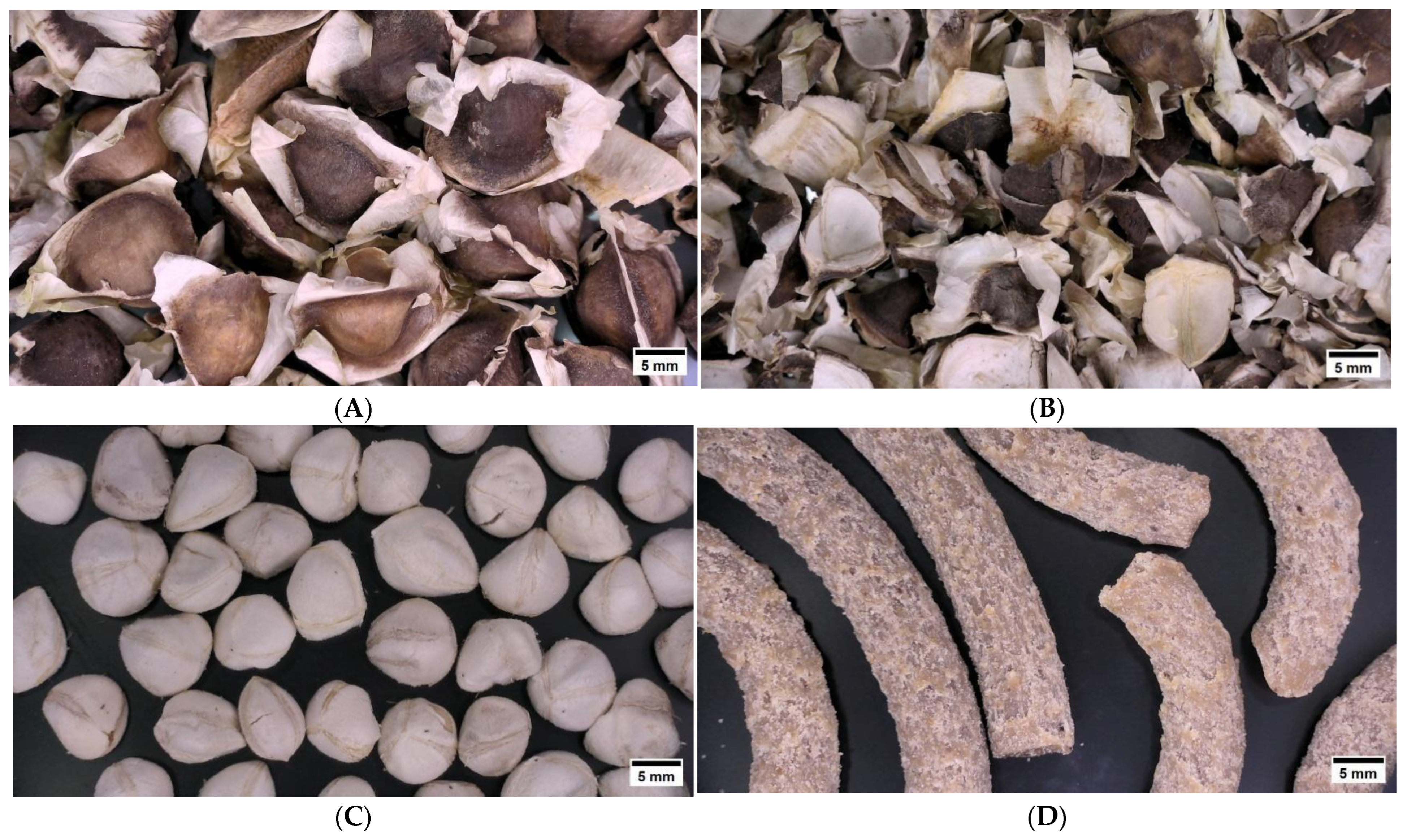
| Component | Units | Dehulled Moringa Seeds | Reference Values | Residual Cake after Oil Extraction | Reference Values |
|---|---|---|---|---|---|
| Moisture | g/100 g | 7.39 ± 0.03 a | 4.70 a; 7.0 g; 5.7–8.9 h | 8.16 ± 0.03 b | 5.03 a; 5.39 d; 8.4 g |
| Proteins f: 5.4975 (2); (f: 6.25) | g/100 g d.m. | 33.7 ± 0.1 a (38.3) | 28.04 (f: NI) a 36.4 (f: 5.4975) g; 41.1 (f:6.25) g; 29.4–33.3 h | 45.83 ± 0.0.02 b (52.1) | 50.80 (f: NI) a; 43.82 (f: NI) d; 47.6 (f: 5.4975) g; 54.1 (f:6.25) g |
| Total dietary fibre (TDF) Insoluble (IDF) Soluble (SDF) | g/100 g d.m. | TDF: 8.28 ± 0.21 a IDF: 5.82 ± 0.29 a SDF: 2.45 ± 0.50 a | 7.73 a (3); 6.8–8.0 (TDF) h; 6.5 (TDF)/5.9 (IDF)/0.6 (SDF) g | TDF: 13.0 ± 0.3 b IDF: 10.48 ± 0.04 b SDF: 2.52 ± 0.28 a | 2.54 d (3); 12.96 a (3); 12.8 (TDF)/11.9 (IDF)/0.89 (SDF) g |
| Lipids | g/100 g d.m. | 38.5 ± 0.5 a | 30–40 b; 39.0 g; 34.7–40.4 h | 9.66 ± 0.06 b | 11.2 b; 26.99 d; 10.5 g |
| Ash | g/100 g d.m. | 3.56 ± 0.04 a | 4.10 a; 3.6 g; 4.4–6.9 h | 6.09 ± 0.13 b | 3.96 d; 10.0 a; 5.0 g |
| Carbohydrates not included in fibre (4) | g/100 g d.m. | 16.7 | 10.59 a; 14.5 g; 16.5–19.8 h | 26.5 | 17.3 d; 18.15 a; 24.1 g |
| Myo-Inositol Phosphate, g/100 g d.m. | Dehulled Moringa Seeds | Reference Values | Residual Cake after Oil Extraction | Reference Values |
|---|---|---|---|---|
| InsP6, (Phytic Acid) | 0.74 ± 0.14 a | 0.059 a; 1.85 f; 0.175–1.381 g | 1.39 ± 0.26 b | 0.149 a; 2.18 f |
| InsP5 | 0.0019 ± 0.0008 | 0.110 f | N.D. | 0.247 f |
| InsP4 | N.D. | 0.020 f | N.D. | 0.043 f |
| InsP3 | N.D. | 0.006 f | N.D. | 0.007 f |
| Mineral, mg/100 g d.m. | ||||
