1. Introduction
Breeding meat chickens is at the heart of the global food industry. These species are fast-growing animals that can reach commercial weight relatively quickly, thus enabling efficient production and rapid stock rotation while minimizing production costs. This activity plays a crucial role in food security by providing high-quality animal proteins easily accessible to broad sections of the world’s population, thus providing an affordable option to meet essential nutritional needs. Furthermore, chicken farming contributes significantly to creating direct and indirect jobs in rural areas, thus enabling it to play an essential role in the economic stimulation of the regions where it is established. Beyond all these considerations, the activity is also known to have a smaller environmental footprint compared to other forms of animal breeding. For this purpose, meat chickens consume less food and water and take up less space for breeding, making it a more environmentally sustainable option.
However, this industry has several challenges, especially the new climate that generates longer hot periods that lead to the installation of chronic thermal stress situations. The effects are amplified on the one hand by the physiology of these animals, known to be without sweat glands and covered with an insulating coating. On the other hand, the activity is usually carried out in facilities that neglect the control of the atmosphere, making the temperature of the buildings largely dependent on that of the outside. This set of conditions can affect the entire production process and, thus, jeopardize the entire food distribution chain.
2. Material and Methods
2.1. Experimental Framework
The study was conducted in northern Algeria during the summer period between August and September. The experiment occurred in two buildings: the first housing the Cobb 500 strain (lot CB) and the second housing the Arbor Acres strain (lot AA). It is worth pointing out that breeding infrastructure does not perfectly meet the construction and environmental control standards and thus represents how chicken breeding is carried out in Algeria in most cases.
The measurements were performed on different ages, namely, on the 10th, 35th, and 50th days of age. The impact of climate data was expressed through ambient temperature and relative humidity, and the repercussions were evaluated, such as the evolution of two thyroid hormones (T3 and T4) and production indicators.
2.2. Measurement Methods
2.2.1. Measurement of Ambient Parameters
The ambient temperature and relative humidity were measured by recording thermo-hygrometers. The latter three were installed in the middle and at the ends of the facilities. The equipment allowed the data to be collected and recorded every half an hour.
2.2.2. Blood Marker Measurement
To measure these parameters, ten chickens per building were selected based on an average living weight representative of the entire band and held a fast for 12 hours before sampling. The blood was collected by bleeding from the jugular vein and then recovered from heparinated tubes. The blood samples were then centrifugated at a speed of 3000 rpm for 15 min. The survivor was recovered using micropipettes with single-use bottles and then placed in Eppendorf-type tubes for analysis.
Hormonal parameters were measured using the enzymatic immunological method [
1]. This was made possible by an automatic AIA 360 analyzer. Triiodothyronine (T3) was determined using the kit (DRG FreeT3 ELISA (EIA-3801), DRG International, Springfield, NJ, USA) and thyroxin (T4) along with the kit.
2.2.3. Measurement of Production Indicators
Bright weight
The live weight of the meat chickens was obtained using a commercial scale.
Average daily gain (GMQ)
The average daily gain was calculated using the following formula:
Consumption Index
The consumption index was measured according to the following formula:
Mortality rate
The mortality rate was calculated as follows:
2.3. Statistical Analysis
The data collected were expressed as average ± standard deviations, and the software used was Microsoft Excel, version 2007. The data were also analyzed using one-factor variance (Anova 1) with the same software. Finally, the data study was completed with three-tiered significance tests where the differences were considered significant, very significant, and highly significant when p < 0.05; p < 0.01; and p < 0.001, respectively.
3. Results
3.1. Atmosphere
Surveys of ambient temperatures revealed that the CB and AA batches were conducted at average temperatures of 33.79 ± 5.87 °C and 32.11 ± 4.77 °C, respectively. The relative humidity was, on average, 58.23 ± 6.54 percent in the CB batch, whereas in the AA batch, it was relatively higher and reached 62.59 ± 4.44 percent.
