Antioxidant Enzymatic Activity of Extracts from Hairy Roots of Root-Lesion-Nematode-Susceptible and -Resistant Cultivars of Medicago sativa †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Growth of Plant Material and Bacteria
2.3. Establishment of Alfalfa Transgenic Roots
2.4. Thiobarbituric Acid-Reactive Substance (TBARS) Assay
2.5. Guaiacol Peroxidase Activity
2.6. Data Treatment and Statistical Analysis
3. Results and Discussion
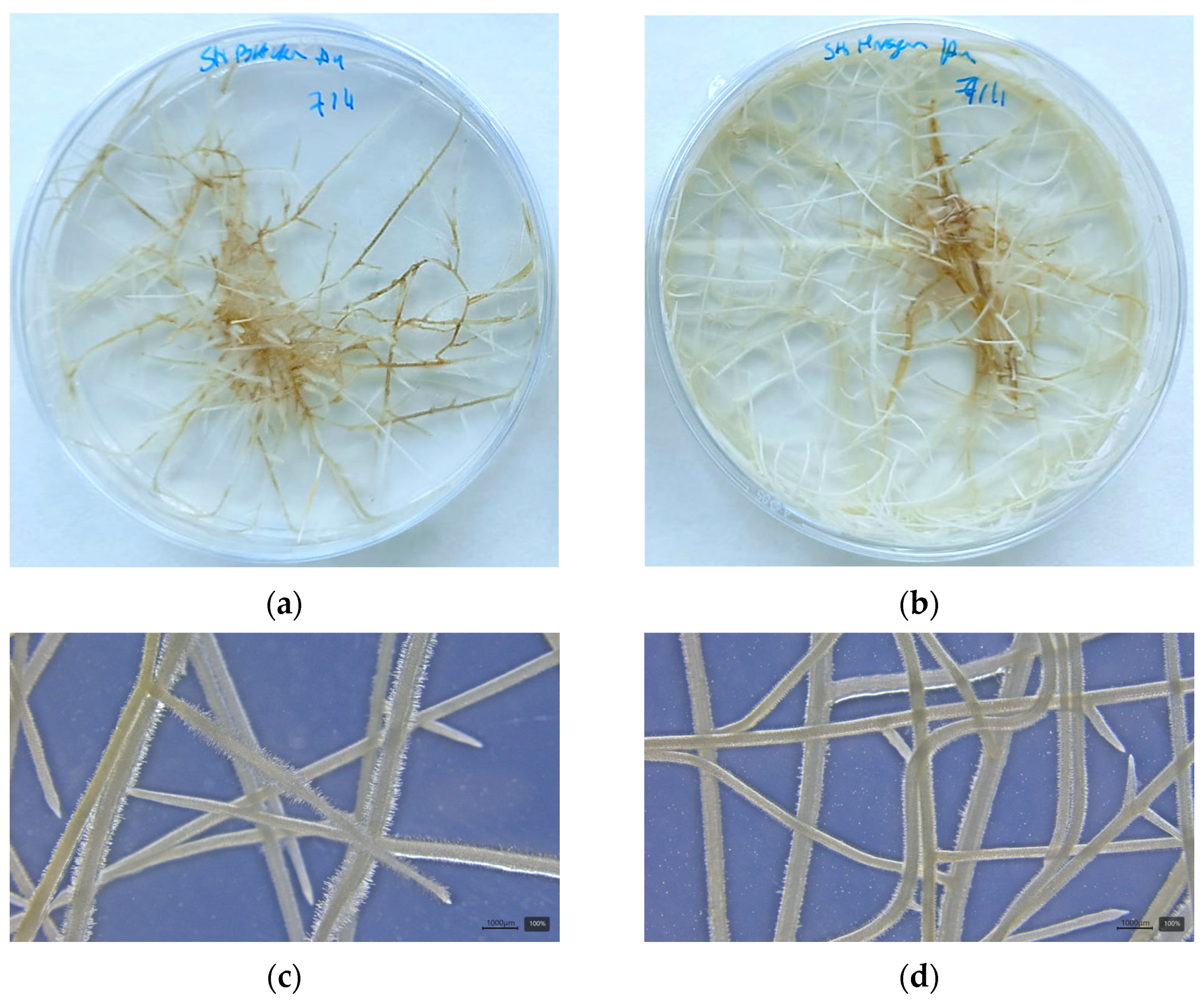
3.1. Susceptible and Resistant Alfalfa Hairy Roots
3.2. Thiobarbituric Acid-Reactive Substances (TBARS) and Activity of Guaiacol Peroxidase
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenicity and Management; BRILL: Leiden, The Netherlands, 2007; Volume 6, ISBN 9789004155640. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 Plant-Parasitic Nematodes in Molecular Plant Pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed]
- Viketoft, M.; Flöhr, A.; Englund, J.-E.; Kardell, J.; Edin, E. Additive Effect of the Root-Lesion Nematode Pratylenchus Penetrans and the Fungus Rhizoctonia Solani on Potato Yield and Damage. J. Plant Dis. Prot. 2020, 127, 821–829. [Google Scholar] [CrossRef]
- MacGuidwin, A.E.; Rouse, D.I. Role of Pratylenchus Penetrans in the Potato Early Dying Disease of Russet Burbank Potato. Phytopathology 1990, 80, 1077. [Google Scholar] [CrossRef]
- Fatemi, E.; Jung, C. Pathogenicity of the Root Lesion Nematode Pratylenchus Neglectus Depends on Pre-Culture Conditions. Sci. Rep. 2023, 13, 19642. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.K.; Fosu-Nyarko, J. Molecular Biology of Root Lesion Nematodes (Pratylenchus spp.) and Their Interaction with Host Plants. Ann. Appl. Biol. 2014, 164, 163–181. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Nijs, L.d.; Hockland, S.; Maafi, Z.T. Current Nematode Threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J.T., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar]