| Ca | 33.3 ± 0.8 a | 20.4 a; 87.6 f; 150–260 g | 61.0 ± 2.3 b | 25.0 a; 265.5 c; 144 f |
| Fe | 1.96 ± 0.01 a | 3.1 a; 4.85 f; 44.8 g | 4.02 ± 0.18 b | 2.5 c; 3.7 a; 16.2 f |
| Zn | 2.36 ± 0.03 a | 0.81 a; 3.83 f; 7.3 g | 3.84 ± 0.12 b | 1.21 a; 7.30 f |
| Na | 0.985 ± 0.002 a | 15.5 a | 3.42 ± 0.05 | 13.8 c; 18.4 a |
| Moringa Material | Total Dietary Fibre g/100 g w.b. b | % Contribution to Adequate Intake c | Ratio SDF/IDF d, g/g |
|---|---|---|---|
| Seeds | 7.61 ± 0.19 a | 30 ± 1 | 1/2.4 |
| Residual Cake | 12.1 ± 0.3 b | 49 ± 1 | 1/4.2 |
| Moringa Material | Proteins g/100 g w.b. a | % Contribution to PRI b, Adults | % Contribution to RDA c, Adults |
|---|---|---|---|
| Seeds | 31.2 ± 0.1 a | 53.7 ± 0.2 | 55.8 ± 0.2 |
| Residual Cake | 42.1 ± 0.0 b | 72.4 ± 0.0 | 75.2 ± 0.0 |
| Mineral | DRI (1) mg/day | % of Contribution to the DRI (2) | Threshold Molar Ratio InsP6/Mineral (3) | Molar Ratio InsP6/Mineral |
|---|---|---|---|---|
| Ca | ||||
| Seeds | Adults, 1000 | 3.3 | >0.24 | 0.15 |
| Residual Cake | 6.1 | 0.15 | ||
| Fe | ||||
| Seeds | Male/Female, | 25/11 | >0.4 | 3.20 |
| Residual Cake | 8/18 | 50/22 | 2.93 | |
| Zn | ||||
| Seeds | Male/Female, | 22/29 | >5 | 3.03 |
| Residual Cake | 11/8 | 35/48 | 3.51 |
| Component | Units | Saline Solution | Ethanolic solution |
|---|---|---|---|
| Moisture | g/100 g | 8.31 ± 0.03 b | 6.89 ± 0.01 a |
| Proteins, f: 5.4975 (2) (f: 6.25) | g/100 g, d.m. | 45.4 ± 1.9 a (51.6) | 49.0 ± 0.0 b (55.7) |
| Total dietary fibre (TDF) Insoluble (FI) Soluble (FS) | g/100 g, d.m. | TDF: 30.5 ± 3.1 b IDF: 27.9 ± 3.3 b SDF: 2.61 ± 1.34 a | TDF: 17.4 ± 1.5 a FI: 14.1 ± 1.7 a SDF: 3.29 ± 0.17 a |
| Lipids | g/100 g, d.m. | 18.9 ± 0.8 b | 6.29 ± 0.66 a |
