Abstract
The African tropical fruits velvet tamarind, African locust beans and baobab are used as foods and for medicinal purposes, and they are important nutrient and bioactive compound sources. This research focused on the nutritional composition and bioactive compounds in the fruit pulps and seeds of these plant species. Quantification and profiling of sugars, organic acids and phenolic compounds (HPLC), soluble and insoluble fibre content analysis (enzymatic method) and elemental analysis (XRF) were performed. The total phenolic compounds and antioxidant capacity contents (spectrophotometry) and fatty acids quantification profiles (GC) were also accessed. Several bioactive compounds were quantified. Generally, fruit pulps are richer in sugars and organic acids, and seeds are richer in protein and fat.
Keywords:
phenolic compounds; organic acids; sugars; protein; fat; amygdalin; velvet tamarind; African locust beans; baobab 1. Introduction
The tropical fruits black velvet tamarind from Dialium guineense Willd, African locust beans from Parkia biglobosa Jacq. and baobab from Andansonia digitata L. are represented by their respective plants in the pictures in Figure 1 and are an important part of the sub-Saharan African culture because of their traditional uses and medicinal properties.

Figure 1.
The fruits of the tropical plants: (1) velvet tamarind (Dialium guineense Willd); (2) African locust beans (Parkia biglobosa Jacq.); and (3) baobab (Andansonia digitata L.) [1,2].
These tropical fruits are mostly used in Africa as foods, usually as raw materials for beverage production, and even for medicinal purposes, acting as important nutrient and bioactive compound sources [1,2]; however, few scientific studies on their compositions and activities can be found in the scientific literature.
Velvet tamarind pulp was studied by Abiodum et al., in 2017, with regard to its sugar content via proximate and sensorial analysis, which showed general acceptability in terms of its taste, appearance and aroma; it was also determined to be a good source of carbohydrates with a high percentage of glucose and fructose, and its use in a beverage lowered its pH, making it acidic [3]. In 2023, Ogbuewu et al. claimed that velvet tamarind fruit is an excellent source of essential oils and a rich source of dietary fibre, minerals and vitamins [4]. The plant possesses beneficial phytochemicals such as alkaloids, tannins, phenols and flavonoids with beneficial pharmacological effects, including antioxidant and antimicrobial properties [4].
In 2021, Aborisade et al. stated that African locust beans from Nigeria had a high sugar content and several micro, macro and trace elements with importance to human health and that the plant was also free of harmful elements that could be hazardous to human health when consumed or used as a herbal medicine [5].
Baobab of several Angolan origins was studied by Monteiro et al. in 2022, showing the influence of abiotic factors on nutrient and bioactive compounds and also the importance of its vitamin C content [6], which is higher than that in oranges or kiwis. In 2009, Chadare et al. claimed that the consumption of 40 g of baobab fruit pulp provided 100% of the recommended daily intake of vitamin C for pregnant women (19–30 years) [7].
Knowledge of the nutritional value of these tropical wild fruits can contribute to a better understanding of their essential role in terms of food and trade, as Kouassi et al. claimed in 2018 in their study on the biochemical characterisation of the juices consumed in Côte d’Ivoire from the wild fruits baobab and African locust beans [8].
These tropical fruits, which are little known and rarely consumed in Europe, are widely consumed among emigrant and Afro-descendant communities, where they are appreciated as a popular and culturally relevant food. Their consumption as novel snacks has been studied, especially for their ability to nutritionally enhance foods [9].
This research focused on the nutritional composition and bioactive compound content in the fruit pulp and seeds of the three tropical plant species Dialium guineense Willd (Fabaceae family), Parkia biglobosa Jacq. (Fabaceae family) and Andansonia digitata L. (Malvaceae family), originating from Guinea-Bissau.
2. Materials and Methods
2.1. Plant Materials
The studied tropical fruit samples from Guinean-origin native plant species Dialium guineense Willd, Parkia biglobosa Jacq. and Andansonia digitata L. were left to dry on their trees before harvesting. The tropical fruits samples were purchased in a market in Guinea-Bissau, placed in hermetic plastic bags and transported to Portugal (Lisbon) by plane. They were manually separated into pulp and seeds (Figure 2 and Figure 3) at the INIAV laboratory.

Figure 2.
The fruits velvet tamarind (1), African locust beans (2) and baobab (3) as received from Guinea-Bissau.

Figure 3.
The pulps (A) and seeds (B) after being manually separated from the tropical fruits velvet tamarind (1), African locust beans, ALBs, (2) and baobab (3).
