1. Introduction
Various methods for isolating milk oligosaccharides illustrate the development of techniques to improve yield, purity, and specificity in the extraction of oligosaccharides in carbohydrate fractions from milk. Each method uses unique steps to isolate oligosaccharides from other milk components, emphasizing different separation principles such as solvent extraction, ion exchange, gel filtration, and membrane-based filtration. The Weiruszeski technique primarily uses ion-exchange chromatography to isolate sialylated oligosaccharides. By using a pyridine acetate buffer gradient, sialyl-oligosaccharides are successfully eluted using the different charges of the molecules. This method is suitable for the precise isolation of charged oligosaccharides, especially sialylated forms. However, the use of ethanol in this procedure may affect sensitivity to lower abundance oligosaccharides, which may be a limitation with limited sample quantities. Urashima’s method focuses on extraction with organic solvents (chloroform/methanol) to remove proteins, followed by Bio-Gel P-2 column chromatography for fractionation. Its focus on distilled water fractionation minimizes contamination and provides an accurate carbohydrate profile. Urashima’s method is particularly characterized by the gentle handling of oligosaccharides, which is of crucial importance for the subsequent analysis. However, organic solvents pose handling risks and potential sample loss, and additional steps are required for specific quantification of sialic acid and hexose levels. The Smith’s method uses ethanol precipitation and gel permeation chromatography using Sephadex G-25 to partially isolate oligosaccharides. By applying the Somogyi method of deproteinization, non-carbohydrate impurities are effectively removed, and a crude oligosaccharide fraction is obtained. Although this method is efficient, there may be problems with yield consistency, particularly since it relies heavily on solvent precipitation and cold centrifugation, which could result in partial loss of oligosaccharides during separation. Membrane technology is a sophisticated, two-stage filtration method that utilizes ultrafiltration [
1] (50 kDa) followed by nanofiltration [
2] (1 kDa) to selectively filter oligosaccharides. Its continuous diafiltration approach effectively separates smaller molecules and retains larger oligosaccharides, ideal for optimized isolation with minimized sample contamination [
3]. However, the use of tangential flow requires complex operational controls (e.g., specific transmembrane pressures) and the required equipment and temperature stability (300 °C) make it more demanding and resource intensive than other methods. Egge’s method combines centrifugation, acetone precipitation, rotary evaporation, and ion-exchange chromatography [
4] for multi-step isolation that includes crystallization to remove lactose. It is able to distinguish sialic acid-containing compounds from neutral oligosaccharides and produce high-purity oligosaccharides suitable for detailed structural analysis. Despite its precision, this approach is time-consuming and can result in losses at each centrifugation and filtration step, potentially reducing the overall yield of oligosaccharides. While each of these methods offer specific advantages such as the simplicity of Urashima [
5] and the scalability of membrane techniques, each of these methods comes with inherent trade-offs. Techniques such as Weiruszeski and Egge et al. provide high specificity in oligosaccharide isolation but can require significant time and resource investment [
6]. Membrane filtration is characterized by continuous separation and minimization of processing steps but requires precise conditions and equipment. In summary, the choice of method depends on the available volume of the sample, the desired purity of the oligosaccharide, and the resources. Future approaches could combine aspects of these methods to achieve high yield, purity, and practicality in a single optimized protocol.
