In-Silico Investigation of Phyllanthus niruri Phytochemicals as Hepatic Fibrosis Modulators †
Abstract
1. Introduction
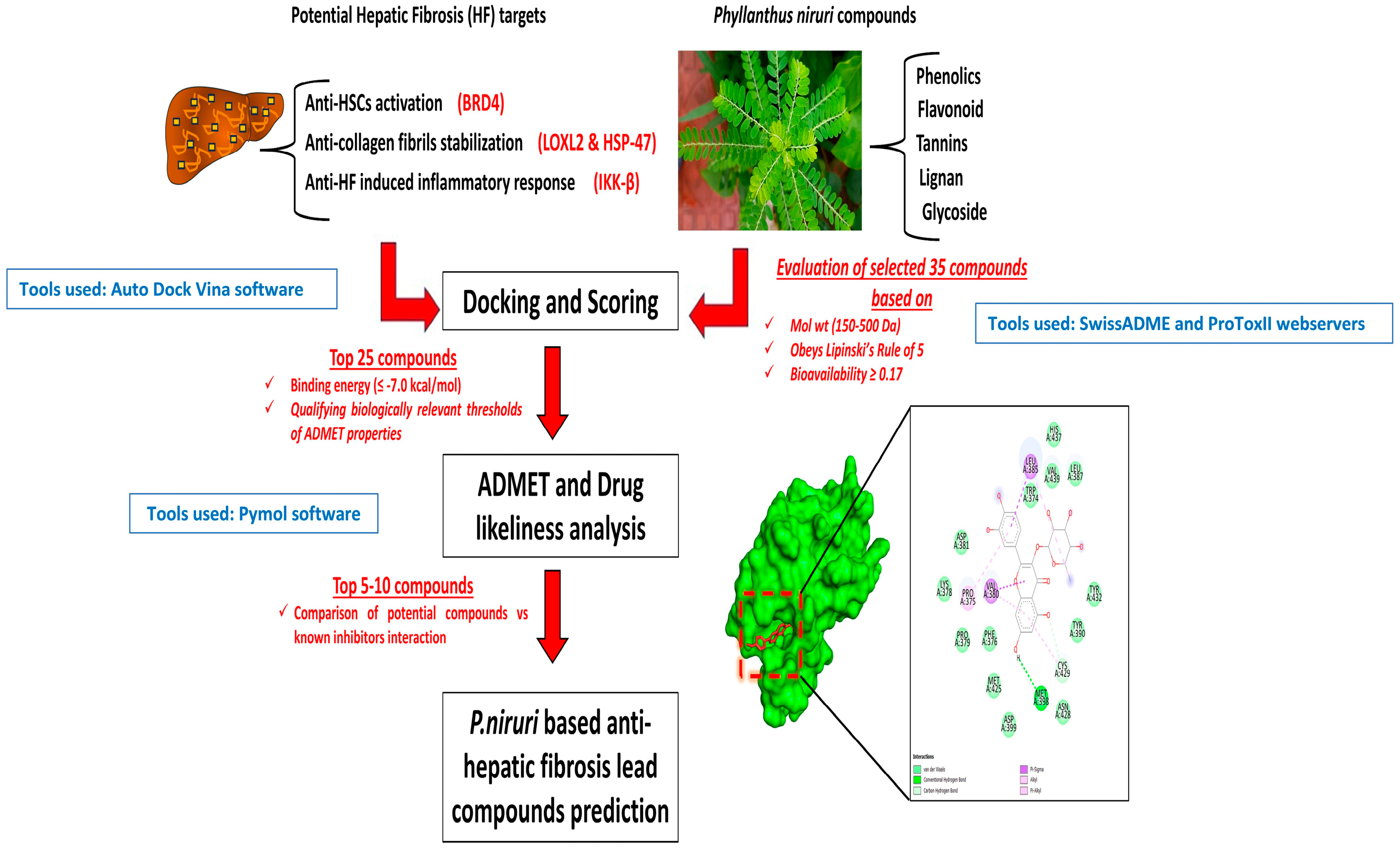
2. Materials and Methods
2.1. Initial Compounds Selection and ADME Studies
2.2. Preparation of Ligands, Protein Structures
2.3. In Silico Molecular Docking Analysis
2.4. In Silico Prediction of Toxicity Parameters
3. Results and Discussion
3.1. ADME/T Profiles of the Best
3.2. Molecular Docking Analysis
3.3. In Silico Toxicity Prediction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsukada, S.; Parsons, C.J.; Rippe, R.A. Mechanisms of liver fibrosis. Clin. Chim. Acta 2006, 364, 33–60. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-T.; Liao, Z.-X.; Ping, J.; Xu, D.; Wang, H. Molecular mechanism of hepatic stellate cell activation and antifibrotic therapeutic strategies. J. Gastroenterol. 2008, 43, 419–428. [Google Scholar] [CrossRef]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef]
- Khomich, O.; Ivanov, A.V.; Bartosch, B. Metabolic Hallmarks of Hepatic Stellate Cells in Liver Fibrosis. Cells 2019, 9, 24. [Google Scholar] [CrossRef]
- Li, R.; Zhang, J.; Liu, Q.; Tang, Q.; Jia, Q.; Xiong, Y.; He, J.; Li, Y. CREKA-modified liposomes target activated hepatic stellate cells to alleviate liver fibrosis by inhibiting collagen synthesis and angiogenesis. Acta Biomater. 2023, 168, 484–496. [Google Scholar] [CrossRef]
- Liu, S.B.; Ikenaga, N.; Peng, Z.; Sverdlov, D.Y.; Greenstein, A.; Smith, V.; Schuppan, D.; Popov, Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J. 2016, 30, 1599–1609. [Google Scholar] [CrossRef]
- Saneyasu, T.; Akhtar, R.; Sakai, T. Molecular Cues Guiding Matrix Stiffness in Liver Fibrosis. BioMed Res. Int. 2016, 2016, 2646212. [Google Scholar] [CrossRef]
