Abstract
Artificial Light at Night (ALAN) is a recent issue of concern for researchers primarily working on the anthropogenic impacts on animal and ecosystem health. Our concern is associated with the ALAN exposure to an aquatic ecosystem by disrupting the natural dark–light cycle, which is essential for maintaining the overall health of the ecosystem and its inhabitants. In this study, we have attempted to understand the adverse consequences of ALAN in inducing neuro-behavioural stress in a freshwater prawn species (aquatic arthropod) Macrobrachium lamarrei by considering grooming behaviour, a well-established indicator of neurological stress in animals. Our results show that continuous ALAN exposure (for seven days) can increase collective grooming activity in Macrobrachium lamarrei over time. In our experiment, we have used two intensities of ALAN (50 and 120 lux). Although the response (in terms collective grooming) to both intensities are apparently different, our fundamental hypothesis is confirmed, where it is evident that prolonged light exposure can induce an elevation in cumulative grooming performances in a freshwater prawn population.
1. Introduction
Artificial Light at Night (ALAN), or prolonged artificial light exposure during the night time, is a very crucial issue in today’s living world. It is an established fact that alterations in the day–night cycle affects the circadian rhythm of animals, which have direct neuro-behavioural issues. Due to increased urbanisation and severe encroachment in the wildlife premises, ALAN has become a very significant anthropogenic concern for circadian biologists, ecologists and evolutionary biologists [1].
ALAN alters circadian rhythms in animals and therefore can be a source of environmental stress affecting their physiology and behaviour. The impact of ALAN can be related to the increased light level, but also to the spectral composition of night lighting [2]. Light cycles assist populations in organising group behaviour and have a significant influence on a community’s interspecies dynamics. ALAN’s ecological consequences have been studied extensively, and they include increased predation, decreased growth and development, and reduced reproductive attempts. Invertebrates, which account for the great majority of species, have an indisputable role in ecosystem functioning. Many animals have been demonstrated to have strong daily rhythms; therefore, it is critical to understand how ALAN may disturb not just their behavioural patterns, but also their circadian clock [3]. Artificial lights are distinguished by different spatial, temporal, and spectral patterns that can disrupt natural rhythms of light and dark, with effects at all levels of biological organisation. Individually, ALAN can evoke a variety of physiological and behavioural responses related to light-mediated processes such diel activity patterns and predator–prey interactions. ALAN has also been found to alter community composition and trophic structure, which has consequences for ecosystem-level processes such as primary production, nutrient cycling, and the energetic links between aquatic and terrestrial systems [4].
ALAN and other anthropogenic influences can have an impact on animal neuroendocrine systems. It is known that an increasing number of studies find light pollution to alter the behaviour of aquatic invertebrates, such as their movements, grooming activity, habitat choice, and foraging behaviour, but the fitness consequences of these behavioural changes are largely unknown, as are their impacts on populations, communities, and ecosystems. Yet, assessing the consequences of behavioural changes for higher ecological levels is of vital importance given the central role of these invertebrates in ecosystems [5]. The neurotransmission pathway is impacted by arsenic, which leads to recurrent behavioural patterns. By examining repetitive grooming and the associated differential gene expression data (acetyl cholinesterase, neurexin-neuroligins), arsenic-induced neurotoxicity was verified [6]. Research suggests that ALAN exposure may cause neurological damage by inhibiting melatonin production, a strong antioxidant. However, the available data are restricted in both quantity and taxonomy. Short-term ALAN exposure has been related to reduced brain structure volumes in the main eye visual pathway, either due to oxidative damage or plastic alterations in neural investment. Although the effects of ALAN were minor, they gave fresh insights into the probable processes behind its behavioural and physiological effects on this important urban predator [7].
Invertebrate community composition is affected by proximity to street lighting independently of the time of day. Five major invertebrate groups contributed to compositional differences, resulting in an increase in the number of predatory and scavenging individuals in brightly lit communities. Results indicate that street lighting changes the environment at higher levels of biological organisation than previously recognised, raising the concern that it can alter the structure and function of ecosystems [8].
