Fungicide-Free Management of Papaya Anthracnose (Colletotrichum gloeosporioides Penz.) Disease Using Combined Bio-Rationales and Bee Wax in Organic Agriculture †
Abstract
:1. Introduction
2. Experiments
2.1. Crude Extraction of Botanicals
2.2. In Vivo Assay of Bio-Rationales against Colletotrichum Gloeosporioides
2.3. Data Analysis
3. Results
3.1. In Vivo Evaluation of Disease Incidence and Disease Severity
3.2. In Vivo Evaluation of Physiochemical (pH, TSS, and Weight Loss) Changes of Papaya Fruits with Different Coatings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Botanicals | pH after Preservation | TSS after Preservation | Weight Loss Percentage after Preservation (WLP) | |||
|---|---|---|---|---|---|---|
| 5th Day | 12th Day | 5th Day | 12th Day | 5th Day | 12th Day | |
| Ocimum basilicum | 5.32 ± 0.35 Aa | 5.42 ± 0.059 Adc | 10.10 ± 0.57 Bbc | 12.44 ± 0.57 Acb | 9.128 ± 1.9 Bba | 23.055 ± 5.87 Abac |
| Ocimum tenuiflorum | 5.28 ± 0.04 Aa | 5.50 ± 0.17 Ac | 9.77 Bbc | 11.48 ± 0.170 Afed | 7.283 ± 1.62 Bb | 17.047 ± 3.86 Ac |
| Allium sativum | 5.11 ± 0.18 Bcd | 5.339 ± 0.29 Abc | 9.10 ± 0.57 Bc | 11.63 ± 0.25 Aced | 8.299 ± 2.99 Bba | 18.797 ± 6.4 Abc |
| Azadiracta indica | 5.01 ± 0.086 Bcd | 5.55 ± 0.036 Abc | 10.77 Bbac | 12.58 ± 0.15 Ab | 11.538 ± 797 Ba | 27.14 ± 8.64 Aa |
| lantana camara | 5.26 ± 0.078 Ba | 5.92 ± 0.022 Aa | 9.73 ± 1.75 Bbc | 12.14 ± 0.63 Acbd | 10.215 ± 4.15 Bba | 20.349 ± 5.02 Abac |
| Ocimumcinnamon | 4.83 ± 0.01 Bfed | 5.23 ± 0.173 Ad | 11.33 ± 2.21 Bba | 13.37 ± 1.22 Aa | 11.472 ± 1.67 Ba | 24.739 ± 4.017 Aba |
| control | 4.88±0.088 Bced | 5.51 ± 0.11 Ac | 11.80 ± 0.15 Ba | 13.67 ± 0.1 Aa | 7.004 ± 0.492 Bb | 16.764 ± 0.322 Ac |
| Ocimum basilium + wax | 4.822 ± 0.002 Bfed | 5.77 ± 0.133 Aba | 10.40 ± 1.09 Bbac | 10.78 ± 0.005 Afeg | 1.6367 ± 0.825 Bc | 4.777 ± 2.98 Ad |
| Ocimum tenuiforum + wax | 4.97 ± 0.061 Bced | 5.78 ± 0.026 Aba | 9.59 ± 0.23 Bc | 10.77 ± 0.05 Afeg | 0.6927 ± 0.226 Bc | 1.58 ± 0.52 Ad |
| Allium sativum + wax | 4.53 ± 0.056 Bg | 5.79 ± 0.26 Aba | 10.103 ± 0.65 Bbc | 11.51 ± 0.22 Afed | 1.1753 ± 0.415 Bc | 2.86 ± 1.6028 Ad |
| Azadiracta indica + wax | 5.02 ± 0.009 Bcd | 5.84 ± 0.0569 Aa | 9.59 ± 0.24 Bc | 11.85 ± 0.085 Acbd | 0.3507 ± 0.044 Bc | 1.657 ± 0.6411 Ad |
| lantana camara + wax | 4.71 ± 0.076 Bf | 5.44 ± 0.0152 Adc | 9.63 ± 0.10 Bc | 10.61 ± 0.33 Acbd | 0.6827 ± 0.33 Bc | 2.416 ± 0.974 Ad |
| Ocimum cinnamon + wax | 4.80 ± 0.072 Bfed | 5.40 ± 0.168 Adc | 9.51 ± 0.42 Bc | 10.53 ± 0.35 Ag | 1.7353 ± 1.20 Bc | 4.118 ± 1.821 Ad |
| control + wax | 4.36 ± 0.02 Bh | 5.77 ± 0.55 Aba | 10.29 ± 0.25 Bc | 11.55 ± 0.69 Afed | 0.0642 ± 0.270 Bc | 1.618 ± 0.0367 Ad |
References
- Chávez-Pesqueira, M.; Núñez-Farfán, J. Domestication and Genetics of Papaya: A Review. Front. Ecol. Evol. 2017, 5, 155. [Google Scholar] [CrossRef] [Green Version]
- Ademe, A.; Ayalew, A.; Woldetsadik, K. Evaluation of Antifungal Activity of Plant Extracts against Papaya Anthracnose (Colletotrichum gloeosporioides). J. Plant Pathol. Microb. 2013, 4, 207. [Google Scholar] [CrossRef] [Green Version]
