Abstract
Annatto, a tropical shrub from Central and South America and parts of India, contains Bixin, an apocarotenoid pigment. Bixin is conventionally used as a natural food colorant and is now receiving attention for its health-promoting nutraceutical properties, particularly in chronic diseases (metabolic syndrome—MetS—and cancers). This study investigates the dose-dependent anti-obesity and anti-steatotic effects of Bixin in in vitro cell culture models. The anti-adipogenic and anti-steatotic effects of Bixin were examined in well-established in vitro models of obesity and non-alcoholic fatty liver disease (NAFLD/steatosis) using 3T3-L1 preadipocytes (by a differentiation protocol) and HepG2cells (steatosis-induced with oleic acid), respectively. Bixin was administered in the following concentration range: 1 μg mL−1–20 μg mL−1 (obesity model) and 2.5 μg mL−1–10 μg mL−1 (NAFLD/steatosis model). The neutral lipid content was estimated by Oil Red O staining; ROS/RNS were quantified by 2′,7′-dichlorofluorescein diacetate (DCFDA) and nitrite assays; and malondialdehyde (MDA), the biochemical marker of lipid peroxidation, was assessed by TBARS assay. At lower concentrations, 5 μg mL−1 in steatotic cells and 10 μg mL−1 in matured adipocytes, Bixin significantly reduced lipid accumulation in both hepatocytes and adipocytes (p < 0.05), demonstrating its potential as an anti-steatotic and anti-obesity agent. This beneficial effect was correlated with a reduction in oxidative stress levels (decreased MDA and ROS/RNS levels). Strikingly, at higher concentrations (>10 μg mL−1), Bixin showed increased lipid accumulation and oxidative stress. Bixin exhibits anti-obesity and anti-steatotic effects at lower doses, which correlates with its antioxidant properties. However, its bioactivity is dose-dependent, meaning that at higher concentrations, it ceases to inhibit adipogenesis. This opposing response is accompanied by elevated oxidative stress levels, indicating a pro-oxidant effect at higher doses, which suggests its anti-cancer potential. The present study highlights the significance of dosage optimization of nutraceuticals and dietary ingredients with respect to their intended biological applications, such as MetS and cancer treatment.
1. Introduction
Metabolic syndrome comprises a cluster of metabolic abnormalities, including obesity, insulin resistance, hypertension, and dyslipidemia, with non-alcoholic fatty liver disease (NAFLD) as a key manifestation [1]. Both NAFLD and obesity are characterized by excessive lipid accumulation in the liver and adipose tissue, contributing to its pathophysiological features [2]. The prevalence of NAFLD is high, ranging from 22.5% to 44.0% in overweight individuals and reaching up to 90% in those with obesity [3]. Current pharmacological interventions for these conditions face significant limitations, including side effects and limited efficacy, highlighting the urgent need for alternative approaches. While obesity is commonly managed with anti-obesity drugs, their potential adverse effects remain a concern. Furthermore, effective and targeted pharmacological treatment for NAFLD has not been established, largely due to its complex and poorly understood pathogenesis. As a result, lifestyle modifications, including dietary interventions, physical activity, and management of associated comorbidities, remain the cornerstone of NAFLD management. These challenges underscore the importance of exploring natural compounds with therapeutic potential as safer and more effective strategies for managing metabolic disorders [4,5].
Bixa orellana L., commonly known as annatto, is a tropical plant that is widely recognized for its seeds, which are rich in Bixin, an apocarotenoid pigment [6]. Out of all the carotenoids, only Bixin presents a 9-cis configuration [7]. Traditionally used as a natural food colorant, Bixin has gained scientific interest due to its potential health-promoting properties, including antioxidant, anti-inflammatory, and lipid-lowering effects [8,9].
Dose optimization is crucial when using phytochemicals, as their biological effects can vary depending on concentration. A study on HL-60 cells demonstrated that curcumin exhibits a biphasic effect on ROS generation. At low doses (10 μM), it reduces ROS levels and acts as an antioxidant, while at high doses (50 μM), it promotes ROS production, functioning as a pro-oxidant and leading to cell death [10]. Similarly, carotenoids such as Bixin, lycopene, and β-carotene are renowned for their antioxidant properties at physiological levels, where they neutralize reactive oxygen species and protect against oxidative damage. However, studies have shown that carotenoids can act as pro-oxidants at higher concentrations, increasing oxidative stress and potential cytotoxicity [11]. These biphasic effects highlight the importance of determining the optimal dosages for therapeutic and dietary applications with different biological effects (anti-cancer, antioxidant, anti-inflammatory, and anti-lipemic). In this study, we investigated the dose-dependent effects of an apocarotenoid, Bixin, in adipogenic and steatotic cell culture models.