A thorough analysis of the light qualities behind those effects is required to better understand and control the effects of this stressor. Amphipods showed moderate activities when exposed to red lights at night, while isopods shifted some of their activity to daylight hours in two experiments when exposed to blue or amber lights, indicating a potential shift in this species’ circadian rhythm. ALAN lowers nocturnal activity, with specific wavelengths having differing impacts on each species. The differences between amphipods and isopods are most likely due to their unique adaptations to natural low-light habitat circumstances, and hence their sensitivity to ALAN [9]. Gammarids (Gammarus jazdzewskii) (Amphipoda) exposed to ALAN in the absence of predation cues consumed less, compared with darkness, mainly due to their lower activity. Moreover, gammarids showed a stronger response to Light-emitting diode (LED), spending more time in the shelter and increasing prey handling time in this treatment. The addition of predation cues did not enhance the negative impact of ALAN on the foraging success. Gammarids maintained similar consumption levels as in the ALAN treatment without predation cues and in darkness with predation cues. However, gammarids in LED light altered their behaviour in response to predation threat; they decreased prey handling time and consumed prey faster, which may have compensated for the higher food demand in stressful conditions [10]. ALAN significantly disrupted the repeatability of risk-related behaviours in ground-dwelling isopod Porcellionides pruinosus, suggesting individual-level behavioural alterations. At the group level, ALAN-exposed isopods exhibited prolonged freezing durations in response to a looming stimulus, increased shelter-seeking behaviour and reduced dispersal in the terrarium [11]. ALAN identified a number of ecological routes that might subject the water flea Daphnia, a crucial ecological interactor, to intricate and cascading selection pressures. According to this conceptual framework, some pathways of ALAN-induced selection pressures may be unique to freshwater habitats or Daphnia model organisms, but other pathways, such as increased predation pressure, can be brought on by ALAN-induced changes in vertical distribution. Such eco-evolutionary feedback will ultimately expand our knowledge of how light pollution affects ecosystem health since evolutionary processes may modify ecological processes [12].
ALAN has grown to be a powerful pollutant that can alter the biology of both plants and animals. Findings unequivocally demonstrated that different light sources can undoubtedly change how the test insects forage. The egg-laying response was the most intriguing discovery, where the preference that a warmer location is needed to lay their eggs was clear. The grasshoppers were seen to deposit more eggs around the edges of the cages, same as in the control settings where the only light source was natural [13]. Artificial light in riparian areas can reduce emergence in aquatic Diptera and hinder dispersal, with effects that vary depending on the taxon. Because many riparian predators rely on adult aquatic insects as prey, these changes can cascade across aquatic–terrestrial ecosystem boundaries. Given the large number of streetlights that are installed along freshwater shorelines, the observed effects are likely to be of relevance to freshwater bodies around the globe [14].
Bioindicators are species or groups whose responses provide insight into the overall status of an environment. The bioindicators require a specific set of physical or chemical variables, and any changes in presence, absence, morphology, physiology, or behaviour of the species indicate that the variables are outside their preferred limits. Bioindicators often refer to species reactions to human-caused environmental changes, while indicators for ‘natural’ environmental conditions are less common [15].
Our study is designed to conduct a research work for the evaluation of possible neuro-behavioural stress caused by ALAN, in terms of collective grooming pattern alteration over a specific time frame. Precisely, the work focuses animal health aimed to determine the unnatural response of Macrobrachium lamarrei (a freshwater prawn) to anthropogenic light pollution.
2. Methodology
A group of freshwater prawns Macrobrachium lamarrei were sampled from a freshwater pond from a rural area of West Bengal. The sampling point was extremely rural without significant urban manifestations. The prawn population was kept in a glass aquaria in clean tap water for acclimatisation for 7 days. During the acclimatisation time and the experimentation time, the prawns were kept in such a position that they were exposed to natural night sources and were maintained in the natural day–night cycle. Food was provided ad libitum.
To set up for ALAN, an electric lamp was placed 1 foot away from the aquarium. During the experimentation time, every evening, during the sunset, the lamp was switched on until the morning time. The prawns were first exposed to 50 lux of ALAN and after a gap of three weeks (with a normal day–night cycle) the same population was exposed to the higher intensity (120 lux). The neurological stress level was evaluated in terms of collective grooming behaviour in the prawns. Collective grooming behaviour was assessed in terms of grooming bouts in the Macrobrachium lamarrei population, quantified as percentage value. That means, what percentage of M. lamarrei population was undergoing grooming behaviour at a certain time point (also mentioned as collective grooming percentage).
3. Results
Our findings clearly demonstrate that ALAN or prolonged artificial light exposure during the night time can have a notable effect on the population of a freshwater prawn, Macrobrachium lamarrei. In this scenario, M. lamarrei is fundamentally representing the biodiversity in the freshwater ecosystem and our objective is to understand the effect of extended artificial light on the underwater (freshwater) organisms in terms of animal health through ethological assessment. In this study, we have considered the collective grooming percentage, which is basically a very simple form of assessing mass self-grooming by quantifying the number of prawns undergoing robust grooming actions at a time (simultaneously). This parameter effectively indicates the alterations in grooming bouts in a prawn population due to artificial light pollution.
