Abstract
Pumpkin (Cucurbita maxima) is a fruit packed with vitamins and nutrients beneficial to human health with numerous therapeutic uses including antiparasitic, antioxidant, it helps to lower bad cholesterol, as an adjuvant in weight loss, improves cancer prevention, etc. Pumpkin is rich in beta-carotene, and contains significant amounts of lutein and zeaxanthin, antioxidants that can considerably prevent cataracts and macular degeneration. Worldwide, five pumpkin species are grown for their edible fruit and seeds. This paper describes the qualitative screening of phytocompounds and the quantitative determination of the main bioactive compounds found in two pumpkin species: Valenciano and Waltham Butternut. The qualitative screening of phytochemicals was based on the visual change in color of aqueous extracts upon adding known reactants. This allowed a preliminary evaluation regarding the presence of different bioactive compounds such as saponins, alkaloids, tannins, flavonoids, etc. In order to determine the specific amount of different phytocompounds (e.g., total content of polyphenols, total content of flavonoids, etc.) UV-Vis spectra were recorded in triplicate at well-established wavelengths, thus obtaining an average absorbance. For example, a method widely applied for the determination of total polyphenolic content is the Folin–Ciocalteu (FC) reaction, which is basically an antioxidant analyses that relies on electron transfer that measures the reductive ability of a specific antioxidant. Briefly, the FC reaction involved mixing 1 mL diluted aqueous extract with 5 mL FC reagent and adding 4 mL Na2CO3 after 8 min After 60 min incubation at room temperature, we recorded the absorptions at 765 nm, which corresponds to the gallic acid curve calibration standard. Also, the antioxidant activity was recorded by using the DPPH method for both aqueous extracts.
1. Introduction
Cucurbita maxima (pumpkin, squash, gourd) is an important representative of the Cucurbitaceae family and is among the top cultivated crops in the world, especially in temperate and subtropical climates [1]. The squash pumpkin has gained greater recognition in the past years, mainly due to the nutritional and health benefits of its seeds, which are packed with nutraceutical compounds such as polysaccharides, carotene, minerals and vitamins [2]. The health benefits of different squash pumpkin species have been reported previously, including that they can lower blood glucose [3] and can even prevent some types of diabetes and reduce their complications [4]. Squash pumpkin contains an impressive amount of vitamin A, that is, 245% of the reference daily intake (RDI), is low in calories as it contains over 94% water, and contains significant amounts of antioxidants (e.g., alpha-carotene, beta-carotene and beta-cryptoxanthin) that can neutralize the action of free radicals [5].
Plants contain multiple and different radical scavenging molecules that are therapeutically active, including alkaloids, amines, betalains, vitamins, phenolic acids, terpenoids. Most phytochemicals are antioxidant agents, which essentially reduce the damages caused in tissue during physiological processes. The pharmaceutic value of plants depends on their bioactive compounds, which exhibit different physiological effects on human health. Therefore, screening for bioactive compounds allows the detection of various components that can be used as a starting point for modern drugs that can treat different diseases [6]. These “metabolic chemicals” are better known as “secondary metabolites” [7] and they include alkaloids, flavonoids, coumarins, tannins, terpenes, terpenoids, phenols, polysaccharides and glycosides [8].
There are many known bioactive compounds, each with their own health action [9,10], including:
- antioxidant, meaning that it protects the human cells from the oxidative stress;
- hormonal, especially isoflavones that can imitate human estrogens;
- antibacterial;
- physical, bioactive compounds can physically attach to cell walls.
This paper describes the qualitative screening of phytocompounds and the quantitative determination of the main bioactive compounds found in two pumpkin species: Valenciano and Waltham Butternut. The fresh samples were air-dried at room temperature for 10 days under no direct sunlight followed by an aqueous extraction at a controlled temperature of 4 °C in the refrigerator. The qualitative screening for bioactive compounds involved the color-changing reaction that results from mixing different extracts with known reagents, while the quantitative screening for bioactive compounds was based on spectrophotometric determinations at specific and well-established wavelengths. The results are presented as a comparison of the two squash pumpkin species and clearly show that both Valenciano and Waltham Butternut are important sources of bioactive compounds with numerous health benefits.
