White Wine Pomace Mitigates Hypoxia in 3D SH-SY5Y Model †
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Gastrointestinal Digestion and Colonic Fermentation of White Wine Pomace Product
2.2. Cell Culture and Treatment
2.3. Cell Death Assessment
2.4. Intracellular Reactive Oxygen Species (ROS) Assessment
2.5. Quantitative Real-Time PCR Analysis (qPCR)
2.6. Statistical Analysis
3. Results and Discussion
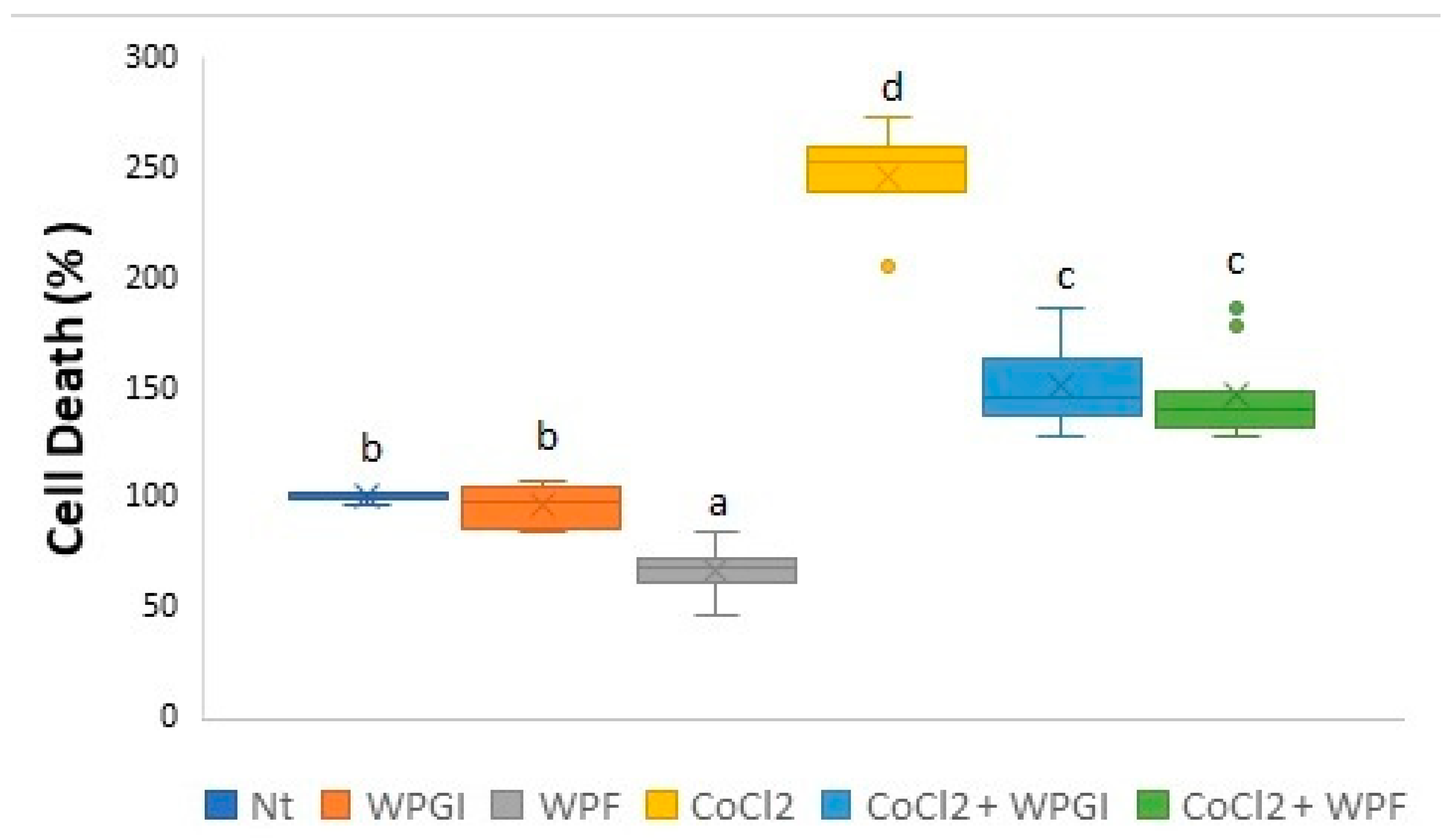
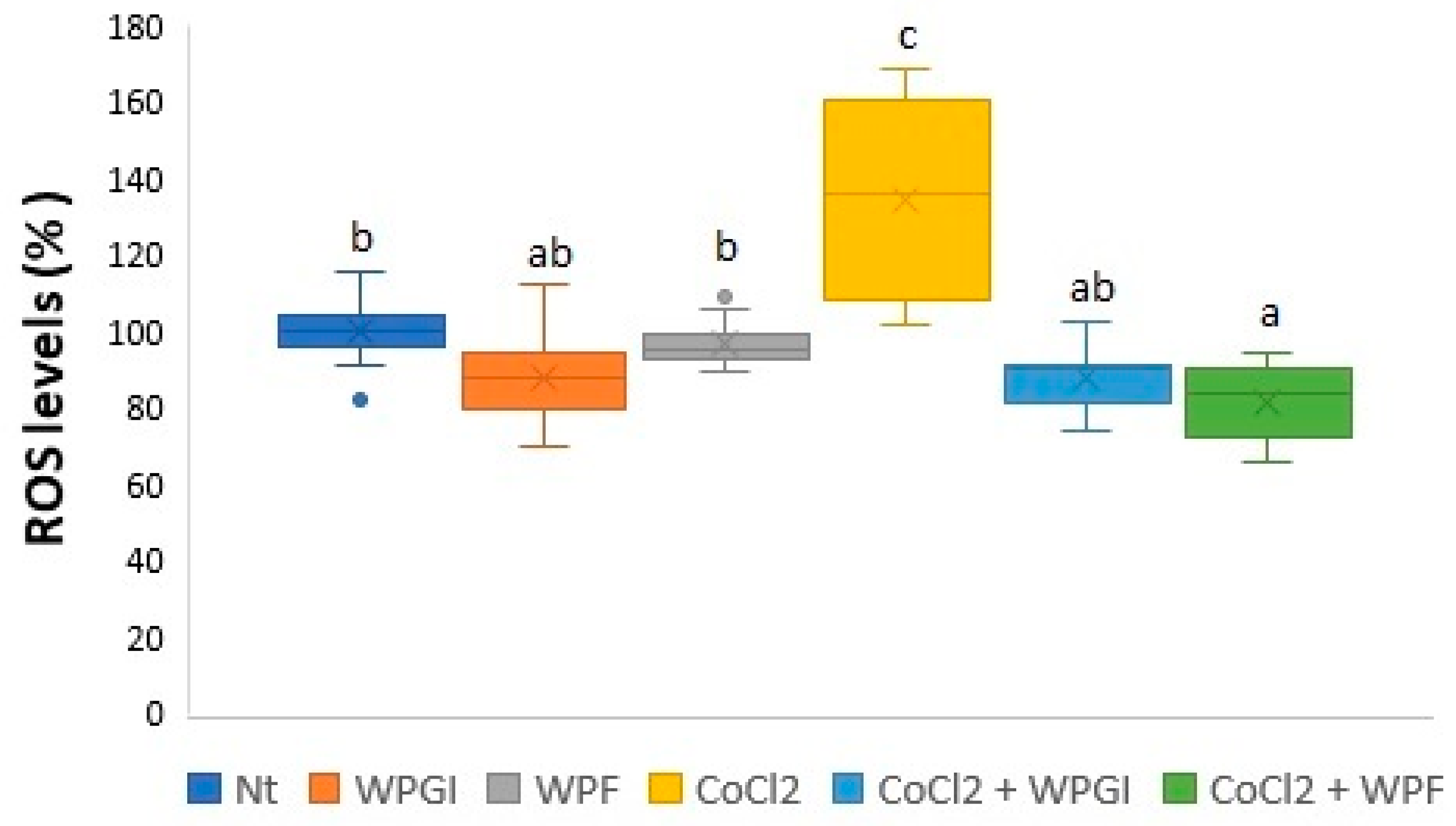
3.1. Hypoxia-Induced Cell Death and ROS Production
3.2. wWPP Modulation of Molecular Pathways in Hypoxia Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef]
- Lee, H.-S.; Jeong, G.-S. Protective Effects of 6,7,4′-Trihydroxyflavanone on Hypoxia-Induced Neurotoxicity by Enhancement of HO-1 through Nrf2 Signaling Pathway. Antioxidants 2021, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L.; Del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz-Rodríguez, P. Antioxidant and antimicrobial properties of wine byproducts and their potential uses in the food industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, G.; Cavia-Saiz, M.; Muñiz, P. From winery by-product to healthy product: Bioavailability, redox signaling and oxidative stress modulation by wine pomace product. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–23. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, V.; Rivero-Perez, M.D.; Gerardi, G.; Muñiz, P.; González-SanJose, M.L.; Jaime, I.; Cavia-Saiz, M. Influence of the packaging systems on the phenolic profile and antioxidant properties of wine pomace used as seasoning in chicken meat. Food Chem. 2023, 427, 136625. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. The protective effects of wine pomace products on the vascular endothelial barrier function. Food Funct. 2020, 11, 7878–7891. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Štampar, M.; Tomc, J.; Filipič, M.; Žegura, B. Development of in vitro 3D cell model from hepatocellular carcinoma (HepG2) cell line and its application for genotoxicity testing. Arch. Toxicol. 2019, 93, 3321–3333. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Sampson, D.A.; Hoehner, J.C. Neuroblastoma survival and death: An in vitro model of hypoxia and metabolic stress. J. Surg. Res. 2004, 116, 288–296. [Google Scholar] [CrossRef]
- Cho, Y.-D.; Choi, S.-H.; Yoon, Y.-H.; Kim, J.-Y.; Park, S.-J.; Lim, C.-S. The Effects of Oxygen and Treatments in Hypoxic Conditions in SH-SY5Y Cells. Shock 2018, 50, 449. [Google Scholar] [CrossRef]
- Yoo, S.-Y.; Yoo, J.-Y.; Kim, H.-B.; Baik, T.-K.; Lee, J.-H.; Woo, R.-S. Neuregulin-1 Protects Neuronal Cells Against Damage due to CoCl2-Induced Hypoxia by Suppressing Hypoxia-Inducible Factor-1α and P53 in SH-SY5Y Cells. Int. Neurourol. J. 2019, 23 (Suppl. S2), S111. [Google Scholar] [CrossRef]
- Park, C.H.; Park, J.Y.; Cho, W.G. Chemical Hypoxia Induces Pyroptosis in Neuronal Cells by Caspase-Dependent Gasdermin Activation. Int. J. Mol. Sci. 2024, 25, 2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Ding, Y.; Piao, M.; Feng, C.; Chi, G.; Luo, Y.; Ge, P. Endoplasmic reticulum stress regulates oxygen-glucose deprivation-induced parthanatos in human SH-SY5Y cells via improvement of intracellular ROS. CNS Neurosci. Ther. 2018, 24, 29–38. [Google Scholar] [CrossRef]
- Marrazzo, P.; Angeloni, C.; Hrelia, S. Combined Treatment with Three Natural Antioxidants Enhances Neuroprotection in a SH-SY5Y 3D Culture Model. Antioxidants 2019, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Goua, M.; Yates, K.; Bermano, G.; Russell, W.R.; Maciel, P.; Lin, P.K.T. Impact of rapeseed pomace extract on markers of oxidative stress and DNA damage in human SH-SY5Y cells. J. Food Biochem. 2021, 45, e13592. [Google Scholar] [CrossRef]