Figure 1A portrays that M. lamarrei, on exposure to 50 lux of intense light (which is approximate to the intensity of normal streetlights), have an irregular collective grooming pattern. After the proximate effect of ALAN, the collective grooming pattern decreased in respect to the control (collective grooming percentage in normal/natural day–night cycle), which was followed by a slight increase and then again a decrease. Up to day 2, 12 h, the collective grooming pattern was below the control level. From day 2, 24 h, the level of collective grooming percentage increases to the level of the control and eventually goes down until day 4, 12 h. From this scenario, there is a sudden notable increase during day 4, 24 h, followed by an alternative slight decrease and increase pattern (Figure 1B). However, from day 4, 24 h onwards, the collective grooming percentage level is notably higher than the control. Contrastingly, M. lamarrei, exposed to the higher artificial light intensity (120 lux), which is more than double the intensity of streetlights, demonstrated a more consistent pattern of increased collective grooming percentage, in respect to the control.
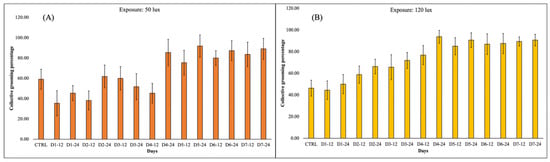
Figure 1.
(A): Demonstration of the collective grooming pattern in Macrobrachium lamarrei, exposed to 50 lux intense light source. (B): Demonstration of the collective grooming pattern in Macrobrachium lamarrei, exposed to 120 lux intense light source.
We found the peak of collective grooming activity for prawns exposed to 50 lux on day 5, 24 h. However, the peak collective grooming activity for prawns exposed to 120 lux was found on day 4, 24 h. It is fact that all the 12 h data were collected during dawn time (after a whole night of ALAN exposure); however, we can confirm that extreme artificial light exposure can increase the neuro-behavioural stress in animals during daytime also. Regression analysis (Figure 2A,B) showed that time versus average collective grooming percentage during 120 lux intensity is more strongly correlated than prawns exposed to 50 lux intensity.
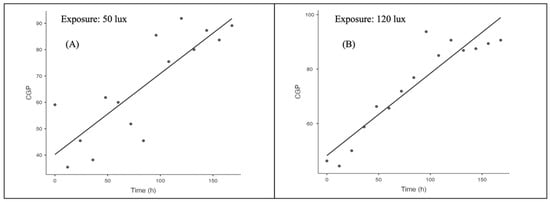
Figure 2.
(A): Demonstration of the regression plot of time versus average collective grooming percentage during 50 lux intensity. (B): Demonstration of the regression plot of time versus average collective grooming percentage during 120 lux intensity. CGP refers to Collective Grooming Percentage.
4. Discussion
Anthropogenic activities in today’s world have significantly impacted the ecosystem and the biodiversity [16,17]. These unnatural anthropogenic activities broadly encompass pollution (environmental contamination), urbanisation, encircling the issues of human-made deforestation, ALAN and artificial sound over exposure, and many more. In fact, considering the broader ecological perspectives, it is of utmost concern for behavioural ecologists and evolutionary biologists to understand the mechanisms of animal response by adjusting their physiology and behaviour, and if it can be predicted how organismal evolution can take a turn in this highly populated human-influenced world, with diverse artificial environmental trepidation. ALAN is a critical anthropogenic threat effectively impacting the biological clock of animals and humans and has significant effect on the brain–behaviour circuit. In today’s world of expanding urbanisation, assessment of the light toxicity in animals of all phyla is necessary for organismal research and is also crucial in understanding how prolonged light exposure induces the adaptiveness to the imbalance in the day–night cycle.
In our study, we consider the grooming (self-grooming) behaviour which is already established to be a biomarker of neuro-behavioural stress in animals [18]. Our research objectives broadly include behavioural ecology and the impact of anthropogenic effects in altering natural behavioural patterns in animals. This proposed work is a population-based study, where the collective grooming pattern was measured as the behavioural marker to assess the effect of ALAN on the mass brain–behaviour circuit.
Our working hypothesis starts with the notion that light pollution can create stress in animals. Hence, it can be postulated that a prolonged artificial light exposure can alter the brain–behaviour interface in aquatic invertebrates in respect to the disruption of the circadian rhythm and thus create neuro-behavioural abnormalities. This work falls under the animal health perspectives and its correlation with environmental pollution or ecological perturbation. Findings strongly indicated that ALAN could induce an elevation in the collective grooming activity (compared to their usual or natural dark/light environment) in our freshwater model organism, Macrobrachium lamarrei. Here, elevated grooming actions mean more physiological stress manifested as behavioural plasticity.