2. Materials and Methods
2.1. Materials
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate), hydrochloric acid (HCl), sulfuric acid (H2SO4), copper sulphate (CuSO4), copper acetate (Cu(CH3COO)2), silver nitrate (AgNO3), aluminum chloride (AlCl3), lead acetate (Pb(Ch3COO)2), catechin standard, gallic acid standard, Folin–Ciocalteu reagent, ferric chloride (FeCl3), glacial acetic acid (CH3COOH), ammonium molybdate, Benedict and Millon reagents were purchased from Sigma-Aldricht. Ethanol (C2H5OH), methanol (CH3OH), chloroform (CHCl3) and sodium hydroxide (NaOH) were purchased from Scharlau. The distilled water was freshly prepared in the laboratory.
2.2. Preparation of the Aqueous Extracts from Cucurbita maxima
Both types of pumpkin (Valenciano and Waltham Butternut, Figure 1) used in the present research study were taken from a local homemaker in Gradistea, Giurgiu, Romania who grew them in an eco-friendly environment with no chemicals or additives.

Figure 1.
Valenciano squash pumpkin (left) and Waltham Butternut squash pumpkin (right).
All the component parts (e.g., shell, core and seeds and equal combinations of these parts) were carefully separated, thoroughly washed twice with tap water, thrice with freshly prepared distilled water, dried at room temperature for 10 days under no direct sunlight, finely ground and used to prepare the corresponding aqueous extracts. The protocol used to prepare the aqueous extracts was the same for both Cucurbita maxima squash pumpkin species and involved the following steps:
- 25 g dried squash parts (e.g., shell, core, seeds and equal mixtures of them) were weighed, transferred into a glass extractor and infused with 250 mL distilled water;
- the solutions were kept for 24 h in a refrigerator (4 °C) to infuse;
- the aqueous extracts were filtered until a clear liquid was obtained;
- the aqueous extracts were stable at 4 °C for more than 4 months.
2.3. Qualitative Screening for Bioactive Compounds
The qualitative screening for bioactive compounds uses standard analytical methods that rely on a color change reaction as a positive response [11].
Test for tannins: 2 mL of 5% FeCl3 was added to 1 mL aqueous extract, and the formation of a dark blue or greenish black solution confirms the presence of tannins.
Test for saponins: 2 mL aqueous extract was mixed with 2 mL distilled water and vigorously shaken lengthwise, using a graduated cylinder, for 15 min The formation of a 1 cm foam layer confirms the presence of saponins.
Test for alkaloids:
- (a)
- Mayer test: 2 mL of concentrated HCl was added to 2 mL aqueous extract, followed by a few drops of Mayer reagent (potassium mercuric iodide). The formation of a green solution or white precipitate indicates the presence of alkaloids.
- (b)
- Wagner test: 1 mL Wagner reagent (iodine in potassium iodide) was added to 3 mL aqueous extract. A reddish-brown precipitate indicated the presence of alkaloids.
- (c)
- Hager test: 1 mL Hager reagent (saturated picric acid solution) was added to 3 mL aqueous extract, and if a yellow precipitate forms, then alkaloids are present.
Test for flavonoids: 2 mL aqueous extract was mixed with 1 mL 2N NaOH. This results in the formation of a yellow color that disappears after adding dilute HCl.
Test for proteins and amino acids:
- (a)
- Millon test: 1 mL aqueous extract reacts with 5–6 drops of Millon reagent and a white precipitate appears that changes its color to red at heating.
- (b)
- Biuret test: 3 mL 4% NaOH solution and few drops of 1% CuSO4 were added to 3 mL aqueous extract, and a purple solution is formed.
- (c)
- Ninhydrin test: 3 drops of 5% Pb(CH3COO)2 were added to 3 mL aqueous extract and heated for 10 min A purple or blue color is a positive response.
- (d)
- Cysteine test: a few drops of 40% NaOH and 5% were added to 5 mL aqueous extract, and boiled for 5 min The solution turns purple or blue or a black precipitate of lead sulphate is formed.