2. Methods
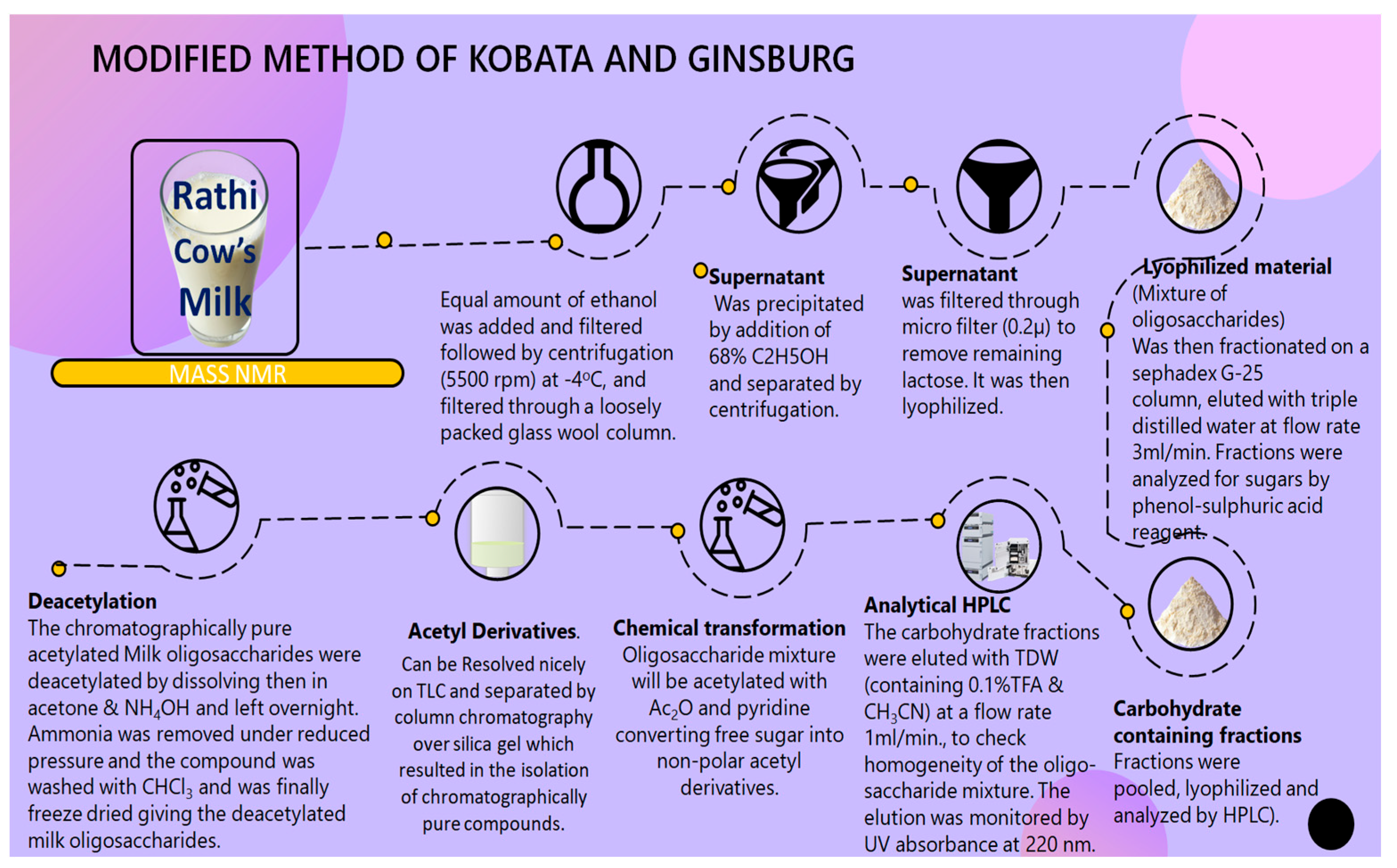
An adequate quantity, which was 10 L [
7], of Rathi cow milk was obtained from a domestic cow in the Panch-Mukhi district of Rajasthan, preserved with ethanol, and centrifuged [
8]. The clear filtrate was mixed with additional ethanol until a concentration of 68% was reached [
9,
10,
11]. The white precipitate, mainly lactose and protein, was purified twice with 68% ethanol. Supernatant and wash water were combined to produce a crude oligosaccharide mixture (283 g). It was then filtered with a micro-filter (0.2 μ) to remove any lactose residues (residue discarded). This oligosaccharide mixture (10 g) was acetylated with Ac
2O and pyridine (1:1,
v/v) to obtain acetyl derivatives that were less polar and could be easily loaded and run on a TLC plate and chromatography column for analysis and isolation, respectively. Individual fractions from column chromatography were examined by TLC and combined fractions were subjected to analysis for sugars using the phenol–sulfuric acid method (
Scheme 1).