- Gerardi, G.; Cavia-Saiz, M.; Rivero-Pérez, M.D.; González-SanJosé, M.L.; Muñiz, P. Wine pomace product modulates oxidative stress and microbiota in obesity high-fat diet-fed rats. J. Funct. Foods 2020, 68, 103903. [Google Scholar] [CrossRef]
- Brum, P.O.; Viola, G.D.; Saibro-Girardi, C.; Tiefensee-Ribeiro, C.; Brum, M.O.; Gasparotto, J.; Krolow, R.; Moreira, J.C.F.; Gelain, D.P. Hypoxia-Inducible Factor-1α (HIF-1α) Inhibition Impairs Retinoic Acid-Induced Differentiation in SH-SY5Y Neuroblastoma Cells, Leading to Reduced Neurite Length and Diminished Gene Expression Related to Cell Differentiation. Neurochem. Res. 2022, 47, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Duong, T.T.H.; Antao, S.; Ellis, N.A.; Myers, S.J.; Witting, P.K. Supplementation with a synthetic polyphenol limits oxidative stress and enhances neuronal cell viability in response to hypoxia–re-oxygenation injury. Brain Res. 2008, 1219, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, O.; Amit, T.; Youdim, M.B.H. The application of proteomics for studying the neurorescue activity of the polyphenol (−)-epigallocatechin-3-gallate. Arch. Biochem. Biophys. 2008, 476, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Yang, Y.; Li, L.; Zhao, Q.; Li, Y.; Liu, Z.; Hao, L.; Guo, B.; Diao, A. The novel prolyl hydroxylase-2 inhibitor caffeic acid upregulates hypoxia inducible factor and protects against hypoxia. Eur. J. Pharmacol. 2022, 934, 175307. [Google Scholar] [CrossRef]
- Smirnova, N.A.; Kaidery, N.A.; Hushpulian, D.M.; Rakhman, I.I.; Poloznikov, A.A.; Tishkov, V.I.; Karuppagounder, S.S.; Gaisina, I.N.; Pekcec, A.; Van Leyen, K.; et al. Bioactive Flavonoids and Catechols as Hif1 and Nrf2 Protein Stabilizers—Implications for Parkinson’s Disease. Aging Dis. 2016, 7, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, Y.; Wang, H.; Zhao, L.; Ma, Z.; Li, T.; Liu, J.; Sun, M.; Jian, Y.; Yao, L.; et al. Edaravone reduces Aβ-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life Sci. 2019, 221, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-C.; Nicol, C.J.B.; Lo, S.