Each pulp (A) and seed (B) of the three vegetal materials was dried in a forced-air circulation stove (MMMed Center Group, Venticell, München, Germany) at 60 °C until constant weight, after which they were ground into a powder (IKA, Yellow line A10, Staufen, Germany) and sieved to less than 500 µm in size.
All reactants, standards and solvents were purchased from Merck, Sigma or Panreac (José Manuel Gomes dos Santos, Lisbon, Portugal) and were of analytical or chromatographic grade. To prepare the solutions, ultrapure water (model Milli-Q 7000, Millipore SAS, Molsheim, France) was used. Mobile phases were filtered under vacuum (pump GAST, model DOAP104-BN, Benton Harbor, MI, USA) with a 0.45 μm nylon membrane and degassed in an ultrasonic bath (Bransonic, Branson 5200, Branson, MO, USA) for at least 25 min.
2.2. Nutricional Analysis
Moisture content analysis was performed in triplicate by a gravimetric method [10], accurately weighing 1 g of sample (balance AG245, Mettler Toledo, Schweiz, Switzerland) to a stainless-steel Petri dish and drying in an air oven at 103 °C (U50, Memmert, Schwabach, Germany) with an open lid, until constant weight.
Ash was also quantified in triplicate by a gravimetric method [10], accurately weighing 1 g of sample (balance AG245, Mettler Toledo, Schweiz, Switzerland) into a ceramic crucible placed on a hotplate (Snijders, Thermal Stirrer 34533, Tilburg, The Netherlands) and, after being carbonised, transferring the sample to a muffle furnace (Thermolyne Muffle Furnace, model 48000, Dubuque, IA, USA) at 550 °C for 5 h and then allowing it to cool in the desiccator before the residue was weighed.
To calculate crude protein content, a conversion factor of 6.25 [6] was used to convert the quantified nitrogen content, determined by the Kjeldahl method using the standard ISO 1871:2009 [11] with the digestor (Foss, Tecator 2020 Digestor, Hillerød, Denmark) and the automatic distiller (Foss, 2300 Kjeltec analyzer unit, Hillerød, Denmark) [12]. Nitrogen analyses were carried out in triplicate.
Fat extraction was performed in duplicate for the pulp and seed samples by hot solvent extraction with petroleum ether in a Soxhlet extractor [6,13]. After cooling and weighing, for the total sample fat determination, a portion of fat was prepared for fatty acid analysis. The fatty acid constituents of the triacylglycerols were derivatised for chromatographic analysis using the cold transesterification method [14].
The fatty acid of the lipid phase was identified and quantified via gas chromatography with a flame ionisation detector (GC-FID, Trace GC 2000, Thermo Quest, CE Instruments, Rodano, Milan, Italy) and a DB 23 separation column (J & W Scientific, Agilent Technologies, Santa Clara, CA, USA) [6]. Due to the pulp’s low fat content, the fatty acid GC profile was only performed in the seed samples.
The total, soluble and insoluble dietary fibre contents (TDF, SDF and IDF) were determined by an enzymatic gravimetric procedure in duplicate and only in the fruit pulps using the Megazyme fibre kit K-TDFR (José Manuel Gomes dos Santos, Lisbon, Portugal) according to the kit procedures and Monteiro et al., 2022 [6].
The free sugars profile identification and quantification were performed using high-performance liquid chromatography with a refractive index detector, HPLC-RI, with an autosampler (model 717plus), binary pump (model 510), column oven (model Col. HTR.) and detector RI (model 2414, Waters, Milford, MA, USA) using a Sugar Pak separation column (Waters, Milford, MA, USA) as used by Panda et al., 2022 [15].
The profile determination and quantification of organic acids was performed using HPLC-PDA (photodiode array detector) (Waters Alliance 2695 with PDA 996, Waters, Milford, MA, USA) with a Rezex™ ROA column (Phenomenex, Torrance, CA, USA) as used by Cartas et al., 2024 [16].
2.3. Biactive Content Analisys
The extraction for the phenolic compounds’ analysis was performed in triplicate for pulp and seed samples with Polytron homogenisation (Ika, Ultra-Turrax T25, Staufen, Germany) for 1 min, using 1 g of sample in 15 mL of methanol (100%), followed by an ultrasonic bath (Bransonic, Branson 5200, Branson, MO, USA), for 5 min and leaving on a rotary shaker (Robbins Scientific, model 16021, Sunnyvale, CA, USA) overnight in the dark at 4 °C (refrigerator Radiber, Sa, UKS5000, Barcelona, Spain), followed by centrifugation (Sigma, model 2K15, rotor 12139H, Osterode am Harz, Germany) for 20 min at 4500× g to allow further analysis of the methanolic extracts’ phenolic compounds [6,16].