3. Isolation Methods of Milk Oligosaccharides
To characterize oligosaccharides, various kinds of isolation, purification, and separation procedures were used. Researchers including Victor Ginsburg, Akira Kobata, Tadasu Urashima, Stephen Thrul, D.F. Smith, Richard Kuhn, Heinz Egge, and Desh Deepak [
9,
12,
13,
14,
15,
16,
17,
18] have developed a variety of methods for isolation of milk oligosaccharides and worked on their structure elucidation. Thanks to advancements in contemporary chromatographic and physicochemical techniques, such as HPLC [
19], gel electrophoresis [
20], ion-exchange chromatography [
21], and graphitized carbon-based solid-phase extraction (S.P.E.) [
22], we can now readily isolate oligosaccharides in their purest form. In our investigation of isolating and revealing the structure of milk oligosaccharides from Rathi cow’s milk, we employed a modified variant of the Kobata and Ginsburg method, which had been developed in our laboratory. The procedure devised in our lab is detailed below.
4. Isolation of Cow Milk Oligosaccharides (CMOs)
In this method by Deepak et al., milk samples of Rathi cow were collected and an equal amount of alcohol was added (for preservation); then, the mixture underwent centrifugation for 15 min at 5000 rpm at −4 °C. The solidified lipid layer was eliminated via filtration through a glass wool column under cold atmospheric conditions. Additional ethanol was added to the clear filtrate (supernatant) to achieve a final concentration of 68% to precipitate the lactose and proteins. The solution was left overnight at 0 °C. A white precipitate formed from lactose and protein was removed by centrifugation for 15 min at 5000 rpm at −4 °C. The 68% ethanol was used twice to wash this precipitate. To ensure complete removal of remaining lactose, after passing through a micro-filter (0–24 mm), the supernatant was lyophilized to extract the crude oligosaccharide mixture (283 g). This lyophilized material (crude oligosaccharide mixture) was analysed using the phenol–sulfuric acid reagent to detect presence of the neutral sugar-containing oligosaccharides. These fractions were subsequently utilized to isolate and purify CMOs using gel filtering (Sephadex chromatography).
5. Gel Filtration or Sephadex Chromatography [23]
The fundamental principle of gel-permeation chromatography is the use of dextran-containing materials to separate macromolecules according to the sizes of their molecules. This technique is primarily employed to determine the protein molecules’ molecular weights and to reduce the salt concentration in protein solutions. In a gel-permeation column, the stationary phase comprises inert molecules with small pores. When a solution containing molecules of different sizes is passed through the column at a constant flow rate, larger molecules, which cannot permeate into the gel particles, are retained between the particles in a restricted area. These larger molecules move rapidly through the spaces between the porous particles and quickly traverse the column. Conversely, smaller molecules diffuse into the pores, and, as their size decreases, they possess greater retention time within the column. The most widely used column material is Sephadex G type, although other materials like dextran, agarose, and polyacrylamide are also used.
6. Acetylation of Cow Milk Oligosaccharides
The process of acetylating oligosaccharides in milk has traditionally used acetic anhydride. Essentially, acetylation confirms the existence of -OH groups in a specific moiety. In this context, acetylation is generally performed using Ac
2O with pyridine. This modification makes the milk oligosaccharides less polar, enabling their conventional isolation through column chromatography. Additionally, acetylation helps in determining the positions of glycosidic linkages, improves the resolution and removes the spectral degeneracy of
1H NMR, and provides better insights into the molecular structure. Non-acetylated oligosaccharides were removed from the mixture of acetylated oligosaccharide (O.S.) mixture via biphasic separation; optimization is provided below. (
Figure 1,
Table 1)
Following acetylation, we proceeded to purify the milk oligosaccharides using chromatographic techniques, as outlined below.
