Effects of Metformin on Antioxidative Response of Lactuca sativa Plants †
Abstract
:1. Introduction
2. Experiments
2.1. Experiment Design
2.2. Hydrogen Peroxide Content and Lipid Peroxidation
2.3. Antioxidant Enzymes Assays
2.4. Statistical Analysis
3. Results
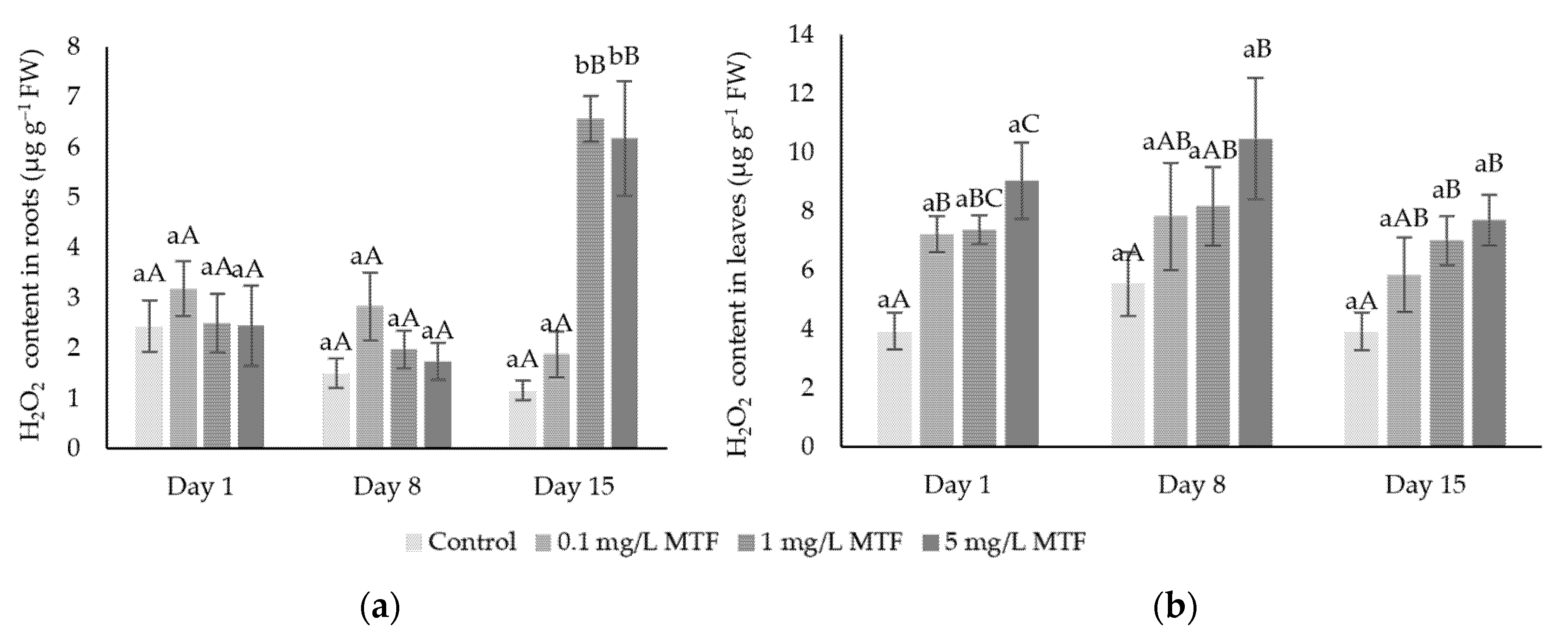
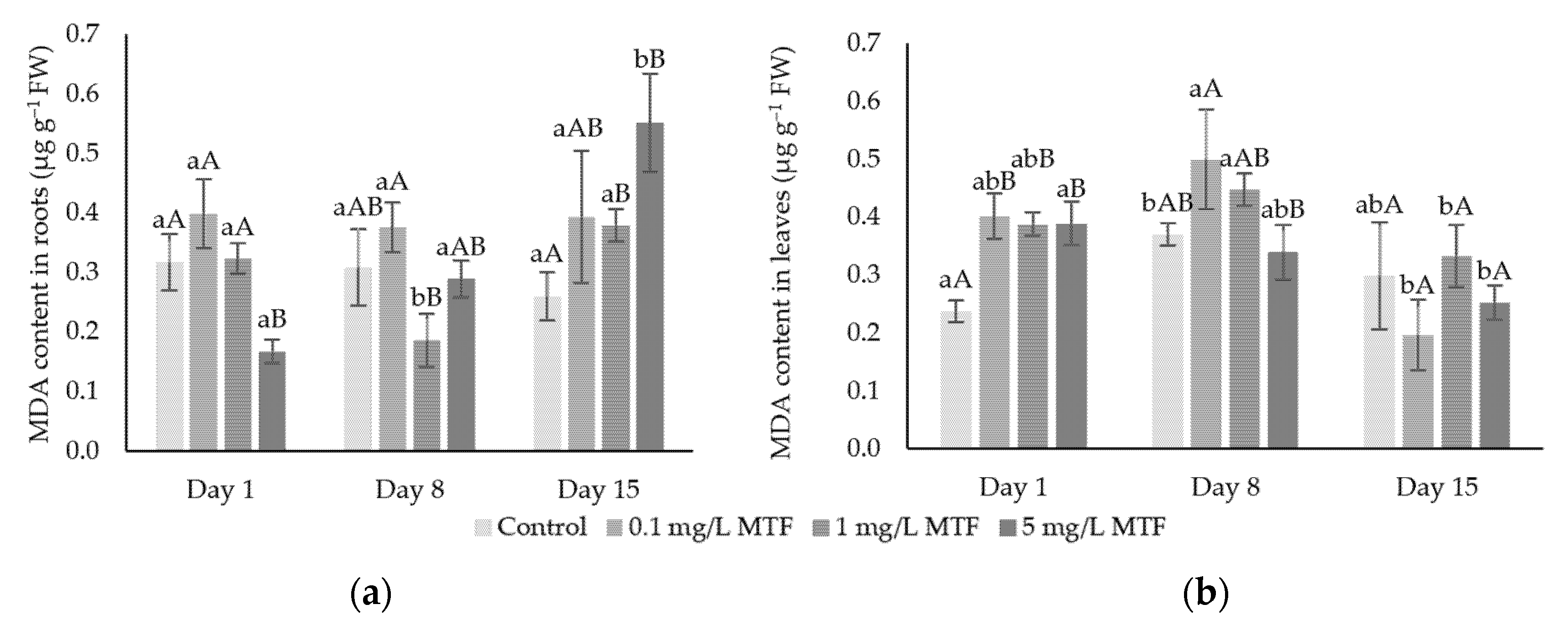
3.1. Oxidative Stress Indicators: H2O2 and MDA
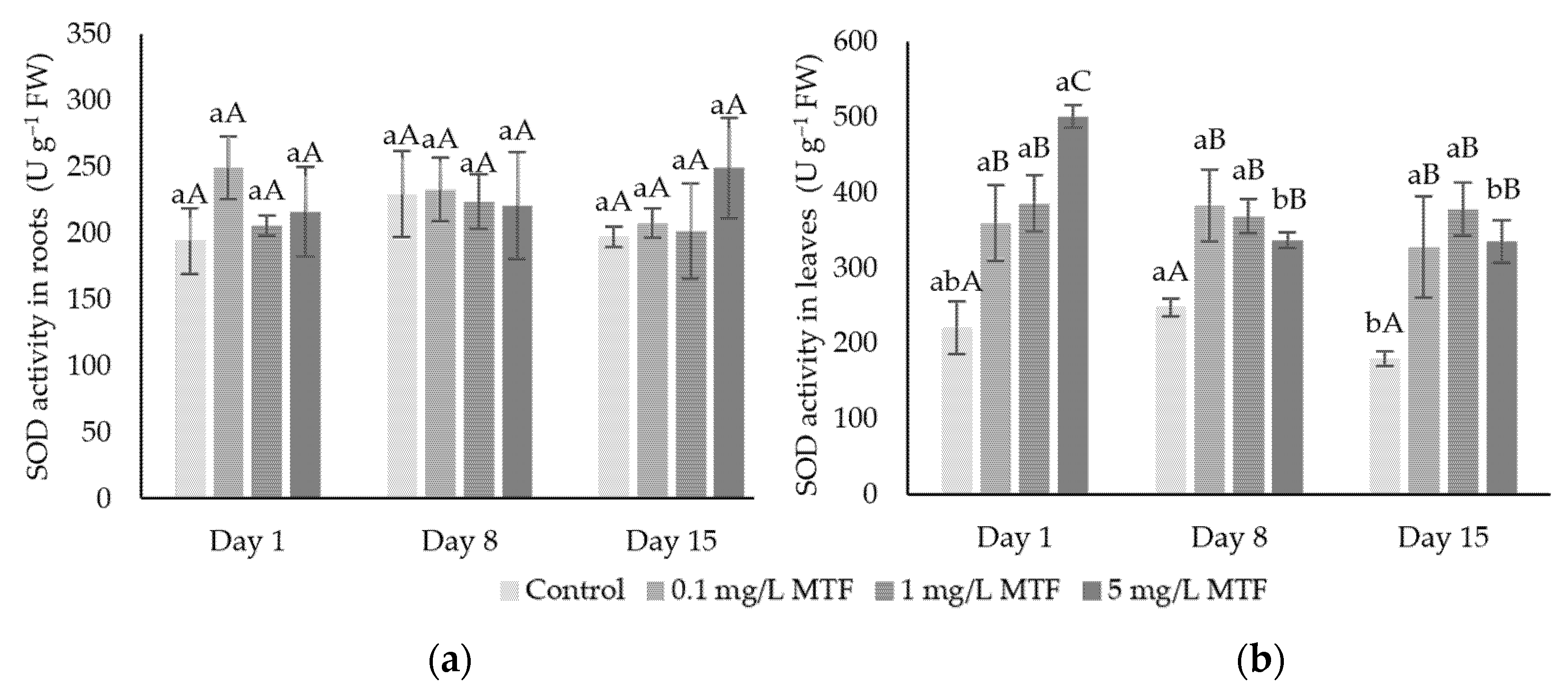
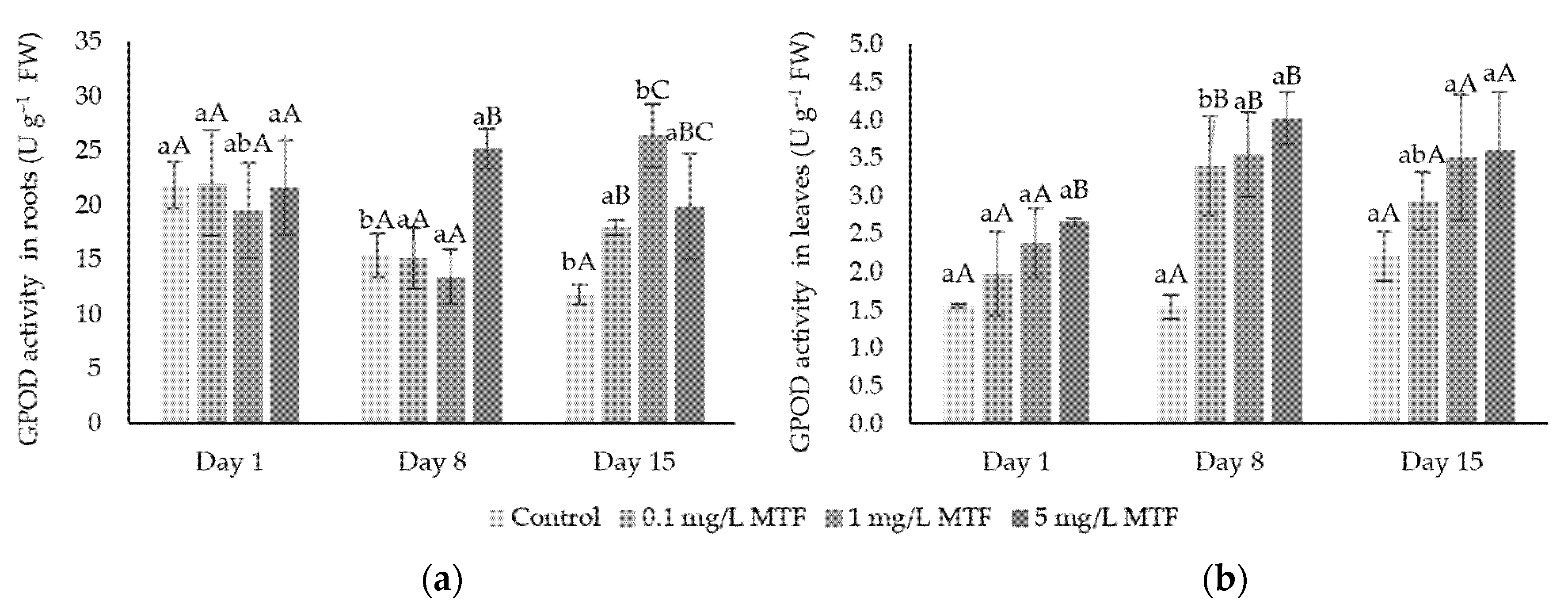
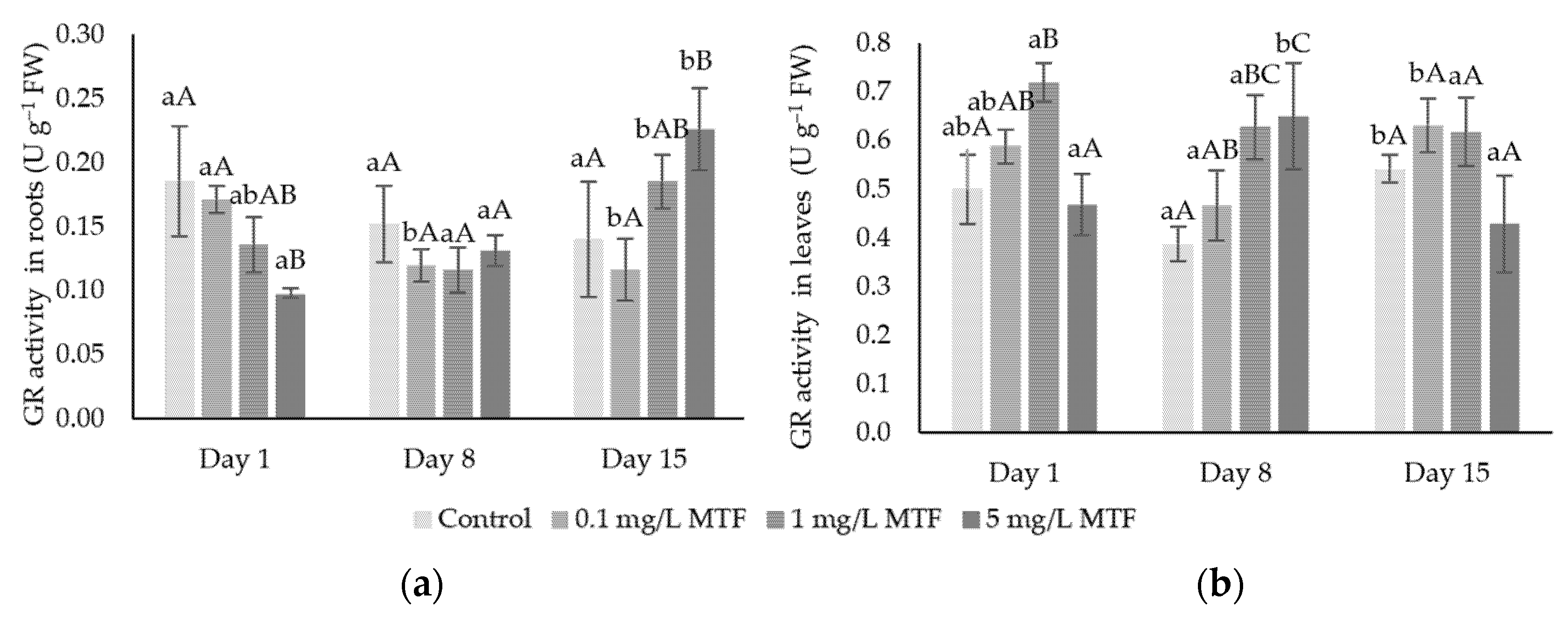
3.2. Enzymatic Mechanisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | ascorbate peroxidase |
| AsA | ascorbarte |
| CAT | catalase |
| GPOD | guaiacol peroxidase |
| GR | glutathione reductase |
| GPX | glutathione peroxidase |
| MDA | malondialdheyde |
| MTF | metformin |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Gaffney, V.D.J.; Cardoso, V.V.; Cardoso, E.; Teixeira, A.P.; Martins, J.; Benoliel, M.J.; Almeida, C.M. Occurrence and behaviour of pharmaceutical compounds in a Portuguese wastewater treatment plant: Removal efficiency through conventional treatment processes. Environ. Sci. Pollut. Res. 2017, 24, 14717–14734. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Picó, Y. Multi-residue determination of 47 organic compounds in water, soil, sediment and fish—Turia River as case study. J. Pharm. Biomed. Anal. 2017, 146, 17–125. [Google Scholar] [CrossRef]