- Anup Kumar, S. Anthracnose diseases of some common medicinally important fruit plants. J. Med. Plants Stud. 2016, 4, 233–236. Available online: https://www.researchgate.net/publication/316889416_Anthracnose_diseases_of_some_common_medicinally_important_fruit_plants (accessed on 25 October 2020).
- Dickman, M.B.; Ploetz, R.C.; Zentmyer, G.A.; Nishijima, W.T.; Rohrbach, K.G.; Ohr, H.D. Papaya Anthracnose: Compendium of Tropical Fruit Diseases; American Phytopathological Society: Minneapolis, MN, USA, 1994; pp. 58–59. [Google Scholar]
- Jayathunge, K.G.L.R.; Prasad, H.U.K.C.; Fernando, M.D.; Palipane, K.B. Prolonging the postharvest life of papaya using modified atmosphere packaging. J. Agric. Technol. 2011, 7, 507–518. Available online: http://www.thaiscience.info/journals/Article/IJAT/10842531.pdf (accessed on 20 January 2020).
- Li, X.; Zhu, X.; Zhao, N.; Fu, D.; Li, J.; Chen, W. Effects of hot water treatment on anthracnose disease in papaya fruit and its possible mechanism. Postharvest Biol. Technol. 2013, 86, 437–446. [Google Scholar] [CrossRef]
- Baker, R.E.D.; Crowdy, S.H.; McKee, R.K. A review of latent infections caused by Colletotrichum gloeosporioides and allied fungi. Trop. Agric. Trinidad. 1940, 17, 128–132. Available online: https://www.cabdirect.org/cabdirect/abstract/19401101455 (accessed on 25 October 2020).
- Gamagaea, S.U.; Sivakumarar, D.; Wilson, S.; Wijeratnama, R.; Wijesundera, R.L.C. Use of sodium bicarbonate and Candida oleophila to control anthracnose in papaya during storage. Crop Prot. 2003, 22, 775–779. [Google Scholar] [CrossRef]
- Siqueira Júnior, C.L.; Freire, M.D.G.M.; Moreira, A.S.N.; Macedo, M.L.R. Control of papaya fruits anthracnose by essential oil of Ricinus communis. Braz. Arch. Biol. Technol. 2012, 55, 75–80. [Google Scholar] [CrossRef] [Green Version]
- AjayKumar, K. Colletotrichum gloeosporioides: Biology, Pathogenicity and Management in India. J. Plant Physiol. Pathol. 2014, 2, 2. [Google Scholar] [CrossRef]
- Muryati Trisyono, Y.A. Effects of citronella grass extract on the oviposition behavior of carambola fruit fly (Bactrocera carambolae) in mango. J. Agric. Biol. Sci. 2012, 7, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Bron, I.U.; Jacomino, A.P. Ripening and quality of ‘Golden’ papaya fruit harvested at different maturity stages. Braz. J. Plant Physiol. 2006, 18, 389–396. [Google Scholar] [CrossRef] [Green Version]
- Sellamuthu, P.; Sivakumar, D.; Soundy, P. Antifungal Activity and Chemical Composition of Thyme, Peppermint and Citronella Oils in Vapor Phase against Avocado and Peach Postharvest Pathogens. J. Food Saf. 2013, 33, 86–93. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; El-Khateeb, A.Y.; Azzaz, N.A. Chemical Composition and Fungicidal Effects of Ocimum basilicum Essential Oil on Bipolaris and Cochliobolus Species. J. Agric. Sci. Technol. 2016, 18, 1143–1152. Available online: http://scholar.google.com/scholar_url?url=https://www.sid.ir/en/VEW-SID/J_pdf/84820160421.pdf&hl=en&sa=X&ei=QhGiX7n_Dde2yATS17rYCQ&scisig=AAGBfm0iFJhZvNSIzSQEhz9t6WvHKfFH-g&nossl=1&oi=scholarr (accessed on 20 January 2020).