2. Materials and Methods
2.1. Chemicals and Reagents
Pure Bixin was obtained from HPC Molecules Analytical Lab Solutions Pvt Ltd. (Hyderabad, India). Chemicals such as DMSO, potassium hydroxide, hydrochloric acid, monopotassium phosphate, sodium hydroxide, tris buffer, TCA (Trichloroacetic acid), and TBA (Thiobarbituric acid) were sourced from Sigma Aldrich (St. Louis, MO, USA).
2.2. In Vitro Disease Models
- Adipogenesis model: The 3T3-L1 preadipocytes were obtained from NCCS (National Centre for Cell Science, Pune, India) and cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2 atmosphere. Adipogenesis was initiated 48 h post-confluence (day 0) using a differentiation cocktail of insulin (10 μg mL−1), dexamethasone (0.25 µM), and (IBMX) 3-isobutyl-1-methylxanthine (500 µM), followed by insulin-only medium from day 2. Different dosages of Bixin (2.5–20 μg mL−1) were administered on day 4 of differentiation [12].
- Hepatic steatosis model: HepG2 cells from NCCS (Pune, India) were cultured in MEM with 10% FBS and 1% penicillin-streptomycin at 37 °C, 5% CO2. For steatotic experiments, cells were seeded in 96-well plates (5 × 105 cells/well), serum-starved for 24 h, and treated with 1 mM oleic acid in MEM with 1% BSA for 48 h to induce steatosis. This was followed by treatment with multiple concentrations of Bixin (2.5–10 μg mL−1) for 24 h [13].
2.3. Assessment of Lipid Accumulation Through Lipid Staining
Oil Red O (ORO) staining was used to assess the dose-dependent impact of Bixin on lipid accumulation in cells following adipogenic and steatotic induction. Cells were fixed with 4% paraformaldehyde for 15 min, washed with PBS, and stained with 0.3% ORO for 20 min. After rinsing with water, lipid droplets were visualized under an inverted microscope. The lipid-bound dye was dissolved in 100% isopropanol, and absorbance was measured at 490 nm to evaluate the effect of varying Bixin concentrations on lipid accumulation.
2.4. TBARS–Lipid Peroxidation Assay
The TBARS assay was used to assess the dose-dependent impact of Bixin on the antioxidant activity in adipogenic and steatotic models by measuring the malondialdehyde (MDA) levels. Cells were treated with varying Bixin concentrations for 24 h, washed, and lysed. After homogenization and centrifugation, the protein content was normalized. The lysate was incubated with Tris buffer and monopotassium phosphate, followed by the addition of TCA and TBA to generate a pink adduct. Absorbance was measured spectrophotometrically at 530 nm and compared to a standard MDA curve.
2.5. Griess Assay
Griess assay was used to measure the nitrate and nitrite concentrations, indicating the Nitric Oxide (NO) levels, in the adipogenic and steatotic models, as well as in the Bixin-treated groups. Nitrate was enzymatically converted to nitrite, and the Griess reagent was added to the cell culture medium. After a 15 min incubation, the optical density (OD) was measured at 540 nm, and the nitrite concentration was determined by comparison to a standard nitrite curve (µM).
2.6. Quantification of Intracellular ROS/RNS Levels
DCFDA assay was used to measure intracellular ROS/RNS levels in steatotic and adipogenic models treated with varying concentrations of Bixin for 24 h. After treatment, cells were incubated with 10 μM DCFDA for 60 min in the dark. The fluorescence intensity (Excitation/Emission = 485/535 nm) was measured, showing a dose-dependent response to Bixin.
2.7. Statistical Analysis
The in vitro experiments were performed at least thrice, in triplicate sets. Statistical analysis was performed using one-way ANOVA to compare the experimental groups, and their statistically significant differences in values were expressed as follows: p ≤ 0.05 (*), p ≤ 0.01 (**), and p ≤ 0.001 (***).
3. Results and Discussion
The effects of Bixin concentrations on controlling adipogenesis and steatosis, and their associated biochemical sequel such as oxidative stress, were assessed using lipid staining and oxidative stress markers. The outcomes are discussed below.