- Vieira, P.; Mowery, J.; Eisenback, J.D.; Shao, J.; Nemchinov, L.G. Cellular and Transcriptional Responses of Resistant and Susceptible Cultivars of Alfalfa to the Root Lesion Nematode, Pratylenchus Penetrans. Front. Plant Sci. 2019, 10, 971. [Google Scholar] [CrossRef]
- Hafez, S.L.; Sundararaj, P.; Miller, D. Reaction of Alfalfa Cultivars to the Root Lesion Nematode Pratylenchus Penetrans. Nematol. Mediterr. 2006, 34, 161–163. [Google Scholar]
- Baldridge, G.D.; O’Neill, N.R.; Samac, D.A. Alfalfa (Medicago sativa L.) Resistance to the Root-Lesion Nematode, Pratylenchus Penetrans: Defense-Response Gene MRNA and Isoflavonoid Phytoalexin Levels in Roots. Plant Mol. Biol. 1998, 38, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, C.; Luo, K. Biosynthesis and Metabolic Engineering of Isoflavonoids in Model Plants and Crops: A Review. Front. Plant Sci. 2024, 15, 1384091. [Google Scholar] [CrossRef]
- Polturak, G.; Misra, R.C.; El-Demerdash, A.; Owen, C.; Steed, A.; McDonald, H.P.; Wang, J.; Saalbach, G.; Martins, C.; Chartrain, L.; et al. Discovery of Isoflavone Phytoalexins in Wheat Reveals an Alternative Route to Isoflavonoid Biosynthesis. Nat. Commun. 2023, 14, 6977. [Google Scholar] [CrossRef]
- Edens, R.M.; Anand, S.C.; Bolla, R.I. Enzymes of the Phenylpropanoid Pathway in Soybean Infected with Meloidogyne Incognita or Heterodera Glycines. J. Nematol. 1995, 27, 292–303. [Google Scholar] [PubMed]