The total phenolic content (TPC) was analysed spectrophotometrically at 725 nm (Jasco V-530 UV/Vis, Tokyo, Japan) [17,18] with a dilution of 150 μL of the obtained extract with 2400 μL of ultrapure water and the addition of 150 μL of Folin–Ciocalteu reactant 0.25 N, followed by mixing and a 3 min incubation in the dark at room temperature, adding 300 μL of sodium carbonate 1 N and leaving the sample for 2 h in the dark at room temperature. The TPC was expressed in mg of gallic acid equivalent (GAE) per 100 g sample [18].
Antioxidant capacity was determined in the methanolic extracts using the DPPH (2,2-diphenyl-1picrylhydrazyl) spectrophotometric method according to the procedures described by Brand-Williams et al. 1995 [19], with modifications by Monteiro et al., 2022, and Pereira et al., 2023 [6,18]. Absorbance was measured at 580 nm using a spectrophotometer (Jasco V-530 UV/Vis, Tokyo, Japan). A calibration curve was prepared using Trolox as a standard in the range of 25 to 800 μM. The results were expressed as mmol of Trolox equivalent (TE) per 100 g sample [16].
The profile determination and quantification of phenolic compounds were performed using HPLC-PDA (Waters Alliance 2695 with a 2996 PDA, Milford, MA, USA) and the separation was performed in a Synergy Hydro column (Phenomenex, Torrance, CA, USA) [18]. The limit of detection was 0.5 mg/100 g dry matter (DM).
The vitamin C content of the fruit pulps and seeds was determined by HPLC as described in the European Standard EN 14130, 2003 [20]. The extractions [20] and the HPLC-PDA assay with a Spherisorb ODS2 separation column (Waters, Milford, MA, USA) were performed in triplicate as described by Monteiro et al., 2022 [6]. The limits of detection and quantification were 15 mg/100 g and 25 mg/100 g of sample, respectively.
HPLC compound identification was performed by comparison of the standards’ retention times and spectra. Samples peak areas were quantified in mg/100 g (DM) and processed with version 2.0 of the Empower Software (Waters, Milford, MA, USA) by comparison with the peak area of the standard linear calibration.
2.4. Toxicological Analysis
Amygdalin, a toxic compound found in fruit seeds, particularly those of Prunus fruits, was analysed by HPLC-PDA [21,22]. In the human body, amygdalin can be metabolised, resulting in the production of cyanide. The reaction involves the β-glucosidase enzyme, which breaks down amygdalin into glucose, cyanide and benzaldehyde. Cyanide is a highly toxic substance that, in sufficiently high doses, interferes with cells’ ability to utilise oxygen, leading to symptoms of cyanide poisoning such as weakness, dizziness, difficulty breathing, and, in extreme cases, convulsions and death.
Amygdaline extraction was performed according to Bolarinwa, 2014 and 2015 [21,23], by ethanol hot extraction at 78.5 °C under reflux, in triplicate. Each extraction was performed in triplicate.
The analysis of amygdalin was performed in the Waters HPLC system equipped with a PDA detector (Waters Alliance 2695, 2996 PDA, Milford, MA, USA). Detection was carried out at 200 nm, with a mobile phase consisting of 25% ethanol and 75% water, at a flow rate of 1 mL/min. Separation was achieved using a reversed-phase C18 Waters Spherisorb ODS2 separation column maintained at 40 °C. Samples were kept at 5 °C, and the injection volume was 20 µL. Quantification was based on the external standard calibration technique, based on a linear relationship between the peak area and standard concentration.
2.5. Elemental Analysis
Calcium, potassium, iron and copper contents were determined in the pulp and the seeds of velvet tamarind, African locust bean, and baobab dried powders using an XRF analyser (model XL3t 950 He GOLDD+), according to Coelho et al. and Fernandes et al., 2022 [24,25]. The analyses were carried out in triplicate.
2.6. Statistical Analysis
Means and standard deviations were calculated by one-way ANOVA and the Scheffé test was applied for p < 0.05, using the Statistica software, v. 8.0 (StatSoft, Tulsa, OK, USA).
3. Results and Discussion
The moisture and ash fruit pulps and seeds results presented in Figure 4 show that the baobab pulp had the lowest water content (followed by the velvet tamarind pulp) and the highest ash content; the values are the range of other research studies. Seeds had a lower water content and higher ash content than pulps. Monteiro et al., 2022, in their study of baobab pulp from different Angolan origins, found ranges from 12 to 13% and from 4.7 to 5.1% for the moisture and ash contents, respectively [6]. Abiodun et al., 2017, in a black velvet tamarind study, found moisture and ash contents of 10.5 and 12.6%, respectively [3]. Kouassi et al., in 2018, studied the African locust bean (ALB) and baobab and found lower protein (0.21–0.28%), ash (0.20–0.47%) and fat (0.26–0.65%) contents with a high level of total carbohydrates (21–30%) and energy (85.83–124.43 Kcal/100 mg) and average total fibre [8].