3.1. Assessment of Lipid Accumulation by Oil Red O Staining
Our previous study identified the non-cytotoxic doses of Bixin for 3T3-L1 preadipocytes and HepG2 cells before optimizing these concentrations for their anti-adipogenic and anti-steatotic effects. We reported that Bixin at concentrations greater than 10 μg mL−1 and 5 μg mL−1 resulted in decreased cell viability in adipocyte and steatotic models [14]. This was a pointer that Bixin could exert cytoprotective and cytotoxic effects at different concentrations. Therefore, multiple dosages below 20 μg mL−1 and 10 μg mL−1 were tested as safe dosages for anti-adipogenic and anti-steatotic effects.
The potential of pure Bixin to inhibit lipid accumulation was assessed in both mature adipocytes and oleic acid-induced HepG2 steatotic cells. Preadipocytes were treated with varying concentrations of Bixin (2.5, 5, 10, 15, and 20 μg mL−1) starting on day 4 of differentiation. For the steatotic model, HepG2 cells were treated with Bixin (2.5, 5, 7.5, and 10 μg mL−1) 24 h after steatosis induction. Both mature adipocytes and steatotic cells showed a significant reduction in lipid accumulation, with a noticeable decrease up to certain concentrations: in adipocytes, this occurred up to 10 μg mL−1, and in steatotic cells, up to 5 μg mL−1. Interestingly, as the concentration increased beyond these values, the lipid accumulation increased, as evidenced by Oil Red O staining (Figure 1). This clearly suggests that there is a dosage threshold, which determines the biphasic nature of Bixin, that is, whether it promotes or supresses adipogenesis and steatosis. In other words, it is important to identify the dosages which would result in reduction in lipid accumulation in order to realize the anti-obesity and anti-NAFLD potential of Bixin. There are mixed reports of the adipogenic property of Bixin. For instance, while certain doses of Bixin (70 µM) promoted adipogenesis in 3T3-L1 adipocytes [15], another study reported that a lower dose of Bixin (20 µM) inhibited the differentiation of 3T3-L1 preadipocytes [16]. Our study characterized this dual nature of Bixin using a low to high dosage range. The present outcomes clearly explain these mixed reports on the basis of the concentration of apocarotenoid.
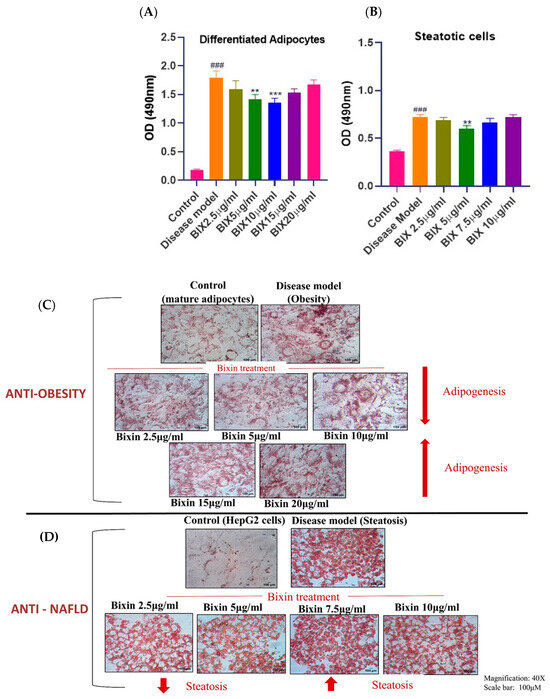
Figure 1.
Inhibitory effects of Bixin on lipid accumulation in in vitro disease models. (A,B) ORO staining quantification reveals the dose-dependent effects of Bixin on lipid accumulation in matured adipocytes and steatotic cells. Preadipocytes and Non-steatotic HepG2 cells were used as the control cells, respectively. Values are expressed as mean ± S.D. of a minimum of three independent experiments, each carried out in triplicate sets. Statistical comparisons are as follows: # with respect to control; * with respect to the disease model. ### p < 0.001, relative to the control group. ** p < 0.01, and *** p < 0.001 relative to the disease model group. (C,D) Photomicrographs (40x) reveal intense ORO staining in untreated disease models, which is contrasted by lipid staining in the Bixin-treated groups.