- Czaja, A.J.; Carpenter, H.A. Progressive fibrosis during corticosteroid therapy of autoimmune hepatitis. Hepatology 2004, 39, 1631–1638. [Google Scholar] [CrossRef]
- Abdel-Sattar, O.E.; Allam, R.M.; Al-Abd, A.M.; Avula, B.; Katragunta, K.; Khan, I.A.; El-Desoky, A.M.; Mohamed, S.O.; El-Halawany, A.; Abdel-Sattar, E.; et al. Cytotoxic and chemomodulatory effects of Phyllanthus niruri in MCF-7 and MCF-7ADR breast cancer cells. Sci. Rep. 2023, 13, 2683. [Google Scholar] [CrossRef]
- Sowjanya, K.; Girish, C.; Bammigatti, C.; Lakshmi, N.C.P. Efficacy of Phyllanthus niruri on improving liver functions in patients with alcoholic hepatitis. Indian J. Pharmacol. 2021, 53, 448–456. [Google Scholar] [CrossRef]
- Cheng, M.; Li, J.J.; Niu, X.N.; Zhu, L.; Liu, J.Y.; Jia, P.C.; Zhu, S.; Meng, H.W.; Lv, X.W.; Huang, C.; et al. BRD4 promotes hepatic stellate cells activation and hepatic fibrosis via mediating P300/H3K27ac/PLK1 axis. Biochem. Pharmacol. 2023, 210, 115497. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lu, S.; Pan, N.; Zhao, F.; Jia, X.; Wang, S.; Zhang, Y.; Zhou, Y. Bromodomain protein 4 is a key molecular driver of TGFβ1-induced hepatic stellate cell activation. Biochim. Et Biophys. Acta BBA—Mol. Cell Res. 2023, 1870, 119569. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Jin, L.; Wang, J. HSP47 and Its Involvement in Fibrotic Disorders; Springer: Berlin/Heidelberg, Germany, 2019; pp. 299–312. [Google Scholar]
- Yuan, W.; Qiu, T.; Yao, X.; Wu, C.; Shi, Y.; Wang, N.; Zhang, J.; Jiang, L.; Liu, X.; Yang, G.; et al. Hsp47 acts as a bridge between NLRP3 inflammasome and hepatic stellate cells activation in arsenic-induced liver fibrosis. Toxicol. Lett. 2022, 370, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Klepfish, M.; Gross, T.; Vugman, M.; Afratis, N.A.; Havusha-Laufer, S.; Brazowski, E.; Solomonov, I.; Varol, C.; Sagi, I. LOXL2 Inhibition Paves the Way for Macrophage-Mediated Collagen Degradation in Liver Fibrosis. Front. Immunol. 2020, 11, 480. [Google Scholar] [CrossRef]