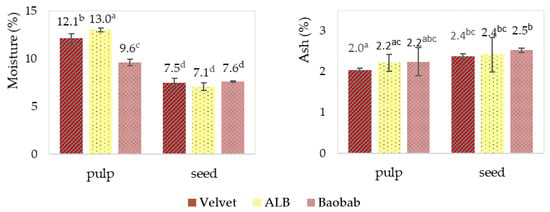
Figure 4.
Fruits’ pulp and seed moisture and ash determinations. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 3. ALB is the African locust bean.
As expected, protein and fat contents were higher in the seeds than in the pulp as shown in the graphic results in Figure 5. Monteiro et al., 2022, in their study of baobab pulp, found very low protein and fat contents [6]. Arogba et al., 1994, studied velvet tamarind and found it to be low in protein and fat [26].
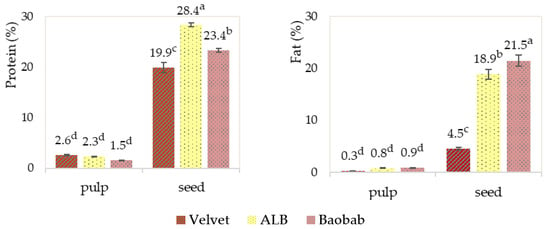
Figure 5.
Fruits’ pulp and seed protein and fat determinations. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 3 for protein and n = 2 for fat.
Analysing the graphics in Figure 6 reveals that the seeds’ fatty acid profiles were quite different. The African locust bean, ALB, stands out for its high polyunsaturated content, and baobab for its high monounsaturated fatty acid content.
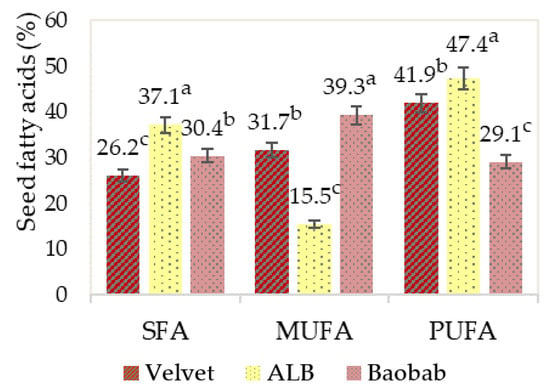
Figure 6.
Fruits’ seed saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 2.
Regarding the sugar profiles presented in Figure 7, the seeds have a very low sugar content when compared with the pulp. The African locust bean, ALB, has the highest sugar content with a high sucrose content followed by glucose and fructose contents. Baobab has the lowest sugar content, with similar levels for its analysed sugars.
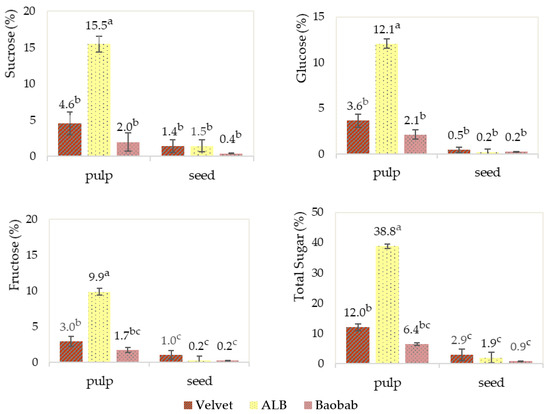
Figure 7.
Fruits’ pulp and seed sugar profile determinations. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 3.
The pulp and seeds’ organic acid profiles, presented in Figure 8, showed high levels of citric acid for ALB and baobab pulps. Considering the total organic acids in the pulps, the ALB pulp stands out due to its high succinic acid content, and in the seeds, velvet tamarind stands out due to its high malic acid content.