- (e)
- Xantoprotein test: 1 mL conc. H2SO4 was added to 3 mL aqueous extract. First a white precipitate is formed, which turns yellow upon boiling and orange after adding 1 mL NH4OH.
2.4. Quantitative Determinations of Bioactive Compounds
The quantitative determination of bioactive compounds was also carried out to determine the total content of tannins (TCF), total content of flavonoids (TCF) and total content of polyphenols (TCP) [12,13].
The antioxidant activity (AA, %) was also determined, and for that a 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) solution was prepared in C2H5OH and 0.5 mL aqueous extract was mixed with 1 mL 0.02 mg/mL DPPH solution. Then, the absorbance was recorded at 517 nm. A blank was also prepared: 0.5 mL distilled water was mixed with 1 mL 0.02 mg/mL DPPH solution. The antioxidant activity was calculated using the formula:
where AControl is the absorbance of the blank DPPH solution and ASample is the absorbance of the aqueous extract mixed with 0.02 mg/mL DPPH solution.
AA % = [(AControl™ ASample)/AControl] × 100
3. Results and Discussions
3.1. Preparation of the AQUEOUS Extracts from Pumpkin Squash Species
The protocol used to prepare the aqueous extracts was the same for both Cucurbita maxima squash pumpkin species. For both species of squash pumpkin, five different aqueous extracts were prepared from three different parts of squash pumpkin as follows:
- three simple aqueous extracts from only one part, e.g., core, shell and seeds;
- two combined aqueous extracts from the core and shell in equal amounts, and the core, shell and seeds in equal amounts.
The resulted aqueous extracts were kept 24 h in a refrigerator (4 °C) to infuse, filtered until a clear liquid was obtained and kept in the refrigerator for more than 4 months.
3.2. Qualitative Screening for Bioactive Compounds
The qualitative screening for tannins clearly showed that they are absent in both squash pumpkin species, and the same conclusion was drawn for steroids and triterpenoids.
The qualitative screening for flavonoids showed a positive response only in the case of the aqueous extract prepared from the seeds of Valenciano pumpkin while in the case of Waltham Butternut pumpkin the presence of flavonoids could be visually observed for the seeds and the complex aqueous extract prepared from all three constituents.
Proteins are the main components of protoplasm and they are involved in all the natural processes that happen in all living cells. In nature, all proteins are colloidal and are irreversibly coagulated at a temperature higher than their boiling point. Proteins are not soluble in neutral salts (e.g., NaCl, MgSO4) and only solubilize once the salts are diluted. On the other hand, the majority of amino acids are soluble in water.
The qualitative analysis of amino acids refers to a color change, precipitation or even ring formation that appears as a result of a modification in the structural configuration upon reacting with a reagent. The results for the qualitative screening of carbohydrates in both squash pumpkin species are detailed in Table 1 for Valenciano pumpkin and Table 2 for Waltham Butternut pumpkin.

Table 1.
Qualitative screening of proteins and amino acids in Valenciano pumpkin.

Table 2.
Qualitative screening of proteins and amino acids in Waltham Butternut pumpkin.
The qualitative screening for proteins and amino acids in Waltham Butternut pumpkin squash clearly reveals that cysteine, a non-essential sulfur-containing amino acid, is absent from all the aqueous extracts while in the case of Valenciano pumpkin squash, cysteine can be found only in the seeds and the complex mixture of core, shell and seeds. The results in Table 2, and specifically for the aqueous extract prepared from the seeds of Waltham Butternut pumpkin squash, clearly show that they do not contain protein or amino acids.
Alkaloids can be analyzed as a bioactive compound by using three different qualitative tests and the results are presented in Table 3 for Valenciano pumpkin squash and Table 4 for Waltham Butternut pumpkin squash.

Table 3.
Qualitative screening of alkaloids for Valenciano pumpkin.

Table 4.
Qualitative screening of alkaloids for Waltham Butternut pumpkin.
The results detailed in Table 3 and Table 4 prove that, whatever the qualitative test used to screen for alkaloids, they are present in all ten aqueous extracts and the only difference is the intensity of the resulting solution.
Saponins represent another bioactive compound that was screened for in all the aqueous extracts and the results show that they are present in 8 out of 10 of the aqueous extracts, the exception being the seeds of both pumpkin squash species.