- Ikenaga, N.; Peng, Z.W.; Vaid, K.A.; Liu, S.B.; Yoshida, S.; Sverdlov, D.Y.; Mikels-Vigdal, A.; Smith, V.; Schuppan, D.; Popov, Y.V. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 2017, 66, 1697–1708. [Google Scholar] [CrossRef]
- Beraza, N.; Malato, Y.; Borght, S.V.; Liedtke, C.; Wasmuth, H.E.; Dreano, M.; de Vos, R.; Roskams, T.; Trautwein, C. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut 2008, 57, 655–663. [Google Scholar] [CrossRef]
- Wei, J. IκB kinase-beta inhibitor attenuates hepatic fibrosis in mice. World J. Gastroenterol. 2011, 17, 5203. [Google Scholar] [CrossRef]
- Şahin, S. 3,4-difluoro-2-(((4-phenoxyphenyl)imino)methyl)phenol with in silico predictions: Synthesis, spectral analyses, ADME studies, targets and biological activity, toxicity and adverse effects, site of metabolism, taste activity. J. Mol. Struct. 2024, 1317, 139136. [Google Scholar] [CrossRef]
- Shah-Abadi, M.E.; Ariaei, A.; Moradi, F.; Rustamzadeh, A.; Tanha, R.R.; Sadigh, N.; Marzban, M.; Heydari, M.; Ferdousie, V.T. In Silico Interactions of Natural and Synthetic Compounds with Key Proteins Involved in Alzheimer’s Disease: Prospects for Designing New Therapeutics Compound. Neurotox. Res. 2023, 41, 408–430. [Google Scholar] [CrossRef]
- Wu, S.; Liang, C.; Xie, X.; Huang, H.; Fu, J.; Wang, C.; Su, Z.; Wang, Y.; Qu, X.; Li, J.; et al. Hsp47 Inhibitor Col003 Attenuates Collagen-Induced Platelet Activation and Cerebral Ischemic–Reperfusion Injury in Rats. Front. Pharmacol. 2022, 12, 792263. [Google Scholar] [CrossRef]