- Faria, J.M.S.; Barbosa, P.; Figueiredo, A.C.; Mota, M.; Vicente, C.S.L. In Vivo and In Vitro Infection of Potato Roots with Plant Parasitic Nematodes for the Assessment of Induced Structural Changes. JoVE 2025, 216, e67756. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Aravind, P.; Prasad, M.N.V. Zinc Alleviates Cadmium-Induced Oxidative Stress in Ceratophyllum demersum L.: A Free Floating Freshwater Macrophyte. Plant Physiol. Biochem. 2003, 41, 391–397. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

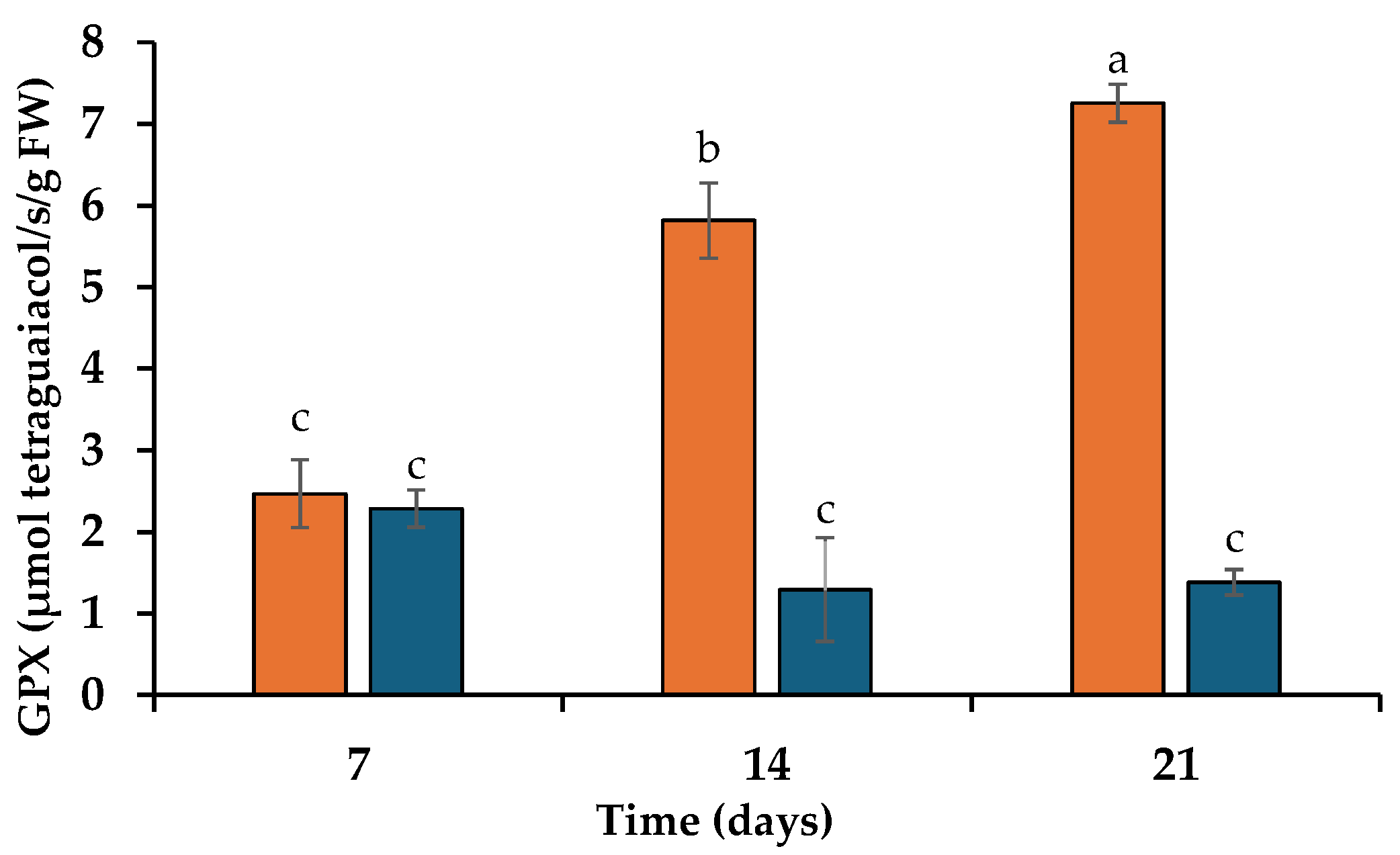
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, G.; Vicente, C.; Faria, J.M.S. Antioxidant Enzymatic Activity of Extracts from Hairy Roots of Root-Lesion-Nematode-Susceptible and -Resistant Cultivars of Medicago sativa . Biol. Life Sci. Forum 2025, 41, 13. https://doi.org/10.3390/blsf2025041013
Pereira G, Vicente C, Faria JMS. Antioxidant Enzymatic Activity of Extracts from Hairy Roots of Root-Lesion-Nematode-Susceptible and -Resistant Cultivars of Medicago sativa . Biology and Life Sciences Forum. 2025; 41(1):13. https://doi.org/10.3390/blsf2025041013
Chicago/Turabian StylePereira, Gonçalo, Cláudia Vicente, and Jorge M. S. Faria. 2025. "Antioxidant Enzymatic Activity of Extracts from Hairy Roots of Root-Lesion-Nematode-Susceptible and -Resistant Cultivars of Medicago sativa " Biology and Life Sciences Forum 41, no. 1: 13. https://doi.org/10.3390/blsf2025041013
APA StylePereira, G., Vicente, C., & Faria, J. M. S. (2025). Antioxidant Enzymatic Activity of Extracts from Hairy Roots of Root-Lesion-Nematode-Susceptible and -Resistant Cultivars of Medicago sativa . Biology and Life Sciences Forum, 41(1), 13. https://doi.org/10.3390/blsf2025041013