Along similar lines with respect to another phytochemical, curcumin, it was shown that low doses of curcumin (15 μM) attenuate adipocyte differentiation, whereas higher concentrations (30 μM) induce apoptotic signaling [17], implying dose-dependent changes in the mechanism. The present study extended the investigation of Bixin’s anti-lipid accumulation efficacy in yet another model, which was steatotic NAFLD. The outcomes in the NAFLD model were in line with the adipogenic model, wherein there was a dosage threshold wherein concentrations of 2.5 μg mL−1 and 5 μg mL−1 led to reductions in steatosis, while a dosage above 5 μg mL−1 resulted in a gradual increase in steatosis.
3.2. Assessment of Oxidative Stress
The assessment of oxidative stress in Bixin-treated cells was carried out using multiple biochemical assays. Through the TBARS assay, DCFDA, and nitrite assays, we observed significantly elevated levels of lipid peroxidation, total intracellular ROS/RNS, and nitrite levels in both the adipogenic and steatotic models compared to the control group (### p < 0.001) (Figure 2). Interestingly, at lower concentrations, Bixin treatment (up to 10 μg mL−1 in adipogenic cells and 5 μg mL−1 in steatotic cells) significantly reduced the total ROS/RNS production and lipid peroxidation (p < 0.01), suggesting its potential antioxidant effects. This supports the potential multifaceted protection that Bixin can exert in obesity and NAFLD by alleviating lipid accumulation and associated oxidative damage. However, oxidative stress was elevated at higher concentrations (>10 μg mL−1 in adipogenic cells and >5 μg mL−1 in steatotic cells), indicating a shift towards a pro-oxidant effect of Bixin. This dose-dependent effect may be partly explained by the increase in lipid accumulation, as shown above, with higher dosages. These results show the importance of dosage optimization in the clinical application of Bixin, particularly in the context of MetS, NAFLD, and cancer, where oxidative stress plays a crucial role in disease progression.
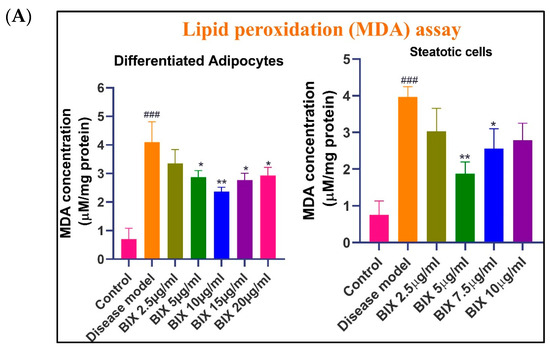
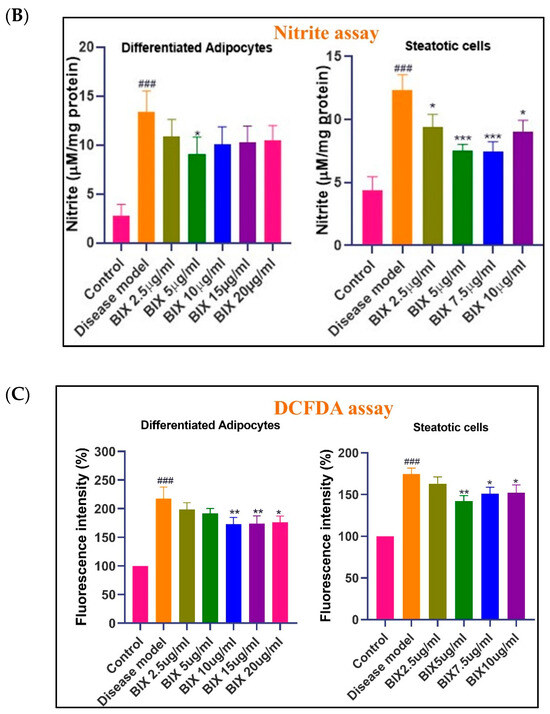
Figure 2.
Quantification of oxidative stress in in vitro disease models. Panel (A) illustrates the dose-dependent effects of Bixin on MDA levels in matured adipocytes and steatotic cells. Panel (B) presents the effects of Bixin on nitrite levels in matured adipocytes and steatotic cells. Panel (C) demonstrates the dose-dependent effects of Bixin on the total ROS/RNS levels in matured adipocytes and steatotic cells. Preadipocytes and Non-steatotic HepG2 cells were used as the control cells, respectively. Values are expressed as mean ± S.D. in three independent experiments, each carried out in duplicate or triplicate sets. Statistical comparisons are as follows: ### p < 0.001, relative to the control group. * p < 0.05, ** p < 0.01, and *** p < 0.001 relative to the disease model group.