- Deshpande, H. Levoleucovorin inhibits LOXL2 (lysyl oxidase like-2) to control breast cancer proliferation: A repurposing approach. J. Biomol. Struct. Dyn. 2024, 42, 5104–5113. [Google Scholar] [CrossRef] [PubMed]
- Popov, S.A.; Semenova, M.D.; Baev, D.S.; Sorokina, I.V.; Zhukova, N.A.; Frolova, T.S.; Tolstikova, T.G.; Shults, E.E.; Turks, M. Lupane-type conjugates with aminoacids, 1,3,4- oxadiazole and 1,2,5-oxadiazole-2-oxide derivatives: Synthesis, anti-inflammatory activity and in silico evaluation of target affinity. Steroids 2019, 150, 108443. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Polito, L.; Djemil, A.; Bortolotti, M. Plant Toxin-Based Immunotoxins for Cancer Therapy: A Short Overview. Biomedicines 2016, 4, 12. [Google Scholar] [CrossRef]
- Tarbiat, S.; Unver, D.; Tuncay, S.; Isik, S.; Yeman, K.B.; Mohseni, A.R. Neuroprotective effects of Cubebin and Hinokinin lignan fractions of Piper cubeba fruit in Alzheimer’s disease in vitro model. Turk. J. Biochem. 2023, 48, 303–310. [Google Scholar] [CrossRef]
- Cunha, N.; Teixeira, G.; Martins, T.; Souza, A.; Oliveira, P.; Símaro, G.; Rezende, K.C.; dos Santos Gonçalves, N.; Souza, D.G.; Tavares, D.C.; et al. (−)-Hinokinin Induces G2/M Arrest and Contributes to the Antiproliferative Effects of Doxorubicin in Breast Cancer Cells. Planta Med. 2016, 82, 530–538. [Google Scholar] [CrossRef]
- Lu, Q.; Zheng, R.; Zhu, P.; Bian, J.; Liu, Z.; Du, J. Hinokinin alleviates high fat diet/streptozotocin-induced cardiac injury in mice through modulation in oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2021, 137, 111361. [Google Scholar] [CrossRef]
- Xiong, W.; Yuan, Z.; Wang, T.; Wu, S.; Xiong, Y.; Yao, Y.; Yang, Y.; Wu, H. Quercitrin Attenuates Acetaminophen-Induced Acute Liver Injury by Maintaining Mitochondrial Complex I Activity. Front. Pharmacol. 2021, 12, 586010. [Google Scholar] [CrossRef]