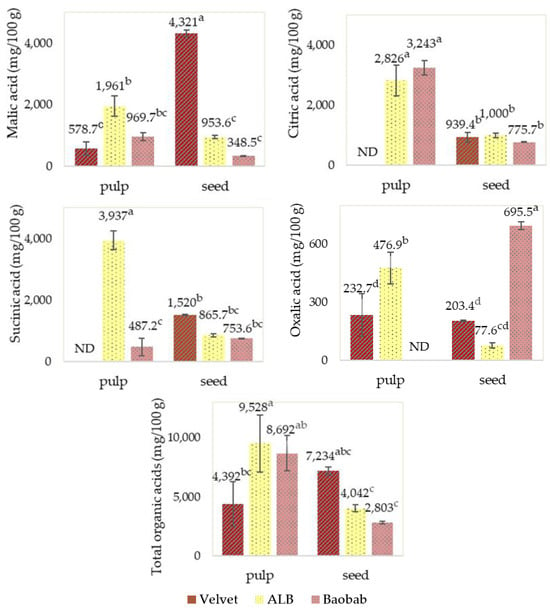
Figure 8.
Fruits’ pulp and seed organic acid profile determinations. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 3. ND: Not detected; the limit of detection was 12.6 mg/100 g for succinic acid and 10.7 mg/100 g for oxalic acid.
Table 1 presents the fruit pulp dietary fibre percentages, with soluble and total dietary fibre being much higher in baobab pulp due to its high pectin content. Velvet tamarind pulp had the highest insoluble dietary fibre.

Table 1.
Fruit pulp dietary fibre: insoluble (IDF), soluble (SDF) and total (TDF).
Baobab and ALB juices were characterised by Kouassi et al., 2018 [8], with an average fibre content of 45.3% similar to the obtained result of 42.5%. Monteiro et al., 2022, in their study of baobab from different Angolan origins, found a TDF range from 51 to 56 g/100 g [6].
Figure 9 shows that velvet tamarind pulp and seeds exhibited the highest total phenolic compound (TPC) content and antioxidant capacity, followed by baobab pulp. In contrast, the fruit seeds of ALB and baobab showed the lowest TPC content and antioxidant capacity.
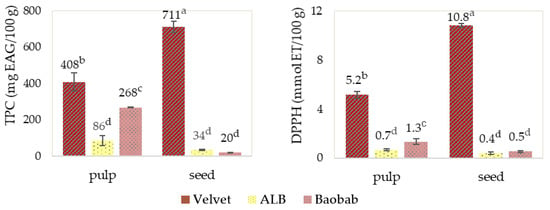
Figure 9.
Fruits’ pulp and seed total phenolic compound (TPC) levels and antioxidant capacity (DPPH). Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 3.
Regarding vitamin C, the quantification shown in the graphic of Figure 10 for the vitamin C in baobab pulp with 80.0 mg/100 g stands out with a higher content per 100 g than the Recommended Dietary Allowance (RDA) for an adult male, which is 70 mg vitamin C, as reported in Vitamin C—Health Professional Fact Sheet, United States Department of Health and Human Services, at https://ods.od.nih.gov (accessed on 20 December 2024). The nutritional importance of vitamin C as an essential water-soluble vitamin is well established, since ascorbic acid has a high redox potential, alone and coupled with other antioxidants, and also acts as a cofactor in numerous physiological reactions [27]. Monteiro et al., in their study of baobab from different Angolan origins, found a higher vitamin C range from 160 to 280 mg/100 g of baobab pulp [6].
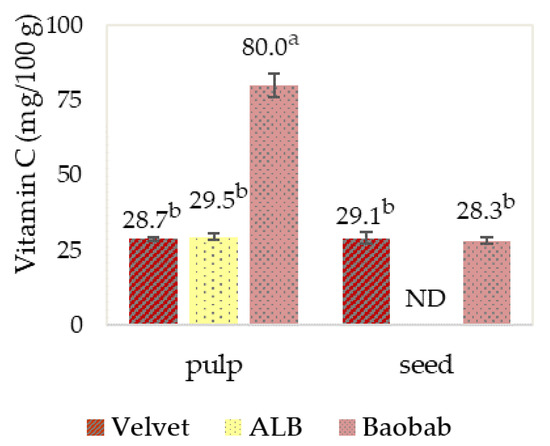
Figure 10.
Fruits’ pulp and seed vitamin C quantification. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there are no significant differences for p ≤ 0.05 and n = 3. ND: Not detected; the limit of detection was 15 mg/100 g.
Concerning the phenolic compound quantification, results showing the quantified phenolic compounds with significant differences between fruit samples are presented in Table 2. Velvet tamarind had the highest values in seeds and higher values than the other fruit pulps, mostly due to epicatechin, catechin, naringin and vanillic acid contents. ALB stands out for rutin, and baobab for procyanidin, rutin and catechin.

Table 2.
Fruits’ pulp and seed phenolic compounds quantified (in mg/100 g) with significant differences between samples (different letter index in each line, after the average, for p ≤ 0.05 and n = 3).