- Oniha, M.; Eqwari, L. Fruit, Leaf and stem diseases of Carica papaya L. J. Agric. Food 2015, 3, 398–407. Available online: http://eprints.covenantuniversity.edu.ng/9164/1/PDF%20Paper%202.pdf (accessed on 21 January 2020).
- Hazarika, T.K.; Lalthanpuii, L.; Mandal, D. Influence of edible coatings on physico-chemical characteristics and shelf-life of papaya (Carica papaya) fruits during ambient storage. Indian J. Agric. Sci. 2017, 87, 1077–1083. Available online: https://www.researchgate.net/publication/319207766_Influence_of_edible_coatings_on_physico-chemical_characteristics_and_shelf-life_of_papaya_Carica_papaya_fruits_during_ambient_storage (accessed on 20 January 2020).
- Bautista-Banos, S.; Dharini, S.; Bello-Perez, A.; Villanueva-Arce, R.; Hernandez-Lopez, M. A review of the management alternatives for controlling fungi on papaya fruit during the postharvest supply chain. Crop Prot. 2012, 49, 8–20. [Google Scholar] [CrossRef]
- Mukherjee, A.; Khandker, S.; Islam, M.R.; Sonia, B.S. Efficacy of some plant extracts on the mycelial growth of Colletotrichum gloeosporioides. J. Bangladesh Agric. Univ. 2011, 9, 43–47. Available online: https://ageconsearch.umn.edu/record/209026/files/8742-31942-1-PB.pdf (accessed on 21 February 2020). [CrossRef] [Green Version]