- Hur, H.J.; Jeong, Y.-H.; Lee, S.H.; Sung, M.J. Quercitrin Ameliorates Hyperlipidemia and Hepatic Steatosis in Ovariectomized Mice. Life 2020, 10, 243. [Google Scholar] [CrossRef]
- Guang Li, H.; Jun Gao, H.; Feng Liu, F.; Liu, J. Quercitrin Suppresses Hepatocellular Carcinoma Metastasis and Angiogenesis by Targeting the Nrf2 Signaling Pathway. 2017. Available online: https://www.oncotarget.com/article/23777/ (accessed on 13 February 2025).
- Giribabu, N.; Karim, K.; Kilari, E.K.; Salleh, N. Phyllanthus niruri leaves aqueous extract improves kidney functions, ameliorates kidney oxidative stress, inflammation, fibrosis and apoptosis and enhances kidney cell proliferation in adult male rats with diabetes mellitus. J. Ethnopharmacol. 2017, 205, 123–137. [Google Scholar] [CrossRef]
- Abu Hassan, M.R.; Hj Md Said, R.; Zainuddin, Z.; Omar, H.; Md Ali, S.M.; Aris, S.A.; Chan, H.K. Effects of one-year supplementation with Phyllanthus niruri on fibrosis score and metabolic markers in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Heliyon 2023, 9, e16652. [Google Scholar] [CrossRef] [PubMed]
- Pahmi, K.; Sidratullah, M. Effect of Leafflower (Phyllanthus niruri Linn.) treatment on kidney and uterus in sodium chloride-induced fibrotic rats. Biosci. Med. J. Biomed. Transl. Res. 2021, 5, 263–267. [Google Scholar]
- Amin, Z.A.; Bilgen, M.; Alshawsh, M.A.; Ali, H.M.; Hadi, A.H.A.; Abdulla, M.A. Protective Role of Phyllanthus niruri Extract against Thioacetamide-Induced Liver Cirrhosis in Rat Model. Evid.-Based Complement. Altern. Med. 2012, 2012, 241583. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.L.; Loh, S.I.; Sattar, M.A.; Muhammad, T.S.T.; Sulaiman, S.F. Cytotoxic, caspase-3 induction and in vivo hepatoprotective effects of phyllanthin, a major constituent of Phyllanthus niruri. J. Funct. Foods 2015, 14, 236–243. [Google Scholar] [CrossRef]