3.3. Quantitative Determination of Bioactive Compounds
The quantitative determination of bioactive compounds was carried out to determine the total content of tannins (TCT), flavonoids (TCF) and total content of polyphenols (TCP) (Table 5).

Table 5.
Quantitative methods for the determination of bioactive compounds.
The number of total tannins and the total content of flavonoids (Figure 2) are described as mg catechin/L and the ten aqueous extracts were analyzed in triplicate. The total content of polyphenols uses gallic acid as a standard calibration curve. The results are detailed in the charts below.
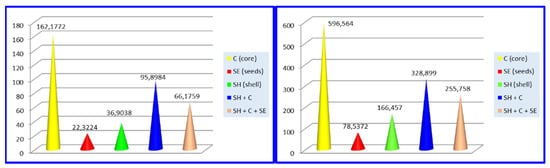
Figure 2.
Total content of flavonoids (TCF) for Valenciano (left) and Waltham Butternut (right).
Based on the detailed charts that represent the total content of flavonoids (TCF) for both pumpkin squash species it can be concluded that in the case of Valenciano pumpkin, the TCF was highest in the aqueous extract prepared from the core, followed by the mixture of core and shell, and the core and shell and seeds while seeds have the lowest concentration of TCF. In the case of Waltham Butternut pumpkin squash, the highest concentration was obtained from the core, followed by the mixture of the core and shell.
The total content of tannins (TCT) was investigated as mg catechin/L and the results are presented in Figure 3.
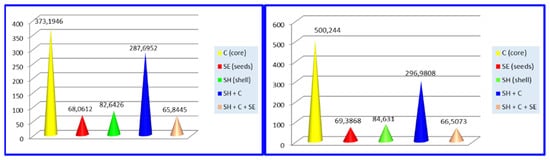
Figure 3.
Total content of tannins (TCT) for Valenciano (left) and Waltham Butternut (right).
By analyzing the charts in Figure 3, it can be concluded that the highest concentration of TCT was found in the core of both Valenciano and Waltham Butternut pumpkin squash (373.1946 mg/L for Valenciano and 500.244 mg/L for Waltham Butternut).
The total content of polyphenols (TCP) uses gallic acid as a standard calibration curve and was investigated as mg gallic acid/L and are detailed in Figure 4.
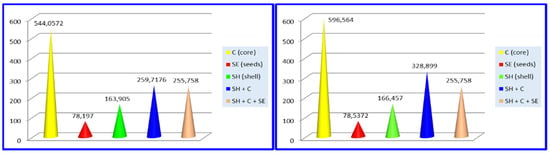
Figure 4.
Total content of polyphenols (TCP) for Valenciano (left) and Waltham Butternut (right).
The antioxidant activity (AA %) was measured using DPPH as the standard:
where AControl is the absorbance of the blank DPPH solution and ASample is the absorbance of the aqueous extract mixed with 0.02 mg/mL DPPH solution. The results are presented in Table 6 as a comparison between the two pumpkin squash species.
AA % = [(AControl − ASample)/AControl] × 100,

Table 6.
Antioxidant activity for pumpkin squash species.
From this table it can be concluded that the highest values were obtained for the shell aqueous extracts prepared from the shell of the two pumpkin squash species.
4. Conclusions
This research study describes two different species of squash pumpkin, Valenciano and Waltham Butternut, which were investigated by means of qualitative and quantitative phytochemical screening to determine the bioactive compounds. The qualitative screening for bioactive compounds involves a color change reaction that appears when mixing different extracts with known reagents and the quantitative screening for bioactive compounds is based on spectrophotometric determinations at well-established wavelengths.
The qualitative screening for tannins clearly showed that they are absent in both squash pumpkin species. The qualitative screening for proteins and amino acids in Waltham Butternut pumpkin squash clearly revealed that cysteine was absent in all the aqueous extracts. Alkaloids were also screened for and it was found that they were present in all ten aqueous extracts.