7. Purification of Cow Milk Oligosaccharides
For qualitative and quantitative analysis, chromatography is an essential biophysical method that makes it easier to separate, identify, and purify the constituents of a mixture. This technique is fundamental in the purification and analysis of milk oligosaccharides. Applying a mixture to a surface or into a solid and liquid stationary phase (stable phase) is the basic idea of chromatography. With the help of the mobile phase, the molecules are separated as they move. Molecular characteristics associated with adsorption (liquid–solid), distribution (liquid–solid), affinity, and fluctuations in molecular weights are key factors that affect this separation process. Because of these variations, certain mixture components stay in the stationary phase longer and pass through the chromatography system more slowly, while others enter the mobile phase more rapidly and leave the system sooner.
Chromatography involves three main components:
- (1)
Stationary Phase: A solid phase or a liquid layer adsorbed on the surface of a solid support makes up this phase;
- (2)
Mobile Phase: A liquid or gaseous component makes up this phase;
- (3)
Purified compounds.
The nature of the interactions among the stationary phase, mobile phase, and the components of the mixture is a critical determinant in the successful separation of molecules. Partition-based chromatographic methods are particularly helpful for separating and identifying small compounds, such as fatty acids, carbohydrates, and amino acids. Below is a description of the different chromatographic methods we used in our laboratory to separate oligosaccharides.
8. Thin-Layer Chromatography (TLC)
Thin-layer chromatography (TLC) is a widely used analytical technique for separating non-volatile mixtures. The process is easy, quick, and affordable and allows chemists to quickly gain insight into the nature of a sample. TLC is extensively employed for qualitative analysis and monitoring the progress of chemical reactions in the fields of chemistry, biochemistry, and pharmacology. TLC operates on the principle of the solid–liquid adsorption chromatography technique. In this method, the stationary phase is a solid adsorbent substance, such as alumina, silica gel, or cellulose, coated onto glass plates. The sample mixture is carried by the mobile phase as it ascends through the stationary phase due to capillary action. The mixture is first applied as small dots to the lower part of the plate using a micropipette tip or a fine capillary tube and it separates the analytes at different speeds as the solvent moves upwards. The solvent, solid phase, and material polarity all affect the upward movement rate. Fluorescence, radioactivity, or certain chemicals (visualizers) can be used to produce a visible coloured spots when the sample molecules are colourless, making them easier to locate in the chromatogram. This colour can be observed in both regular and UV light. The position of each molecule in the mixture is measured by calculating the ratio between the distance travelled by the molecule and the solvent. This measurement is called the relative mobility and is expressed with the symbol Rf; molecules are qualitatively described using the Rf value. The Rf values usually differ for different molecules, so their analysis or separation is possible using this technique. If the spots appear streaky or not well-separated, gradually increase the proportion of acetic acid, for example, an optimum ratio of chloroform, methanol, and acetic acid is 96:4:3.
9. Column Chromatography (CC)
Column chromatography is a widely utilized technique for the purification of various substances, including fatty acids, carbohydrates, and amino acids. These compounds exhibit distinct characteristics, such as binding affinity, molecular size, geometric configuration, interactions with the stationary phase, and overall charge. The effectiveness of this method lies in its ability to accommodate the diverse properties of these biomolecules. In the process of column chromatography, a glass column is filled with a stationary phase. Initially, the sample intended for separation is introduced into this stationary phase, followed by the application of a wash buffer that serves as the mobile phase. As the sample components traverse the glass column, they are separated based on their specific interactions with the stationary phase and their varying affinities for the mobile phase. This chromatographic approach facilitates the efficient purification of a range of biomolecules by capitalizing on their unique chemical and physical attributes.
10. Acetylated Milk Oligosaccharide Purification from Rathi Cow Milk on Silica Gel Column [24]
The acetylated cow milk oligosaccharides (CMOs) were purified by packing a silica gel column and eluting with a solvent system of chloroform and methanol in gradient. The oligosaccharide mixture was loaded to the column, and fractions were collected as the solvents eluted the components based on their polarity. The purity of collected fractions was monitored using TLC and then the fractions were pooled, followed by drying under reduced pressure for further analysis. An average ratio of ~1:77 of OS mixture with silica gel (
Figure 2) was found useful in chromatography 1–5 (results/tables are provided in the
Supplementary File).