- Krithika, R.; Jyothilakshmi, V.; Prashantha, K.; Verma, R.J. Mechanism of protective effect of phyllanthin against carbon tetrachloride-induced hepatotoxicity and experimental liver fibrosis in mice. Toxicol. Mech. Methods 2015, 25, 708–717. [Google Scholar] [CrossRef]
- Krithika, R.; Jyothilakshmi, V.; Verma, R.J. Phyllanthin inhibits CCl4-mediated oxidative stress and hepatic fibrosis by down-regulating TNF-α/NF-κB, and pro-fibrotic factor TGF-β1 mediating inflammatory signaling. Toxicol. Ind. Health 2016, 32, 953–960. [Google Scholar] [CrossRef]
- Hernández-Ortega, L.D.; Alcántar-Díaz, B.E.; Ruiz-Corro, L.A.; Sandoval-Rodriguez, A.; Bueno-Topete, M.; Armendariz-Borunda, J.; Salazar-Montes, A.M. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J. Gastroenterol. Hepatol. 2012, 27, 1865–1872. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Zhang, W.; Chen, X. Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochem. Cell Biol. 2018, 96, 742–751. [Google Scholar] [CrossRef]
- Marcolin, E.; San-Miguel, B.; Vallejo, D.; Tieppo, J.; Marroni, N.; González-Gallego, J.; Tuñón, M.J. Quercetin Treatment Ameliorates Inflammation and Fibrosis in Mice with Nonalcoholic Steatohepatitis3. J. Nutr. 2012, 142, 1821–1828. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Al Zarzour, R.; Ahmad, M.; Asmawi Mohd Kaur, G.; Saeed, M.; Al-Mansoub, M.; Saghir, S.A.; Usman, N.S.; Al-Dulaimi, D.W.; Yam, M.F. Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague–Dawley Rats. Nutrients 2017, 9, 766. [Google Scholar] [CrossRef]
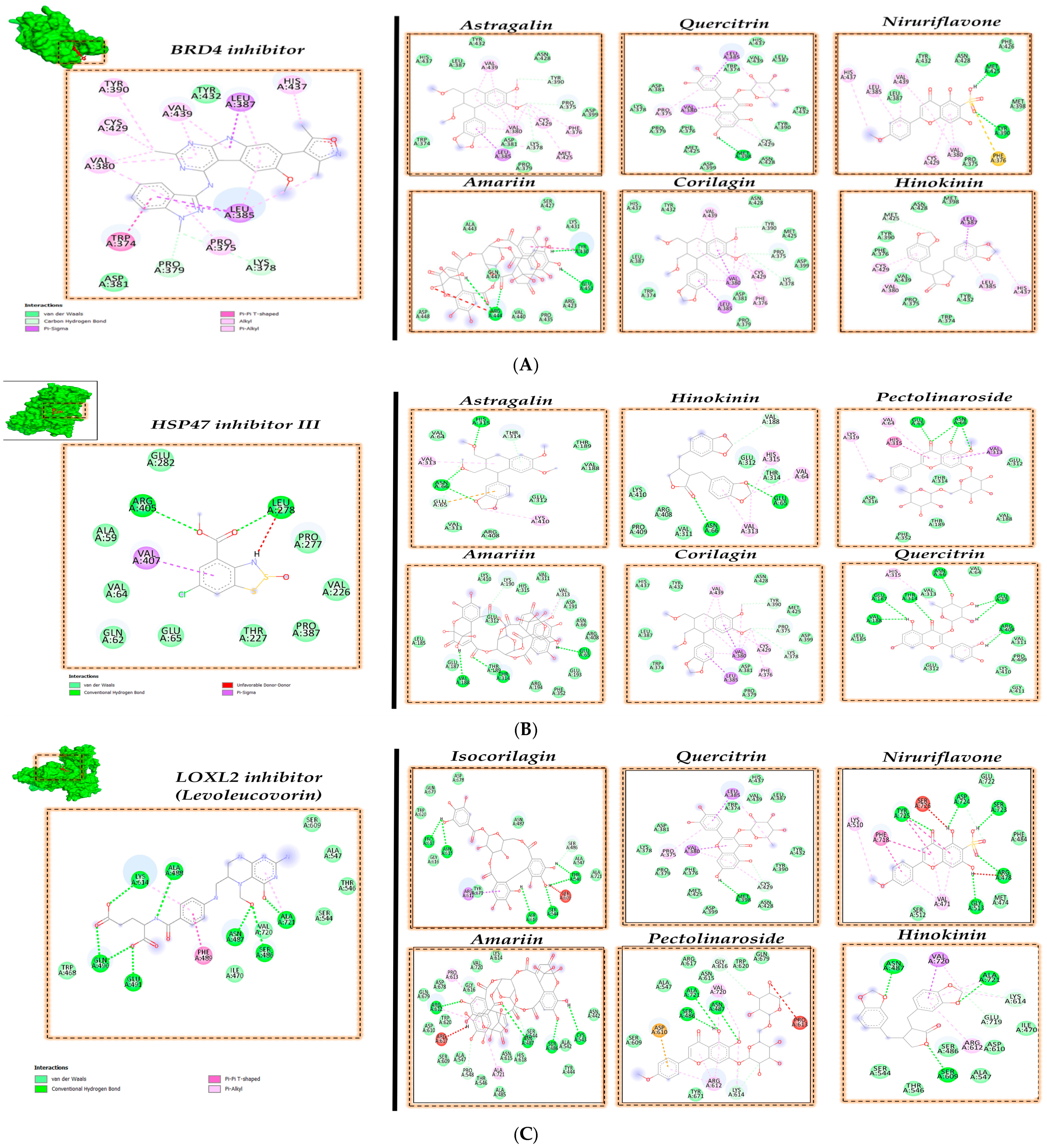
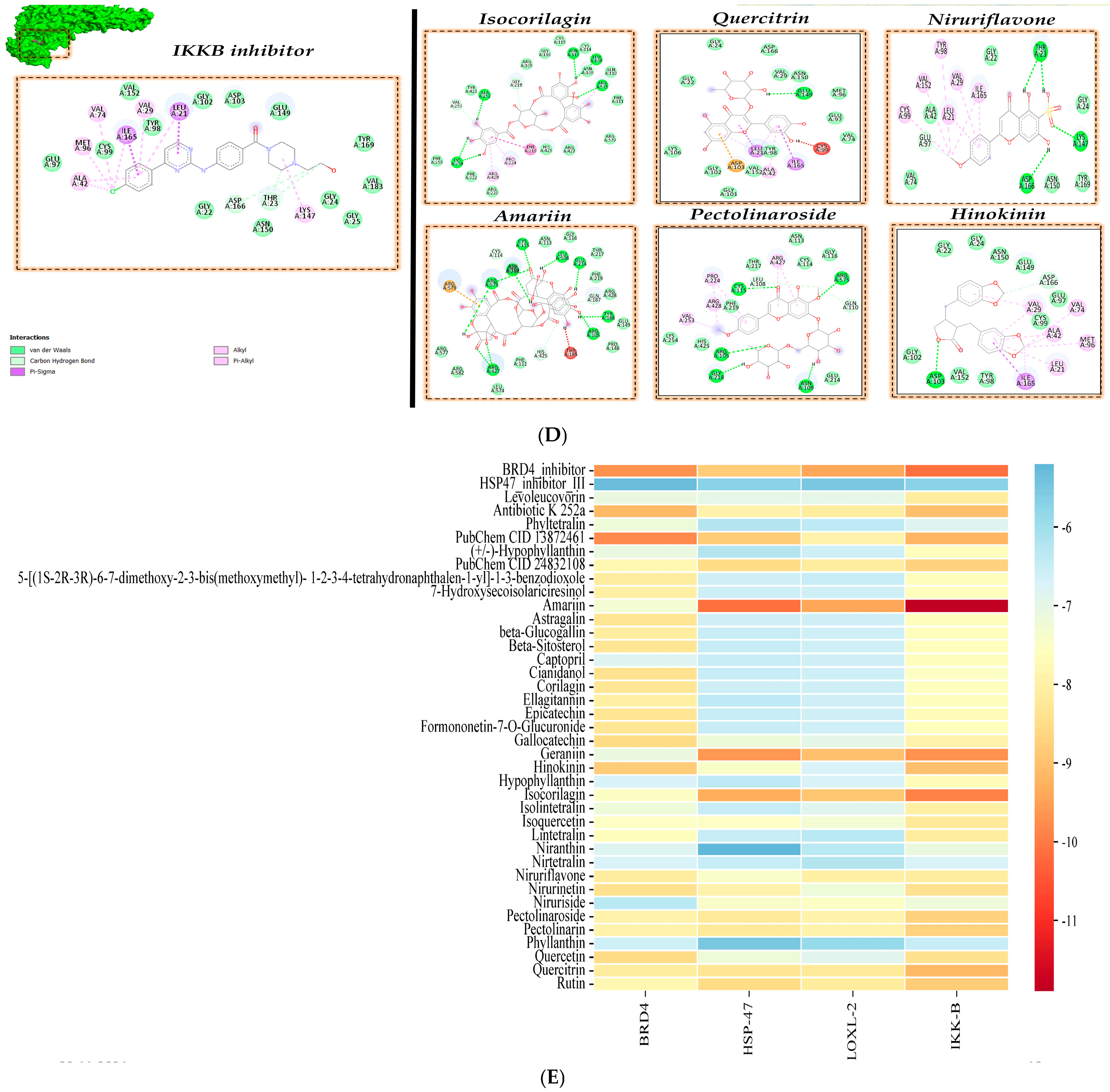
| Target Protein | PDB Id | Inhibitor Compound | Reference for Inhibitor Compound |
|---|---|---|---|