The antioxidant properties of carotenoids are attributed to their ability to scavenge free radicals [18]. Bixin, with its conjugated double bonds, acts as an antioxidant by donating electrons to neutralize free radicals, thereby preventing oxidative damage [19]. However, studies have demonstrated that at higher concentrations, carotenoids such as β-carotene, lycopene, and lutein lose their antioxidant activity and exhibit pro-oxidant effects [20,21]. Another study investigated the effects of β-carotene supplementation in HT29 cells. It demonstrated that at low doses (1–3 μM), β-carotene effectively scavenged radicals within the membrane, thereby preventing lipid peroxidation. In contrast, it increased the membrane fluidity at higher concentrations (4–10 μM), potentially facilitating greater oxidant damage [22].
4. Conclusions
The biphasic behavior of Bixin concentrations underscores the necessity of dose optimization in its anti-obesity and anti-NAFLD applications. The present outcomes, taken together with other related reports, clearly implicate that the efficacy and safety of nutraceuticals are closely dependent on their concentrations. Therefore, it is imperative to understand the specific biological context and intended health outcomes before standardizing the dosage of the phytochemicals. Further, the nature of administration, such as a single phytochemical or combinations, pure chemical or botanical extracts, and different delivery systems such as encapsulations, would all need corresponding dosage optimization with respect to the intended biological effect and actual bioavailability of the phytochemical.
Author Contributions
P.R.D. and S.P.: Conceptualization; S.P. and S.K.M.: Methodology and Experimentation; S.P. and S.K.M.: Data curation and Software; S.P., S.K.M. and P.R.D.: Writing and original draft preparation; P.R.D. and P.K.S.: Visualization, Investigation, and Supervision; S.P., S.K.M., P.R.D. and P.K.S.: Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from the Indian Council of Medical Research (ICMR), New Delhi (Grant No. 52/13/2022-BIO/BMS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
S.P. and S.K.M. acknowledge Institute Fellowships from BITS Pilani. The authors also thank Birla Institute of Technology and Science (BITS), Pilani—Pilani Campus, for providing research support and instrumentation facilities.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Swarup, S.; Ahmed, I.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2024. [Google Scholar]
- Parekh, S.; Anania, F.A. Abnormal Lipid and Glucose Metabolism in Obesity: Implications for Nonalcoholic Fatty Liver Disease. Gastroenterology 2007, 132, 2191–2207. [Google Scholar] [CrossRef]
- Lau, L.H.S.; Wong, S.H. Microbiota, Obesity and NAFLD. In Obesity, Fatty Liver and Liver Cancer; Springer: Singapore, 2018; pp. 111–125. [Google Scholar]
- Godoy-Matos, A.F.; Silva Júnior, W.S.; Valerio, C.M. NAFLD as a Continuum: From Obesity to Metabolic Syndrome and Diabetes. Diabetol. Metab. Syndr. 2020, 12, 60. [Google Scholar] [CrossRef]
- Sanna, C.; Fais, A.; Era, B.; Delogu, G.L.; Sanna, E.; Dazzi, L.; Rosa, A.; Marengo, A.; Rubiolo, P.; De Agostini, A.; et al. Promising Inhibition of Diabetes-Related Enzymes and Antioxidant Properties of Ptilostemon Casabonae Leaves Extract. J. Enzyme Inhib. Med. Chem. 2023, 38, 2274798. [Google Scholar] [CrossRef]
- Islam, S.U.; Rather, L.J.; Mohammad, F. Phytochemistry, Biological Activities and Potential of Annatto in Natural Colorant Production for Industrial Applications—A Review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Raddatz-Mota, D.; Pérez-Flores, L.J.; Carrari, F.; Mendoza-Espinoza, J.A.; de León-Sánchez, F.D.; Pinzón-López, L.L.; Godoy-Hernández, G.; Rivera-Cabrera, F. Achiote (Bixa Orellana L.): A Natural Source of Pigment and Vitamin E. J. Food Sci. Technol. 2017, 54, 1729–1741. [Google Scholar] [CrossRef]