- Kot-Wasik, A.; Jakimska, A.; Śliwka-Kaszyńska, M. Occurrence and seasonal variations of 25 pharmaceutical residues in wastewater and drinking water treatment plants. Environ. Monit. Assess. 2016, 188, 661. [Google Scholar] [CrossRef]
- Huber, S.; Remberger, M.; Kaj, L.; Schlabach, M.; Jörundsdóttir, H.Ó.; Vester, J.; Arnórsson, M.; Mortensen, I.; Schwartson, R.; Dam, M. A first screening and risk assessment of pharmaceuticals and additives in personal care products in waste water, sludge, recipient water and sediment from Faroe Islands, Iceland and Greenland. Sci. Total Environ. 2016, 562, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Eggen, T.; Lillo, C. Antidiabetic II drug metformin in plants: Uptake and translocation to edible parts of cereals, oily seeds, beans, tomato, squash, carrots, and potatoes. J. Agric. Food Chem. 2012, 60, 6929–6935. [Google Scholar] [CrossRef]
- Eggen, T.; Asp, T.N.; Grave, K.; Hormazabal, V. Uptake and translocation of metformin, ciprofloxacin and narasin in forage-and crop plants. Chemosphere 2011, 85, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Briones, R.M.; Sarmah, A.K.; Padhye, L.P. A global perspective on the use, occurrence, fate and effects of anti-diabetic drug metformin in natural and engineered ecosystems. Environ. Pollut. 