- Cha, D.S.; Chinnan, M. Biopolymer-based antimicrobial packaging: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Avena-Bustillos, R.J.; Krochta, J.M.; Saltveit, M.E.; Rojas-Villegas, R.J.; Sauceda-Perez, J.A. Optimization of edible coating formulations on zucchini to reduce water loss. J. Food Eng. 1994, 21, 197–214. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Osman, A.; Tan, C.P.; Rahman, R.A. Effects of edible surface coatings (Sodium carboxymethyl cellulose, sodium caseinate and glycerol) on storage quality of berangan banana (Musa sapientum cv. Berangan) using response surface methodology. J. Food Process. Preserv. 2012, 36, 252–261. [Google Scholar] [CrossRef]
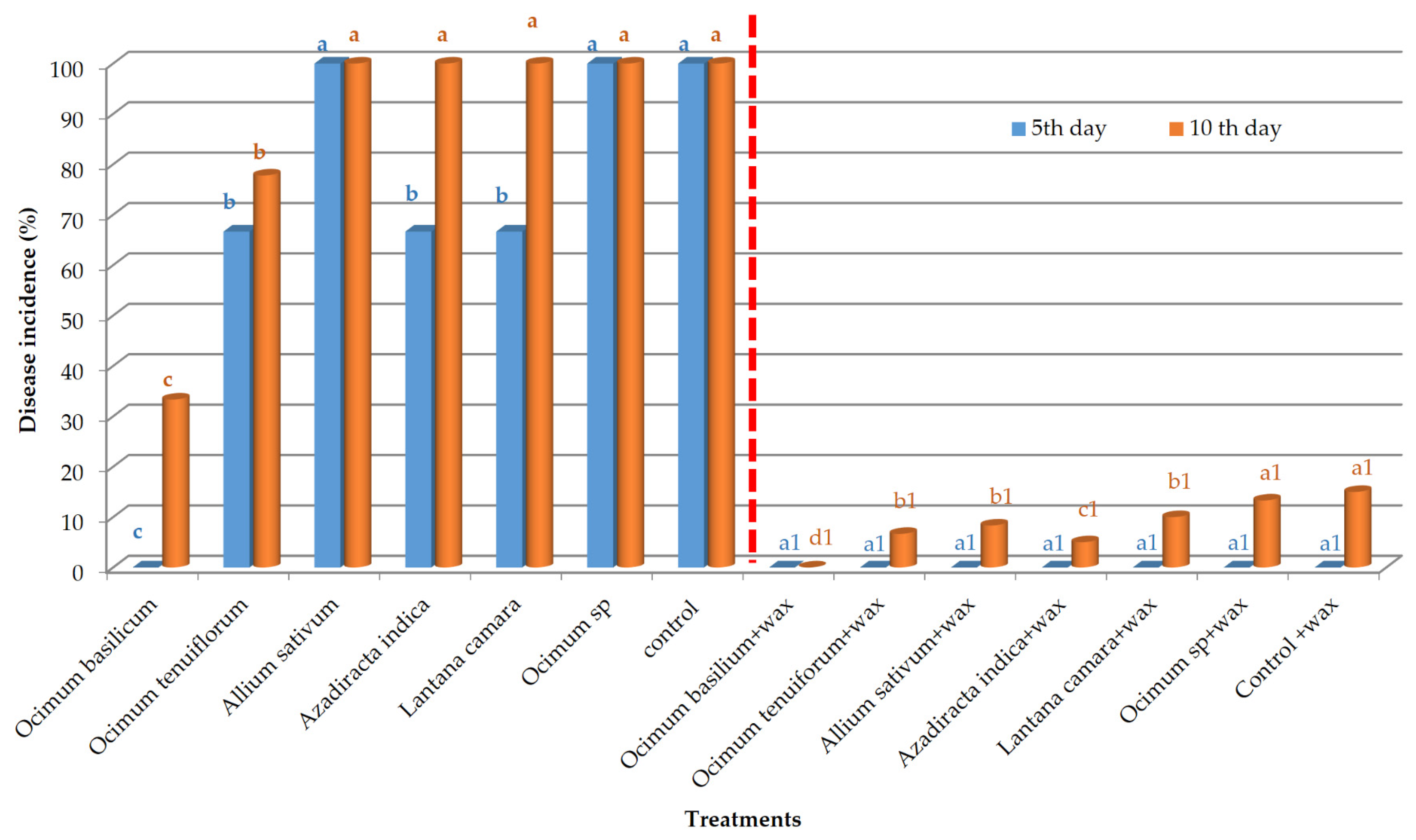
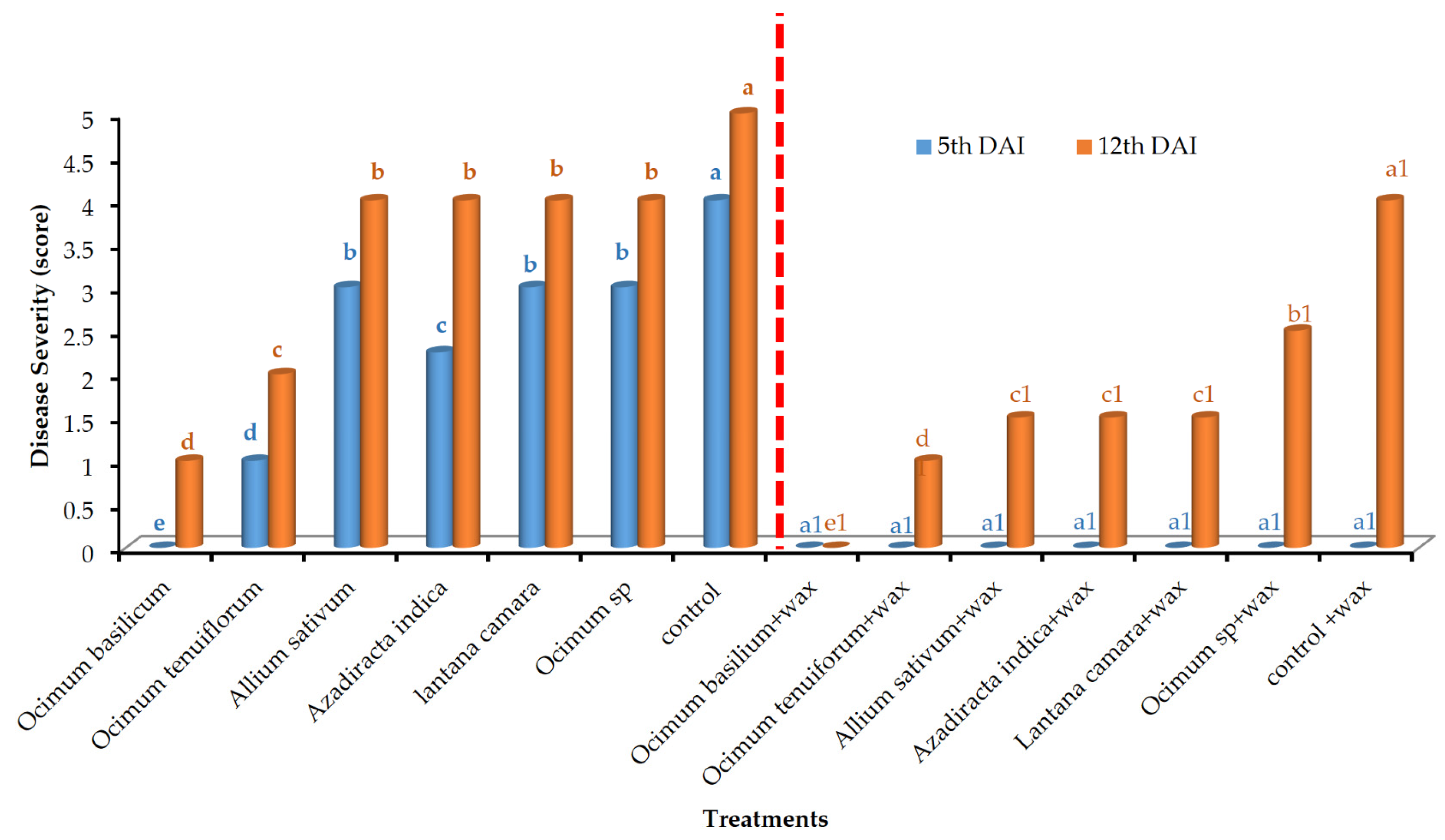
| Treatment Number | Treatment-Set 1 | Treatment-Set 2 |
|---|---|---|
| T1 | Ocimum basilicum | Ocimum basilicum + wax |
| T2 | Ocimum tenuiforum | Ocimum tenuiforum + wax |
| T3 | Allium sativum | Allium sativum + wax |
| T4 | Azadiracta indica | Azadiracta indica + wax |
| T5 | Lantana camara | Lantana camara + wax |
| T6 | Ocimum cinnamon | Ocimum cinnamon + wax |