-S.; Hung, S.-W.; Wang, C.-J.; Lin, C.-H. Resveratrol Mitigates Oxygen and Glucose Deprivation-Induced Inflammation, NLRP3 Inflammasome, and Oxidative Stress in 3D Neuronal Culture. Int. J. Mol. Sci. 2022, 23, 11678. [Google Scholar] [CrossRef]
- Wu, L.; Xu, H.; Cao, L.; Li, T.; Li, R.; Feng, Y.; Chen, J.; Ma, J. Salidroside Protects against MPP+-Induced Neuronal Injury through DJ-1-Nrf2 Antioxidant Pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 5398542. [Google Scholar] [CrossRef] [PubMed]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell. Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Khatri, R.; Jaiswal, A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014, 66, 36–44. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez-González, V.; Gerardi, G.; Sendra, M.; Muñiz, P.; Cavia-Saiz, M. White Wine Pomace Mitigates Hypoxia in 3D SH-SY5Y Model. Biol. Life Sci. Forum 2024, 40, 31. https://doi.org/10.3390/blsf2024040031
Gutiérrez-González V, Gerardi G, Sendra M, Muñiz P, Cavia-Saiz M. White Wine Pomace Mitigates Hypoxia in 3D SH-SY5Y Model. Biology and Life Sciences Forum. 2024; 40(1):31. https://doi.org/10.3390/blsf2024040031
Chicago/Turabian StyleGutiérrez-González, Víctor, Gisela Gerardi, Marta Sendra, Pilar Muñiz, and Mónica Cavia-Saiz. 2024. "White Wine Pomace Mitigates Hypoxia in 3D SH-SY5Y Model" Biology and Life Sciences Forum 40, no. 1: 31. https://doi.org/10.3390/blsf2024040031
APA StyleGutiérrez-González, V., Gerardi, G., Sendra, M., Muñiz, P., & Cavia-Saiz, M. (2024). White Wine Pomace Mitigates Hypoxia in 3D SH-SY5Y Model. Biology and Life Sciences Forum, 40(1), 31. https://doi.org/10.3390/blsf2024040031