11. Conclusions
This research successfully refined the purification and modification techniques for extracting oligosaccharides from Rathi cow’s milk, establishing a straightforward, reliable, and cost-efficient method for their analysis. The ethanol-based preservation method addresses the challenges associated with sample collection in remote areas, where immediate access to laboratory facilities is limited, ensuring the integrity of oligosaccharide content during long-distance transportation, combined with a sequence of microfiltration, lyophilization, and silica gel column chromatography. The implementation of gradient elution using chloroform and methanol enabled efficient fractionation, ensuring optimized use of both solvents and silica gel. This approach not only minimized wastage but also enhanced time efficiency by yielding optimal results in the separation process, while the phenol–sulfuric acid method confirmed the sugar content. Additionally, the acetylation process yields less polar acetyl derivatives, which prevent analytes from adhering to the silica gel column or TLC, thereby facilitating efficient separation and analysis, respectively. These advancements not only improve analytical accuracy but also carry significant implications for nutritional and medicinal research on cow’s milk. Furthermore, this contributes to a deeper understanding of cow milk’s bioactive components and their potential applications in health and wellness.
Author Contributions
Conceptualization, D.D. and A.S.; software, D.D.A.P.S.C.; validation, S.C., A.S., and D.D.; formal analysis, D.D.A.P.S.C.; investigation, D.D.; data curation, S.A.U.; writing—original draft preparation, D.D.; writing—review and editing, S.A.U.; visualization, S.C.; supervision, S.C.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
A.S. acknowledges funding support from the Department of Biotechnology (BT/PR38505/MED/29/1513/2020), Govt. of India, Department of Science and Technology (CRG/2022/001047), Govt. of India, ICMR (No. 52/08/2019-BIO/BMS), DST-PURSE program (SR/PURSE Phase 2/29(C)), UP Higher Education (No. 10/2021/281/-4-Sattar-2021-04(2)/2021), No. 39/2024/242/Sattar-4-2024-001-4(33)/2023, and the University of Lucknow.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/
Supplementary Material, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jelen, P. Standardization of Fat and Protein Content. In Encyclopedia of Dairy Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 545–549. [Google Scholar] [CrossRef]
- de Moura Bell, J.M.L.N.; Cohen, J.L.; de Aquino, L.F.; Lee, H.; de Melo Silva, V.L.; Liu, Y.; Domizio, P.; Barile, D. An integrated bioprocess to recover bovine milk oligosaccharides from colostrum whey permeate. J. Food Eng. 2018, 216, 27–35. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Hwang, I.K. Optimization of the extraction and purification of oligosaccharides from defatted soybean meal. Int. J. Food Sci. Technol. 2003, 38, 337–342. [Google Scholar] [CrossRef]
- Yin, H.; Du, Y. Research Progress in Oligosaccharins; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Urashima, T.; Asakuma, S.; Fukuda, K.; Saito, T. Recent Advances of the Study on Milk Oligosaccharides of Domestic Farm Animals Including Cows. Kagaku Seibutsu 2012, 50, 498–509. [Google Scholar] [CrossRef]
- Wieruszeski, J.-M.; Chekkor, A.; Bouquelet, S.; Montreuil, J.; Strecker, G.; Peter-Katalinic, J.; Egge, H. Structure of two new oligosaccharides isolated from human milk: Sialylated lacto-N-fucopentaoses I and II. Carbohydr. Res. 1985, 137, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Singh Chauhan, D.D.A.P.; Chauhan, S.; Shukla, M.; Mishra, A.; Deepak, D. Isolation and Structure Elucidation of a New Tetrasssaccharide ‘Hisose’ from Rathi Cow’s Milk by 2D NMR. Dairy Vet. Sci. J. 2024, 16, 1–12. [Google Scholar]