2016, 219, 1007–1020. [Google Scholar] [CrossRef] [PubMed]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S. Multi-residue analytical method for human pharmaceuticals and synthetic hormones in river water and sewage effluents by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 6791–6806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartha, B.; der Naturwissenschaften, D. Uptake and Metabolism of Human Pharmaceuticals in Plants. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2012. [Google Scholar]
- Dordio, A.; VBelo, M.; Teixeira, D.M.; Carvalho, A.P.; Dias, C.; Picó, Y.; Pinto, A. Evaluation of carbamazepine uptake and metabolization by Typha spp., a plant with potential use in phytotreatment. Bioresour. Technol. 2011, 102, 7827–7834. [Google Scholar]
- Huber, C.; Bartha, B.; Harpaintner, R.; Schröder, P. Metabolism of acetaminophen (paracetamol) in plants-two independent pathways result in the formation of a glutathione and a glucose conjugate. Environ. Sci. Pollut. Res. 2009, 16, 206–213. [Google Scholar] [CrossRef] [Green Version]
- Mukhtar, A.; Manzoor, M.; Gul, I.; Zafar, R.; Jamil, H.I.; Niazi, A.K.; Ali, M.A.; Park, T.J.; Arshad, M. Phytotoxicity of different antibiotics to rice and stress alleviation upon application of organic amendments. Chemosphere 2020, 258, 127353. [Google Scholar] [CrossRef]