| BRD4 | 6C7Q | Compound CE277 (BRD4 inhibitor) | Used in the PDB 6C7Q |
| HSP47 | 3ZHA | Hsp47 inhibitor Col003 (HSP47 inhibitor III) | [21] |
| LOXL2 | 5ZE3 | Levoleucovorin | [22] |
| IKKβ | 3RZF | Ligand K-252A | [23] |
| Compound Name | Molecular Weight (150–500 Da) | Bio Availability (>0.17) | Lipinski’s Rule Violation | Drug Likeliness (>0.18) |
|---|---|---|---|---|
| Phyltetralin | 416.51 | 0.55 | 0 | 0.70 |
| PubChem CID 13872461 | 434.39 | 0.55 | 1 | 1.08 |
| (+/−)-Hypophyllanthin | 430.49 | 0.55 | 0 | 0.81 |
| PubChem CID 24832108 | 430.49 | 0.55 | 0 | 0.81 |
| 5-((1S-2R-3R)-6-7-dimethoxy-2-3-bis(methoxymethyl)-1-2-3-4-tetrahydronaphthalen-1-yl)-1-3-benzodioxole | 400.46 | 0.55 | 0 | 0.37 |
| 7-Hydroxysecoisolariciresinol | 378.42 | 0.55 | 0 | 0.41 |
| Amariin | 968.64 | 0.17 | 3 | 0.49 |
| Astragalin | 448.38 | 0.17 | 2 | 0.67 |
| Beta-Glucogallin | 332.26 | 0.55 | 1 | 0.81 |
| Beta-Sitosterol | 414.71 | 0.55 | 1 | 0.78 |
| Captopril | 217.29 | 0.56 | 0 | −0.20 |
| Cianidanol | 290.27 | 0.55 | 0 | 0.64 |
| Corilagin | 634.45 | 0.17 | 3 | 0.64 |
| Ellagitannin | 992.71 | 0.17 | 3 | 0.29 |
| Epicatechin | 290.27 | 0.55 | 0 | 0.64 |
| Formononetin-7-O-Glucuronide | 444.39 | 0.11 | 0 | 0.10 |
| Gallocatechin | 306.27 | 0.55 | 1 | 0.57 |
| Geraniin | 268.48 | 0.55 | 1 | 0.30 |
| Hinokinin | 354.35 | 0.55 | 0 | −0.74 |
| Hypophyllanthin | 430.49 | 0.55 | 0 | 0.81 |
| Isocorilagin | 634.45 | 0.17 | 3 | 0.64 |
| Isolintetralin | 400.46 | 0.55 | 0 | 0.60 |
| Isoquercetin | 464.38 | 0.17 | 2 | 0.68 |
| Lintetralin | 400.46 | 0.55 | 0 | 0.37 |
| Niranthin | 432.51 | 0.55 | 0 | −0.19 |
| Nirtetralin | 430.49 | 0.55 | 0 | 0.84 |
| Niruriflavone | 364.33 | 0.56 | 0 | 0 |
| Nirurinetin | 356.37 | 0.55 | 0 | 0.77 |
| Niruriside | 770.73 | 0.17 | 2 | −0.63 |