| T7 | Control | Control + wax |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikantharajah, N.; Pakeerathan, K.; Mikunthan, G. Fungicide-Free Management of Papaya Anthracnose (Colletotrichum gloeosporioides Penz.) Disease Using Combined Bio-Rationales and Bee Wax in Organic Agriculture. Biol. Life Sci. Forum 2021, 4, 16. https://doi.org/10.3390/IECPS2020-08906
Srikantharajah N, Pakeerathan K, Mikunthan G. Fungicide-Free Management of Papaya Anthracnose (Colletotrichum gloeosporioides Penz.) Disease Using Combined Bio-Rationales and Bee Wax in Organic Agriculture. Biology and Life Sciences Forum. 2021; 4(1):16. https://doi.org/10.3390/IECPS2020-08906
Chicago/Turabian StyleSrikantharajah, Niveka, Kandiah Pakeerathan, and Gunasingham Mikunthan. 2021. "Fungicide-Free Management of Papaya Anthracnose (Colletotrichum gloeosporioides Penz.) Disease Using Combined Bio-Rationales and Bee Wax in Organic Agriculture" Biology and Life Sciences Forum 4, no. 1: 16. https://doi.org/10.3390/IECPS2020-08906
APA StyleSrikantharajah, N., Pakeerathan, K., & Mikunthan, G. (2021). Fungicide-Free Management of Papaya Anthracnose (Colletotrichum gloeosporioides Penz.) Disease Using Combined Bio-Rationales and Bee Wax in Organic Agriculture. Biology and Life Sciences Forum, 4(1), 16. https://doi.org/10.3390/IECPS2020-08906