- Kusmita, L.; Franyoto, Y.D.; Mutmainah, M.; Puspitaningrum, I.; Nurcahyanti, A.D.R. Bixa orellana L. Carotenoids: Antiproliferative Activity on Human Lung Cancer, Breast Cancer, and Cervical Cancer Cells in Vitro. Nat. Prod. Res. 2022, 36, 6421–6427. [Google Scholar] [CrossRef] [PubMed]
- Pinzón-García, A.D.; Orellano, L.A.A.; de Lazari, M.G.T.; Campos, P.P.; Cortes, M.E.; Sinisterra, R.D. Evidence of Hypoglycemic, Lipid-Lowering and Hepatoprotective Effects of the Bixin and Bixin: β-CD Inclusion Compound in High-Fat-Fed Obese Mice. Biomed. Pharmacother. 2018, 106, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Da, W.; Zhang, D.; Liu, Q.; Kang, J. Water-Soluble Antioxidants Improve the Antioxidant and Anticancer Activity of Low Concentrations of Curcumin in Human Leukemia Cells. Die Pharm. Int. J. Pharm. Sci. 2005, 60, 57–61. [Google Scholar]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Mandal, S.K.; Kumar, B.K.; Sharma, P.K.; Murugesan, S.; Deepa, P.R. In Silico and in Vitro Analysis of PPAR—α/γ Dual Agonists: Comparative Evaluation of Potential Phytochemicals with Anti-Obesity Drug Orlistat. Comput. Biol. Med. 2022, 147, 105796. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Kirad, S.; Muzaffar-Ur-Rehman, M.; Mandal, S.K.; Sharma, P.K.; Sankaranarayanan, M.; Deepa, P.R. Lipogenic Stearoyl-CoA Desaturase-1 (SCD1) Targeted Virtual Screening for Chemical Inhibitors: Molecular Docking/Dynamics Simulation and in Vitro Assessment of Anti-NAFLD Efficacy. RSC Adv. 2024, 14, 31797–31808. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Mandal, S.K.; Joshi, T.; Nikita; Srivastava, A.; Sharma, P.K.; Deepa, P.R. Anti-Adipogenic and Anti-Steatotic Potential of Bixin and Annatto Seed Extracts: LC-MS Profiling and in Vitro Validation (Under Revision). Food Biosci. 2024. In process. [Google Scholar]
- Takahashi, N.; Goto, T.; Taimatsu, A.; Egawa, K.; Katoh, S.; Kusudo, T.; Sakamoto, T.; Ohyane, C.; Lee, J.-Y.; Kim, Y. Bixin regulates mRNA expression involved in adipogenesis and enhances insulin sensitivity in 3T3-L1 adipocytes through PPARγ activation. Biochem. Biophys. Res. Commun. 2009, 390, 1372–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-E.; Fan, J.; Gao, R.; Ngoc, N.B. Suppressive effects of carotenoids on proliferation and differentiation of 3T3-L1 preadipocytes. J. Food Nutr. Res. 2017, 5, 129–136. [Google Scholar]
- Wu, L.-Y.; Chen, C.-W.; Chen, L.-K.; Chou, H.-Y.; Chang, C.-L.; Juan, C.-C. Curcumin Attenuates Adipogenesis by Inducing Preadipocyte Apoptosis and Inhibiting Adipocyte Differentiation. Nutrients 2019, 11, 2307. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.G.; Young, A.J. Carotenoid Radical Chemistry and Antioxidant/pro-Oxidant Properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chisté, R.C.; Benassi, M.T.; Mercadante, A.Z. Effect of Solvent Type on the Extractability of Bioactive Compounds, Antioxidant Capacity and Colour Properties of Natural Annatto Extracts. Int. J. Food Sci. Technol. 2011, 46, 1863–1870. [Google Scholar] [CrossRef]
- Palozza, P.; Calviello, G.; Serini, S.; Maggiano, N.; Lanza, P.; Ranelletti, F.O.; Bartoli, G.M. β-Carotene at High Concentrations Induces Apoptosis by Enhancing Oxy-Radical Production in Human Adenocarcinoma Cells. Free Radic. Biol. Med. 2001, 30, 1000–1007. [Google Scholar]
- Eichler, O.; Sies, H.; Stahl, W. Divergent Optimum Levels of Lycopene, β-Carotene and Lutein Protecting Against UVB Irradiation in Human Fibroblasts. Photochem. Photobiol. 2002, 75, 503–506. [Google Scholar] [CrossRef]
- Lowe, G.M.; Booth, L.A.; Young, A.J.; Bilton, R.F. Lycopene and β-Carotene Protect against Oxidative Damage in HT29 Cells at Low Concentrations but Rapidly Lose This Capacity at Higher Doses. Free Radic. Res. 1999, 30, 141–151. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).