Author Contributions
Conceptualization, formal analysis, writing, supervision, C.M.; Methodology, writing, data curation, F.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was done strictly following the “Guidelines for the ethical treatment of nonhuman animals in behavioural research and teaching” published by ASAB Ethical Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
Analyzed data is provided in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Munshi, C. ALAN (Artificial Light At Night): A possible anthropogenic hazard to alter grooming activity in prawns. In Proceedings of the European Conference on Behavioural Biology, Zurich, Switzerland, 16–19 July 2024. [Google Scholar]
- Czarnecka, M.; Jermacz, Ł.; Glazińska, P.; Kulasek, M.; Kobak, J. Artificial light at night (ALAN) affects behaviour, but does not change oxidative status in freshwater shredders. Environ. Pollut. 2022, 306, 119476. [Google Scholar] [CrossRef] [PubMed]
- Underwood, C. The Impacts of Artificial Light at Night on Gammarid Crustaceans. Ph.D. Thesis, University of Southampton, Southampton, UK, 2024. [Google Scholar]
- Zapata, M.J.; Sullivan, S.M.P.; Gray, S.M. Artificial lighting at night in estuaries—Implications from individuals to ecosystems. Estuaries Coasts 2019, 42, 309–330. [Google Scholar] [CrossRef]
- Ganguly, A.; Candolin, U. Impact of light pollution on aquatic invertebrates: Behavioral responses and ecological consequences. Behav. Ecol. Sociobiol. 2023, 77, 104. [Google Scholar] [CrossRef]
- Munshi, C.; Mukhuty, A.; Bandyopadhyay, A.; Mondal, P.; Bhowmik, A.D.; Shaw, P.; Bhattacharya, S. Arsenic-induced neurotoxicity: A study on the brain–behaviour circuit. Proceedings 2024, 102, 33. [Google Scholar]
- Willmott, N.J.; Black, J.R.; McNamara, K.B.; Wong, B.B.; Jones, T.M. The effects of artificial light at night on spider brains. Biol. Lett. 2024, 20, 20240202. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.W.; Bennie, J.; Gaston, K.J. Street lighting changes the composition of invertebrate communities. Biol. Lett. 2012, 8, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Ahumada, D.; Quijón, P.A.; Jahnsen-Guzmán, N.; Lynn, K.D.; Pulgar, J.; Palma, J.; Manríquez, P.H.; Duarte, C. Splitting light pollution: Wavelength effects on the activity of two sandy beach species. Environ. Pollut. 2024, 356, 124317. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Kobak, J.; Czarnecka, M. Artificial light at night alters foraging behavior of freshwater amphipods depending on the light spectrum and the presence of predation cues. Curr. Zool. 2024, 70, zoae061. [Google Scholar] [CrossRef]
- Dissegna, A.; Chiandetti, C. Artificial light at night alters risk-related behaviors of the ground-dwelling isopod Porcellionides pruinosus. J. Exp. Biol. 2025, 228, JEB249626. [Google Scholar] [CrossRef] [PubMed]
- Tüzün, N.; De Meester, L.; Hölker, F. Eco-evolutionary feedbacks under artificial light at night. iScience 2025, 28, 107123. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A. Light dependent host plant choice, foraging and oviposition site selection in common Indian grasshopper Spathosternum prasiniferum (Orthoptera: Acrididae). Int. J. Trop. Insect Sci. 2024, 44, 2863–2868. [Google Scholar] [CrossRef]
- Manfrin, A.; Hölker, F.; Teurlincx, S.; Baranov, V.; van Grunsven, R.H.A.; Bundschuh, M.; Monaghan, M.T. Artificial light at night reduces emergence and attracts flying adults of aquatic Diptera. Aquat. Sci. 2025, 87, 38. [Google Scholar] [CrossRef]
- Gerhardt, A. Bioindicator species and their use in biomonitoring. Environ. Monit. 2002, 1, 77–123. [Google Scholar]
- Marangoni, L.F.; Davies, T.; Smyth, T.; Rodríguez, A.; Hamann, M.; Duarte, C.; Pendoley, K.; Berge, J.; Maggi, E.; Levy, O. Impacts of artificial light at night (ALAN) in marine ecosystems—A review. Glob. Change Biol. 2024; in press. [Google Scholar]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The biological impacts of artificial light at night: The research challenge. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140133. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Stewart, A.M.; Song, C.; Berridge, K.C.; Graybiel, A.M.; Fentress, J.C. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 2016, 17, 45–59. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).