- Riaz, L.; Mahmood, T.; Coyne, M.; Khalid, A.; Rashid, A.; Hayat, M.T.; Gulzar, A.; Amjad, M. Physiological and antioxidant response of wheat (Triticum aestivum) seedlings to fluoroquinolone antibiotics. Chemosphere 2017, 177, 250–257. [Google Scholar] [CrossRef]
- Fernández, R.; Bertrand, A.; Reis, R.; Mourato, M.P.; Martins, L.L.; González, A. Growth and physiological responses to cadmium stress of two populations of Dittrichia viscosa (L.) Greuter. J. Hazard. Mater. 2013, 244–245, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.L.; Mourato, M.P.; Cardoso, A.I.; Pinto, A.P.; Mota, A.M.; Maria de Lurdes, S.G.; de Varennes, A. Oxidative stress induced by cadmium in Nicotiana tabacum L.: Effects on growth parameters, oxidative damage and antioxidant responses in different plant parts. Acta Physiol. Plant. 2011, 33, 1375–1383. [Google Scholar]
- Pinto, F.R.; Mourato, M.P.; Sales, J.R.; Moreira, I.N.; Martins, L.L. Oxidative stress response in spinach plants induced by cadmium. J. Plant Nutr. 2016, 40, 268–276. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Gao, C.-J.; Ogweno, J.O.; Zhou, Y.; Xia, X.-J.; Mao, W.-H.; Shi, K.; Yu, J.Q. Brassinosteroids induce plant tolerance against phenanthrene by enhancing degradation and detoxification in Solanum lycopersicum L. Ecotoxicol. Environ. Saf. 2012, 80, 28–36. [Google Scholar] [CrossRef]