The three tropical fruits showed different phenolic compound profiles.
Kaempferol, chlorogenic acid, quercetin, ferulic acid, coumaric acid and caffeic acid were quantified but showed no significant differences between the fruit samples, and the obtained average contents are presented in Table 3.

Table 3.
Fruits’ pulp and seed phenolic compound quantified average contents without differences between fruit samples.
As far as elemental analysis is concerned, based on the data presented in Figure 11, significant differences were observed between the pulp and seeds of the three fruits. The calcium content in the pulp of the fruits was lower compared to the seeds. African locust beans exhibited the highest content of potassium, iron and copper in the pulp. On the other hand, compared to the other fruits, velvet tamarind showed the lowest content of calcium, potassium and iron in the pulp but has a higher calcium content in the seed. Baobab pulp stands out for its calcium content.
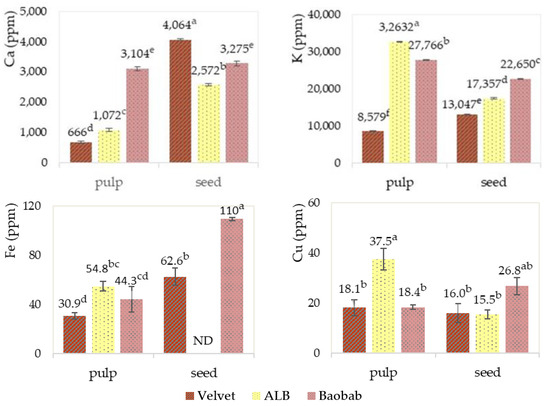
Figure 11.
Calcium, potassium, iron and copper content (in mg/kg, considering the dry weight) in pulp and seeds of velvet tamarind (velvet), African locust bean (ALB) and baobab. Graphics with averages and standard deviation error bars. The same letter index, after the average, means that there is no significant difference for p ≤ 0.05 and n = 3. ND stands for not detected—the value was under the detection limit of the equipment (<25 mg/kg).
No toxic levels were quantified for the elements analysed.
Regarding the toxicological analysis, amygdalin—a toxic compound present mostly in fruit seeds—was analysed because amygdalin can be metabolised in the human body to cyanide, a highly toxic substance, which in sufficiently high doses interferes with the cells’ ability to utilise oxygen, leading to symptoms of cyanide poisoning such as weakness, dizziness, difficulty of breathing and, in extreme cases, convulsions and death. The reaction involves the β-glucosidase enzyme which breaks down amygdalin into glucose, cyanide and benzaldehyde.
Amygdalin analysis was performed by HPLC-PDA [21,22,23] in the seeds, and the toxic compound amygdalin was not detected. The method limit of detection was 6.34 mg/100 g and the limit of quantification was 19.22 mg/100 g. The lethal amygdalin dosage is 0.5 mg/kg of the person’s weight [22], and the ingestion of 500 mg amygdalin corresponds to 30 mg cyanide [28].
The tropical fruits velvet tamarind, African locust beans and baobab can be considered functional foods, since several bioactive compounds with medicinal properties were quantified, namely vitamin C, hydroxycinnamic and hydroxybenzoic acids (like caffeic, coumaric, chlorogenic and ferulic; and gallic, vanillic and hydroxybenzoic), procyanidins and flavonoids (such as quercetin, catechin, epicatechin, rutin, naringin, procyanidin and kaempferol) and essential fatty acids from the ω-3 and ω-6 series.
The highest contents obtained were as follows: for sugar and organic acid, ALB pulp was highest (due to sucrose and succinic acid); for total phenolic content and antioxidant capacity, velvet tamarind was highest (due to the phenolic compounds epicatechin, rutin and naringin); for microelements, baobab pulp was highest in calcium and potassium, and ALB pulp was highest in potassium and copper. Vitamin C in baobab pulp, at 80.0 mg/100 g, stands out with a higher content than the Recommended Dietary Allowance for an adult (the RDA for an adult male is 70 mg of vitamin C).
4. Conclusions
Generally, fruit pulps are richer in sugars and organic acids, and seeds are richer in protein and fat. The fruits’ profiles are similar in terms of sugars but exhibit differences in their organic acids. African locust beans stand out for their sugars and organic acids, due to their sucrose and succinic acid levels, and baobab has the lowest sugar content and stands out for its vitamin C and citric acid levels.
Several bioactive compounds were quantified, such as vitamin C, various hydroxycinnamic acids (caffeic, coumaric, chlorogenic and ferulic), hydroxybenzoic acids (gallic, vanillic and p-hydroxybenzoic) and polyphenols (quercetin, catechin, epicatechin, rutin, naringin, procyanidin and kaempferol), as well as fatty acids from the ω-3 and ω-6 series.