| Pectolinaroside | 622.57 | 0.17 | 3 | 0.87 |
| Phyllanthin | 418.52 | 0.55 | 0 | −0.56 |
| Quercetin | 302.24 | 0.55 | 0 | 0.52 |
| Quercitrin | 448.38 | 0.17 | 2 | 0.82 |
| Rutin | 610.52 | 0.17 | 3 | 0.91 |
| Compounds | Predicted LD50 (mg/kg) | Predicted Toxicity Class | Hepato-Toxicity | Carcino-Genicity | Immunogenicity | Tox21-Nuclear Receptor Signaling Pathways | ||
|---|---|---|---|---|---|---|---|---|
| PPAR-γ | MMP | p53 | ||||||
| BRD4 inhibitor | 3000 | 5 | ||||||
| HSP47 inhibitor III | 1600 | 4 | ||||||
| Levoleucovorin | 135 | 3 | ||||||
| Antibiotic K 252a | 11 | 2 | ||||||
| Amariin | 620 | 4 | ||||||
| Astragalin | 5000 | 5 | ||||||
| Corilagin | 2260 | 5 | ||||||
| Hinokinin | 1500 | 4 | ||||||
| Isocorilagin | 2260 | 5 | ||||||
| Niruriflavone | 3919 | 5 | ||||||
| Pectolinaroside | 5000 | 5 | ||||||
| Quercitrin | 5000 | 5 | ||||||
 ,
,  .
.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raju, C.; Sankaranarayanan, K. In-Silico Investigation of Phyllanthus niruri Phytochemicals as Hepatic Fibrosis Modulators. Biol. Life Sci. Forum 2024, 38, 7. https://doi.org/10.3390/blsf2024038007
Raju C, Sankaranarayanan K. In-Silico Investigation of Phyllanthus niruri Phytochemicals as Hepatic Fibrosis Modulators. Biology and Life Sciences Forum. 2024; 38(1):7. https://doi.org/10.3390/blsf2024038007
Chicago/Turabian StyleRaju, Chithra, and Kavitha Sankaranarayanan. 2024. "In-Silico Investigation of Phyllanthus niruri Phytochemicals as Hepatic Fibrosis Modulators" Biology and Life Sciences Forum 38, no. 1: 7. https://doi.org/10.3390/blsf2024038007
APA StyleRaju, C., & Sankaranarayanan, K. (2024). In-Silico Investigation of Phyllanthus niruri Phytochemicals as Hepatic Fibrosis Modulators. Biology and Life Sciences Forum, 38(1), 7. https://doi.org/10.3390/blsf2024038007