- Kapoor, D.; Singh, S.; Kumar, V.; Romero, R.; Prasad, R.; Singh, J. Antioxidant enzymes regulation in plants in reference to reactive oxygen species (ROS) and reactive nitrogen species (RNS). Plant Gene 2019, 19, 100182. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Peng, X.; Xu, S.; Zhang, H.; Gao, J.; Xi, Z.-M. Exogenous 24-epibrassinolide regulates antioxidant and pesticide detoxification systems in grapevine after chlorothalonil treatment. Plant Growth Regul. 2017, 81, 455–466. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Sharma, I.; Bhardwaj, R.; Pati, P.K. Stress modulation response of 24-epibrassinolide against imidacloprid in an elite indica rice variety Pusa Basmati-1. Pestic. Biochem. Physiol. 2013, 105, 144–153. [Google Scholar] [CrossRef]
- Kolahi, M.; Kazemi, E.M.; Yazdi, M.; Goldson-Barnaby, A. Oxidative stress induced by cadmium in lettuce (Lactuca sativa Linn.): Oxidative stress indicators and prediction of their genes. Plant Physiol. Biochem. 2020, 146, 71–89. [Google Scholar] [PubMed]
- Islam, T.; Manna, M.; Kaul, T.; Pandey, S.; Reddy, C.S.; Reddy, M.K. Genome-Wide Dissection of Arabidopsis and Rice for the Identification and Expression Analysis of Glutathione Peroxidases Reveals Their Stress-Specific and Overlapping Response Patterns. Plant Mol. Biol. Report. 2015, 33, 1413–1427. [Google Scholar] [CrossRef]