3.2. Blood Markers
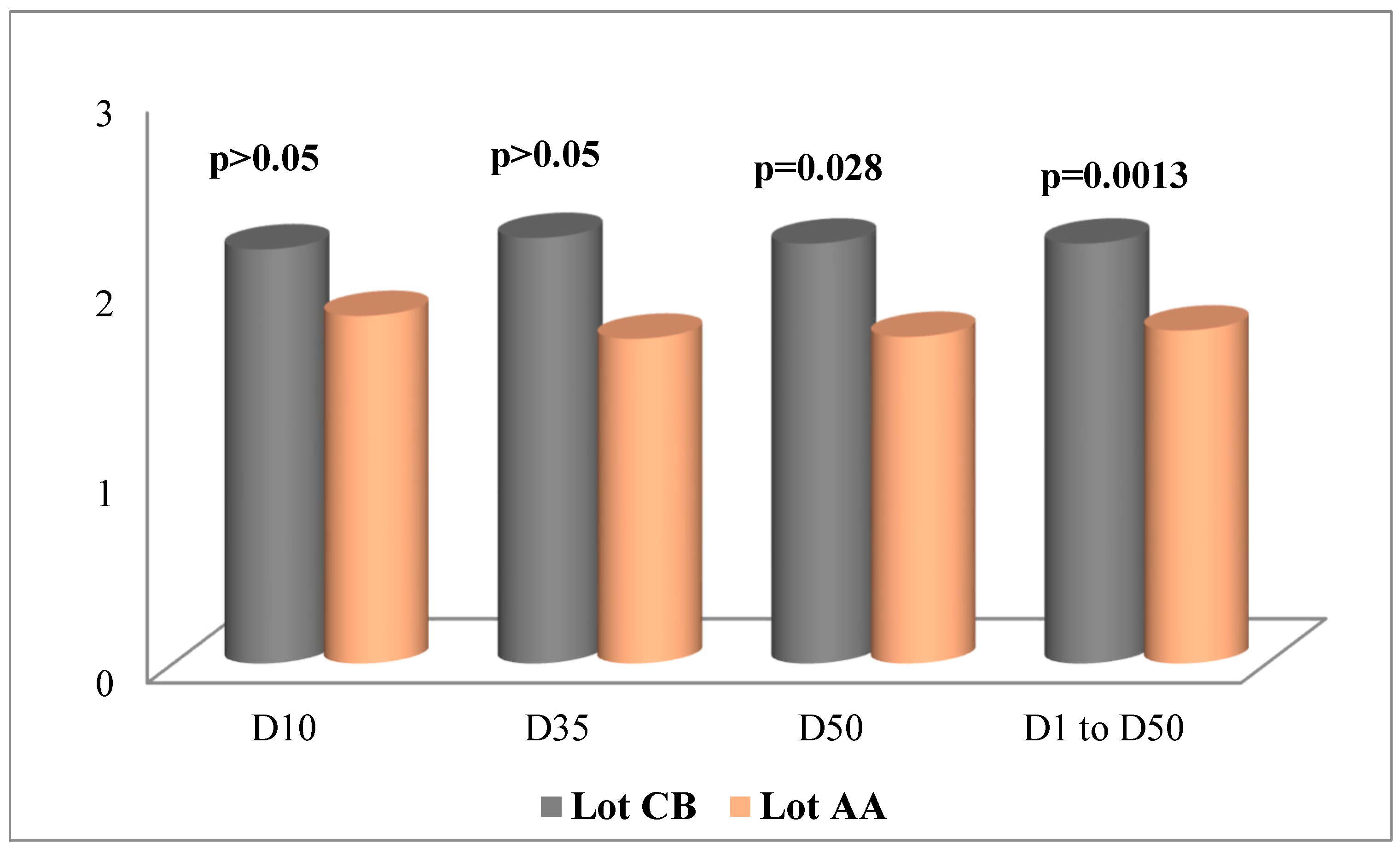
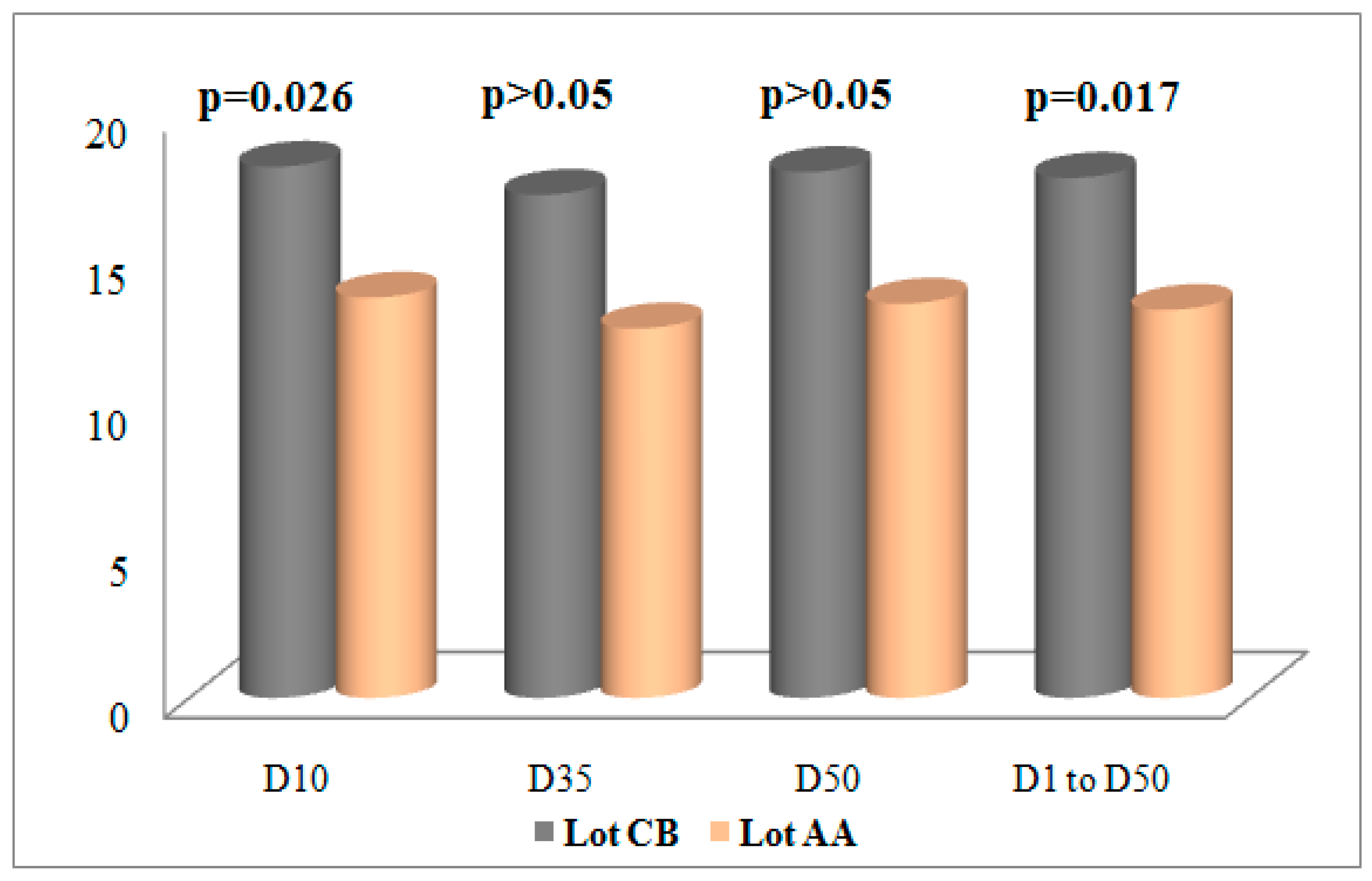
Figure 1 and
Figure 2 show the evolution of serum thyroid hormone concentrations during the experimental trial.
A review of the results revealed that chickens of the Cobb 500 strain had higher serum concentrations of T3 and T4 compared to the Arbor Acres strain, regardless of the sample. For T3, the differences were significant in the end-of-breeding period (J50) and over the entire production period, where they were established in proportions at +22.17% and +20.66%, respectively. In contrast, in J10 and J35, the differences were +16.05 and +23.66 but were not statistically significant. For T4, the differences were significant at the first sampling (+24.63%) and for all samplings, from J1 to J50 (+25.40%). Furthermore, at J35 and J50, the respective differences were +26.58% and +25.04% but remained statistically insignificant.
3.3. Production Indicators
Table 1 summarizes the production indicators measured during the experimental test.
Production indicators were significantly higher in the CB batch compared to the AA batch. This resulted in significant increases in living weight and average daily gain, parallel to a significant decrease in the consumption index and the mortality rate.
4. Discussion
Regarding the experimental conditions, the temperature records revealed that they were significantly superior to the recommendations for the two strains studied. Indeed, the optimal temperature should be between 18 and 20 °C from the 27th day of age [
2,
3,
4]. The temperature rise, in turn, led to the drying of the atmosphere, as evidenced by the humidity levels recorded when they were expected to turn around 70%. These experimental conditions also confirm the installation of chronic thermal stress since some authors indicated that beyond 24 °C, chickens began to show signs of suffering [
5].