- Gangwar, L.; Kumar, A.; Deepak, D. Original Article Isolation and Structure Elucidation of Biologically Active Novel Pentasaccharide from the milk of Bubalus bubalis. Int. J. Carbohydr. Res. 2017, 7, 9–13. [Google Scholar]
- Ginsburg, V.; Zopf, D.A.; Yamashita, K.; Kobata, A. Oligosaccharides of human milk. Isolation of a new pentasaccharide, lacto-N-fucopentaose V. Arch. Biochem. Biophys. 1976, 175, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Kobata, A.; Ginsburg, V.; Tsuda, M. Oligosaccharides of Human Milk. Arch. Biochem. Biophys. 1969, 130, 509–513. [Google Scholar] [CrossRef]
- Verma, P.; Sarkar, J.; Deepak, D. Isolation of a Novel Oligosaccharide Biological and Chemical Research. J. Biol. Chem. Res. 2017, 34, 221–230. [Google Scholar]
- Kobata, A. Isolation of Oligosaccharides from Human Milk. Methods Enzymol. 1972, 28, 262–271. [Google Scholar] [CrossRef]
- Verma, P.; Sarkar, J.; Deepak, D. Isolation, purification and structure elucidation of novel hexasaccharide mesose from Camel milk by NMR. Trends Carbohydr. Res. 2019, 11, 52–58. [Google Scholar]
- Urashima, T.; Saito, T.; Ohmisya, K.; Shimazaki, K. Structural determination of three neutral oligosaccharides in bovine (Holstein-Friesian) colostrum, including the novel trisaccharide; GalNAcαl-3Galβ1-4Glc. Biochim. Biophys. Acta—Gen. Subj. 1991, 1073, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Egge, H.; Dell, A.; Von Nicolai, H. Fucose containing oligosaccharides from human milk. I. Separation and identification of new constituents. Arch. Biochem. Biophys. 1983, 224, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Henker, J.; Taut, H.; Tovar, K.; Sawatzki, G. Variations of neutral oligosaccharides and lactose in human milk during the feeding. Z. Fur Ernahrungswissenschaft 1993, 32, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Zorf, D.A.; Ginsburg, V. Fractionation of sialyl oligosaccharides of human milk by ion-exchange chromatography. Anal. Biochem. 1978, 85, 602–608. [Google Scholar] [CrossRef]
- Kuhn, R.; Baer, H.H.; Gauhe, A. Kristallisierte Fucosido-lactose. Eur. J. Inorg. Chem. 1956, 89, 2513. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Sharma, C.B. Goat milk oligosaccharides: Purification and characterization by HPLC and high-field 1H-NMR spectroscopy. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1988, 967, 115–121. [Google Scholar] [CrossRef]
- Szigeti, M.; Meszaros-Matwiejuk, A.; Molnar-Gabor, D.; Guttman, A. Molnar-Gabor, and A. Guttman, “Rapid capillary gel electrophoresis analysis of human milk oligosaccharides for food additive manufacturing in-process control. Anal. Bioanal. Chem. 2021, 413, 1595–1603. [Google Scholar] [CrossRef]
- Mariño, K.; Lane, J.A.; Abrahams, J.L.; Struwe, W.B.; Harvey, D.J.; Marotta, M.; Hickey, R.M.; Rudd, P.M. Method for milk oligosaccharide profiling by 2-aminobenzamide labeling and hydrophilic interaction chromatography. Glycobiology 2011, 21, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Pyles, M.B.; Brock, K.; Schendel, R.R.; Lawrence, L.M. Improved methods for mare milk analysis: Extraction and quantification of mare milk carbohydrates and assessment of FTIR-based macronutrient quantification. Front. Nutr. 2023, 10, 1066463. [Google Scholar] [CrossRef]
- Messer, M.; Mossop, G. Milk carbohydrates ofmarsupials i. Partial separation and characterization of neutral milk oligosaccharides of the eastern grey kangaroo. Aust. J. Biol. Sci. 1977, 30, 379–388. [Google Scholar] [CrossRef]
- Shahi, S.; Gangwar, L.; Verma, P.; Deepak, D. Isolation, Purification and NMR Study of a Novel Nonasaccharide (Rieose) from ‘Gaddi Sheep’ Milk. J. Biol. Chem. Res. 2017, 34, 569–582. [Google Scholar]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).