Velvet tamarind had the highest antioxidant capacity; African locust beans had the highest sugar, potassium, iron and copper contents; and baobab had the highest vitamin C, calcium and soluble dietary fibre and total dietary fibre contents.
Amygdalin was not detected in any seeds and no toxic levels were quantified for the analysed elements.
The tropical fruits velvet tamarind, African locust beans and baobab have several bioactive compounds recognised for their beneficial pharmacological effects and can be considered functional foods.
Author Contributions
Conceptualisation, M.M.L.; methodology, M.M.L., J.F., A.R.F.C., A.S., A.C.M. and A.M.C.P.; validation, M.M.L., J.F. and A.R.F.C.; formal analysis, M.M.L.; investigation, M.M.L., J.F., A.R.F.C. and A.M.C.P.; resources, M.M.L.; writing—original draft preparation, M.M.L.; writing—review and editing, M.M.L., J.F. and A.R.F.C.; visualisation, M.M.L., J.F. and A.R.F.C.; supervision and project administration, M.M.L. and A.M.C.P.; funding acquisition, M.M.L. and A.R.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the FCT—Fundação para a Ciência e a Tecnologia, I.P., Research Unit GEOBIOTEC: UIDB/04035/2020, https://doi.org/10.54499/UIDB/04035/2020.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Acknowledgments
Thanks are due to Maria José Rodrigues for providing samples of velvet tamarind, African locust beans and baobab fruits with seeds from Guinea-Bissau, and to Viyaletta Brandes (Erasmus trainee at INIAV-UTI, Project 2022-1-DE02-KA121-VET-000063714) for her laboratory work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Catarino, L.; Indjai, B. Árvores Florestais da Guiné-Bissau; IBAP-Instituto da Biodiversidade e das Áreas Protegidas: Bissau, Guinea-Bissau, 2019; ISBN 978-989-96831-8-1. [Google Scholar]
- Catarino, L.; Frazão-Moreira, A.; Bessa, J.; Parathian, H.; Hockings, K. Plantas Usadas por CHIMPANZÉS e HUMANOS no Cantanhez, Guiné-Bissau. Guia de Campo; LAE/CRIA Laboratório de Antropologia Ambiental e Ecologia Comportamental Centro em Rede de Investigação em Antropologia: Lisbon, Portugal, 2020; ISBN 978-989-97179-8-5. [Google Scholar]
- Abiodun, O.A.; Dauda, A.O.; Adebisi, T.T.; Alonge, C.D. Physico-Chemical, Microbial and Sensory Properties of Kunu Zaki Beverage Sweetened with Black Velvet Tamarind (Dialium guineense). Croat. J. Food Sci. Technol. 2017, 9, 46–56. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Modisaojang, M.M.C.; Mokolopi, B.G.; Mbajiorgu, C.A. Nutritional and Chemical Composition of Black Velvet Tamarind (Dialium guineense Willd) and Its Influence on Animal Production: A Review. Open Agric. 2023, 8, 20220174. [Google Scholar] [CrossRef]
- Aborisade, C.A.; Bakare, L.M.; Balogun, F.O. Elemental Characterization of African Locust Beans (Parkia biglobosa) in Igbope, Oyo State of Nigeria. Asian J. Appl. Sci. 2021, 9, 127–132. [Google Scholar] [CrossRef]
- Monteiro, S.; Reboredo, F.H.; Lageiro, M.M.; Lourenço, V.M.; Dias, J.; Lidon, F.; Abreu, M.; Martins, A.P.L.; Alvarenga, N. Nutritional Properties of Baobab Pulp from Different Angolan Origins. Plants 2022, 11, 2272. [Google Scholar] [CrossRef] [PubMed]
- Chadare, F.J.; Linnemann, A.R.; Hounhouigan, J.D.; Nout, M.J.R.; Van Boekel, M.A.J.S. Baobab Food Products: A Review on Their Composition and Nutritional Value. Crit. Rev. Food Sci. Nutr. 2009, 49, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, K.A.; Beugré, G.A.M.; Kouassi, K.N.; N’Dri, Y.D.; Amani, N.’G.G.; Gnakri, D. Biochemical Characterization of Juices from Three Wild Fruit Species Consumed in Côte d’Ivoire “Adansonia digitata, Parkia biglobosa and Tamarindus indica”. OSJ 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Monteiro, S.; Dias, J.; Lourenço, V.; Partidário, A.; Lageiro, M.; Lampreia, C.; Fernandes, J.; Lidon, F.; Reboredo, F.; Alvarenga, N. Development of a Functional Dark Chocolate with Baobab Pulp. Foods 2023, 12, 1711. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of the Association of Official Analytical Chemists, 13th ed.; Association of Official Analytical Chemists, Ed.; Association of Official Analytical Chemists: Washington, WA, USA, 1980; ISBN 978-0-935584-14-1. [Google Scholar]
- ISO 1871:2009; Food and Feed Products-General Guidelines for the Determination of Nitrogen by the Kjeldahl Method. ISO-International Organization for Standardization: Geneva, Switzerland, 2009.