The quantitative determination of bioactive compounds was carried out to determine the total content of tannins (TCF), total content of flavonoids (TCF) and total content of polyphenols (TCP). In the case of Valenciano pumpkin, the TCF was highest in the aqueous extract prepared from the core (162.1772 mg/L), followed by the mixture of core and shell (95.8984 mg/L), and the core and shell and seeds (66.1759 mg/L) while seeds had the lowest concentration of TCF (22.3224 mg/L). In the case of Waltham Butternut pumpkin squash, the highest concentration was obtained for the core, followed by the mixture of core and shell.
The highest concentration for total content of tannins (TCT) was found in the core of both Valenciano and Waltham Butternut pumpkin squash (373.1946 mg/L for Valenciano and 500.244 mg/L for Waltham Butternut). From the results obtained for the antioxidant activity it can be concluded that the highest values were found in the shell aqueous extracts prepared from the shell of the two pumpkin squash species (54.52716% for Valenciano pumpkin and 54.12475% for Waltham Butternut).
The results presented clearly show that both Valenciano and Waltham Butternut are important sources of bioactive compounds with numerous health benefits.
Author Contributions
Conceptualization, A.-A.S. and A.N.; methodology, A.-A.S., A.N. and R.-M.I.; physical—chemical investigations, A.-A.S. and A.N.; writing—original draft preparation, A.-A.S.; writing—review and editing, A.-A.S. and A.N.; visualization, A.-A.S., A.N. and R.-M.I.; supervision, A.N. and R.-M.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tadmor, Y.; Paris, H.S.; Meir, A.; Schaffer, A.A.; Lewinsohn, E. Dual role of the pigmentation gene B in affecting carotenoid and vitamin E content in squash (Cucurbita pepo) mesocarp. J. Agric. Food Chem. 2005, 53, 9759–9763. [Google Scholar] [CrossRef] [PubMed]
- Zhemerichkin, D.A.; Ptichkina, N.M. The composition and properties of pumpkin and sugar beet pectins. Food Hydrocoll. 1995, 9, 147–149. [Google Scholar] [CrossRef]
- Li, Q.H.; Tian, Z.; Cai, T.Y. Study on the hypoglycaemic action of pumpkin extract in diabetic rat. Acta Nutr. Sin. 2001, 25, 34–36. [Google Scholar]
- Makni, M.; Fetoui, H.; Gargouri, N.K. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in x-3 and x-6 fatty acids in hypercholeserolemic rats. Food Chem. Toxicol. 2008, 46, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Fishier, R. Analysis of carotenoids with emphasis on 9-cis-β-carotene in vegetables and fruits commonly consumed in Israel. Food Chem. 1998, 62, 515–520. [Google Scholar] [CrossRef]
- Sheikh, N.; Kumar, Y.; Misra, A.K.; Pfoze, L. Phytochemical screening to validate the ethnobotanical importance of root tubers of Dioscorea species of Meghalaya, North East India. J. Med. Plants Stud. 2013, 1, 62–69. [Google Scholar]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; Chapman and Hall: London, UK, 1973. [Google Scholar]
- Okwu, D.E. Phytochemicals and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain. Agric. Environ. 2004, 6, 30–34. [Google Scholar]
- Chah, K.F.; Eze, C.A.; Emuelosi, C.A.; Esimone, C.E. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol. 2006, 104, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Kisseih, E.; Lechtenberg, M.; Petereit, F.; Sendker, F. Phytochemical characterization and in vitro wound healing activity of leaf extracts from Combretum mucronatum Schum. & Thonn.: Oligomeric procyanidins as strong inductors of cellular differentiation. J. Ethnopharmacol. 2015, 174, 628–636. [Google Scholar] [PubMed]
- Tona, L. Anti ameobic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 2005, 61, 57–65. [Google Scholar] [CrossRef]
- Biju, J.; Sulaiman, C.T.; Satheesh, G.; Reddy, V.R.K. Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharmacol. Sci. 2014, 6, 406–408. [Google Scholar]
- Alam, N.; Hossain, M.; Khalil, M.I.; Moniruzzaman, M.; Sulaiman, S.A.; Gan, S.H. High catechin concentrations detected in Withania somnifera (Ashwagandha) by high performance liquid chromatography analysis. BMC Complement. Altern. Med. 2011, 11, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).