- Bartha, B.; Huber, C.; Schröder, P. Uptake and metabolism of diclofenac in Typha latifolia—How plants cope with human pharmaceutical pollution. Plant Sci. 2014, 227, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Bartha, B.; Huber, C. Effects of acetaminophen in Brassica juncea L. Czern. investigation of uptake, translocation, detoxification, and the induced defense pathways. Environ. Sci. Pollut. Res. 2010, 17, 1553–1562. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leitão, I.; Mourato, M.P.; Sales, J.; Marques, M.M.; Oliveira, M.C.; Martins, L.L. Effects of Metformin on Antioxidative Response of Lactuca sativa Plants. Biol. Life Sci. Forum 2021, 4, 63. https://doi.org/10.3390/IECPS2020-08771
Leitão I, Mourato MP, Sales J, Marques MM, Oliveira MC, Martins LL. Effects of Metformin on Antioxidative Response of Lactuca sativa Plants. Biology and Life Sciences Forum. 2021; 4(1):63. https://doi.org/10.3390/IECPS2020-08771
Chicago/Turabian StyleLeitão, Inês, Miguel P. Mourato, Joana Sales, Maria Matilde Marques, Maria Conceição Oliveira, and Luisa L. Martins. 2021. "Effects of Metformin on Antioxidative Response of Lactuca sativa Plants" Biology and Life Sciences Forum 4, no. 1: 63. https://doi.org/10.3390/IECPS2020-08771
APA StyleLeitão, I., Mourato, M. P., Sales, J., Marques, M. M., Oliveira, M. C., & Martins, L. L. (2021). Effects of Metformin on Antioxidative Response of Lactuca sativa Plants. Biology and Life Sciences Forum, 4(1), 63. https://doi.org/10.3390/IECPS2020-08771