As far as hormones are concerned, a decrease in serum T3 and T4 levels would respond to thermal stress situations [
6] and result in a slowdown in the biological conversion of T4 to T3. This is controlled by the Outer-Ring Deiodinase type I (ORD-1), whose action is slowed down under thermal stress [
7], which promotes a slowdown of the body to generate less heat.
As for the indicators of output, they remained highly degraded as per the recommendations of the guide [
3] and [
8]; living weights were reported between 3641 and 4099 g/s, with average daily gains between 89 and 98/s/d, consumption indices between 1.787 and 1.707 and a mortality rate that did not exceed 6%. Several authors have already undertaken this development [
5,
6,
7,
9,
10], which resulted from thermal stress adaptation. Adaptation depends on productivity and, in extreme cases, on sustainability.
5. Conclusions
This study highlights the metabolic responses of two strains of meat chickens, the Cobb 500 and the Arbor Acres, to thermal stress situations generated by the new climate in the context of Algerian poultry farming. The results revealed that the CB batch responded relatively better to these situations than the AA batch. This is significant for serum hormones expressed by levels of T3 and T4, which could even be used as tools to diagnose stress states. However, despite these responses, both strains showed significant degradation in terms of production and sustainability.
Finally, given all these results, it is clear that the new climate may trigger a chain of adaptive reactions that result, among other things, in a new hormonal balance in birds, thereby promoting survival at production.
Author Contributions
Conceptualization, A.K.M., D.H., R.M. and H.I.; methodology, formal analysis A.K.M. and D.H.; writing—review and editing, A.K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study does not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
Our thanks are expressed to the operators who allowed us access to the facilities and who were responsible for the handling of the animals and the conduct of the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eastman, C.J.; Corcoran, J.M.; Ekins, R.P.; Williams, E.S.; Nabarro, J.D. The radioimmunoassay of triiodothyronine and its clinical application. J. Clin. Pathol. 1975, 28, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Arbor Acres Guide D’élevage du Poulet de Chair; 1118-AVNAA-041; Aviagen United Kingdom Ltd.: Newbridge, UK, 2018; p. 162.
- Arbo rAcres Plus. Broiler Performance Objectives; 0419-AVNAA-042; Aviagen United Kingdom Ltd.: Newbridge, UK, 2019; p. 15.
- COBB 500. Broiler Performance and Nutrition Supplement; Cobb-Vantress, Inc.: Lyme Regis, UK, 2015; L-2114-07; p. 14.
- Nawaz, M.S.; Sami, S.A.; Bano, M.; Khan, M.R.Q.; Anwar, Z.; Ijaz, A.; Ahmed, T. Impact of salt stress on cotton. Int. J. Agric. Biosci. 2023, 12, 98–103. [Google Scholar]
- Javad Cheraghi, E.H.; Taheri, S.S.; Taherpour, K.; Kaviani, K.Z.; Rezazadeh, L. Thyroid hormones investigation under heat stress in broilers administered with probiotic (BIO-SAF) and prebiotic (BIO-MOS). Eur. J. Exp. Biol. 2013, 3, 562–567. [Google Scholar]
- Tao, X.; Zhang, Z.Y.; Dong, H.; Zhang, H.; Xin, H. Responses of thyroïd hormones of market-size broilers to thermoneutral constant and warm cyclic temperatures. Poult. Sci. 2006, 85, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- COBB 500. Broiler Performance and Nutrition Supplement; Cobb-Vantress, Inc.: Lyme Regis, UK, 2022; L-054-01-22; p. 16.
- Hamidi, O.; Chamani, M.; Ghahri, H.; Sadeghi, A.A.; Malekinejad, H. Effects of using different levels of chromium picolinate on performance, some blood biochemical and intestinal morphology and microflora in ross 308 broiler chicks exposed to the heat stress condition. Int. J. Pharm. Sci. Res. 2019, 10, 4494–4500. [Google Scholar]
- Torrent, J.; Arce Menocal, J.; Lopez Coello, C.; Avila Gonzalez, E. Effects of functional oils on performance and carcass characteristics of broilers under two different temperature environments. Poult. Sci. 2019, 98, 5855–5861. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).