- Pereira, P.; Palma, M.L.; Palma, C.; Borges, C.; Maurício, E.; Fernando, A.L.; Duarte, M.P.; Lageiro, M.; Fernandes, A.; Mateus, N.; et al. Exploring the Benefits of Nutritional and Chemical Characteristics of Touriga Nacional and Arinto Varieties (Vitis vinifera L.). Foods 2024, 13, 1535. [Google Scholar] [CrossRef] [PubMed]
- NP ISO 6492; Animal Feeding Stuffs. Determination of Fat Content. Determinação do teor de Gordura em Alimentos para Animais. Instituto Português da Qualidade: Lisbon, Portugal, 2014.
- NP EN ISO 5509; Animal and Vegetable Fats and Oils Preparation of Methyl Esters of Fatty Acids. Gorduras e Óleos Comestíveis. Determinação Ácidos Gordos. Instituto Português Da Qualidade: Lisbon, Portugal, 2003.
- Panda, A.; Alvarenga, N.; da Silva, J.L.; Partidário, A.; Lageiro, M.; Roseiro, C.; Dias, J. Influence of Cocoa Origin on the Nutritional Characterization of Chocolate. Eur. Food Res. Technol. 2022, 248, 2569–2577. [Google Scholar] [CrossRef]
- Cartas, J.; Alvarenga, N.; Partidário, A.; Lageiro, M.; Roseiro, C.; Gonçalves, H.; Leitão, A.E.; Ribeiro, C.M.; Dias, J. Influence of Geographical Origin in the Physical and Bioactive Parameters of Single Origin Dark Chocolate. Eur. Food Res. Technol. 2024, 250, 2569–2580. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 978-0-12-182200-2. [Google Scholar]
- Pereira, N.; Farrokhi, M.; Vida, M.; Lageiro, M.; Ramos, A.C.; Vieira, M.C.; Alegria, C.; Gonçalves, E.M.; Abreu, M. Valorisation of Wasted Immature Tomato to Innovative Fermented Functional Foods. Foods 2023, 12, 1532. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- EN 14130; Quantitative Determination of Vitamin C by High Performance Liquid Chromatography. European Committee for Standardization (CEN): Brussels, Belgium, 2003.
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of Amygdalin in Apple Seeds, Fresh Apples and Processed Apple Juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Shragg, T.A.; Albertson, T.E.; Fisher, C.J. Cyanide Poisoning after Bitter Almond Ingestion. West. J. Med. 1982, 136, 65–69. [Google Scholar] [PubMed]
- Bolarinwa, I.; Orfila, C.; Morgan, M. Amygdalin Content of Seeds, Kernels and Food Products Commercially-Available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.R.F.; Lidon, F.C.; Pessoa, C.C.; Daccak, D.; Luís, I.C.; Marques, A.C.; Ramalho, J.C.; Semedo, J.M.N.; Silva, M.M.; Pais, I.P.; et al. Mineral Monitorization in Different Tissues of Solanum Tuberosum L. during Calcium Biofortification Process. Horticulturae 2022, 8, 1020. [Google Scholar] [CrossRef]
- Fernandes, J.; Reboredo, F.H.; Luis, I.; Silva, M.M.; Simões, M.M.; Lidon, F.C.; Ramalho, J.C. Elemental Composition of Commercial Herbal Tea Plants and Respective Infusions. Plants 2022, 11, 1412. [Google Scholar] [CrossRef] [PubMed]
- Arogba, S.S.; Ajiboro, A.A.; Odukwe, I.J. A Physico-Chemical Study of Nigerian Velvet Tamarind (Dialium guineense L.) Fruit. J. Sci. Food Agric. 1994, 66, 533–534. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.-M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.-C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of Wild Strawberry-Tree Fruits (Arbutus Unedo L.) through Nutritional Assessment and Natural Production Data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Dang, T.; Nguyen, C.; Tran, P.N. Physician Beware: Severe Cyanide Toxicity from Amygdalin Tablets Ingestion. Case Rep. Emerg. Med. 2017, 2017, 4289527. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).