| Ash | g/100 g, d.m. | 4.68 ± 0.15 a | 5.86 ± 0.12 b |
| Carbohydrates not included in fibre (3) | g/100 g, d.m. | 0.52 | 21.5 |
| InsP6 | g/100 g, d.m. | 2.98 ± 1.2 b | 1.71 ± 1.3 a |
| InsP6, not accounting for solid losses, % reduction | g/100 g, d.m. % | 1.22 ± 0.29 a 12 | 1.32 ± 0.28 a 5 |
| Ca | mg/100 g d.m. | 99.2 ± 28 b | 65.5 ± 3.7 a |
| Fe | mg/100 g d.m. | 5.50 ± 0.20 b | 3.72 ± 0.21 a |
| Zn | mg/100 g d.m. | 8.55 ± 0.27 b | 4.33 ± 0.29 a |
| Na | mg/100 g d.m. | 73.3 ± 6.7 b | 2.58 ± 0.16 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Gómez, N.; Escobar-García, J.D.; Álvarez, A.A.; Haros, C.M. Revalorization of the Residual Cake from Moringa Seeds as an Alternative Source of Plant-Based Proteins. Biol. Life Sci. Forum 2025, 50, 2. https://doi.org/10.3390/blsf2025050002
Peña-Gómez N, Escobar-García JD, Álvarez AA, Haros CM. Revalorization of the Residual Cake from Moringa Seeds as an Alternative Source of Plant-Based Proteins. Biology and Life Sciences Forum. 2025; 50(1):2. https://doi.org/10.3390/blsf2025050002
Chicago/Turabian StylePeña-Gómez, Nataly, Juan David Escobar-García, Andrea Alonso Álvarez, and Claudia Monika Haros. 2025. "Revalorization of the Residual Cake from Moringa Seeds as an Alternative Source of Plant-Based Proteins" Biology and Life Sciences Forum 50, no. 1: 2. https://doi.org/10.3390/blsf2025050002
APA StylePeña-Gómez, N., Escobar-García, J. D., Álvarez, A. A., & Haros, C. M. (2025). Revalorization of the Residual Cake from Moringa Seeds as an Alternative Source of Plant-Based Proteins. Biology and Life Sciences Forum, 50(1), 2. https://doi.org/10.3390/blsf2025050002






