Abstract
Actinobacteria species, especially Streptomyces, are well known and widely studied as promising biocontrol and phytostimulation agents. They constitute an eco-friendly substitute for chemical fungicides. Streptomyces-like strains were isolated from wheat fields to suppress the growth of Fusarium, the leading wheat root-rot-associated fungi, and to stimulate plant growth. The isolates were characterized morphologically and biochemically and subjected to a comprehensive in vitro screening for various plant-growth-promoting (PGP) traits. The potential beneficial effects of these strains on wheat plants were evaluated upon their inoculation (germination rate, shoot and root lengths). Among 32 isolates, the strain Act 02 was positive in inhibiting Fusarium growth and showing vigorous antifungal activity. In vitro assays demonstrated the ability of Act 02 to produce ammonia and indole-acetic acid (IAA). The strain showed extracellular enzyme production, such as Chitinases, Cellulases, and solubilized phosphate (Ca3PO4). The strain Act 02 tolerated high concentrations of NaCl with a considerable interval of [1–8]% (w/v), with optimum ranges between [1–3]%. 16s RNA gene barcoding and phylogenetic analysis showed that the strain Act 02 belongs to S. lividans with a 99.04% similarity. Seed germination and pot experiments were conducted by inoculating Triticum durum seeds with a selected isolate extract. Act 02 was able to significantly increase plant lengths.
1. Introduction
Population growth is increasing global food demand, forcing agriculture to improve productivity. However, limited soil macronutrients necessitate agrochemicals, posing environmental and health risks. Innovative, cost-effective, and environmentally friendly strategies are needed to achieve similar agricultural goals [1,2]. Actinobacteria species, especially Streptomyces, constitute a group of abundant beneficial microorganisms, have significant biotechnological potential due to their ability to enhance soil fertility through mineral solubilization, nitrogen fixation, organic matter decomposition, and nutrient storage. This study explores Actinobacteria’s role as promising biocontrol and phytostimulation agents and their growth-promoting traits, providing insights into their future potential as an eco-friendly substitute for chemical fungicides.
2. Materials and Methods
2.1. Isolation and Identification of Streptomyces-like Strains
Soil samples were collected from the wheat rhizosphere in the Taoura region of Souk-Ahras, Algeria (36°9′48.41″ N, 8°2′37.65″ E). Actinobacteria were isolated on International Streptomyces project (ISP2) and selected according to their morphology [2].
2.2. Antagonism Assay by Diffusible Compounds
The antifungal activity of the Streptomyces-like isolates was assessed against phytopathogenic Fusarium culmorum obtained from the LaMyBAM culture collection using the dual culture method and calculating the inhibition percentage (I%) of fungal growth [3].
2.3. In Vitro Assessment of Plant Growth Promotion (PGP) Traits
- -
- Ammonia production (NH3) and Hydrogen Cyanide (HCN) production were evaluated according to Farda et al. [4] and Alloun et al. [2], respectively.
- -
- Indole 3-acetic acid (IAA) production was assessed on yeast–tryptone broth supplemented with 0.2% (w/v) L-tryptophan after eight days of incubation. The IAA quantification process involved centrifuging the culture supernatants for 30 min at 8000 rpm and using the Salkowski reagent with a ratio of (1:2) (v/v). The pink-red colour indicates indole compounds. A standard curve was generated using synthetic IAA, and optical density was measured at 530 nm using a UV–vis spectrophotometer [5,6].
- -
- Phosphate solubilisation was investigated on Pikovskaya medium (PVK) amended with Ca3PO4 [7].
2.4. Enzymatic Profile of Selected Strains
- -
- Protease production, cellulolytic and amylolytic activity, and chitinolytic activity were evaluated according to [1], [8,9], and [2], respectively.
- -
- The ability of the isolate Act 02 to tolerate NaCl concentrations from 0 to 10% (w/v) (at intervals of 1.0 NaCl unit) was assessed on GYMA after 8 days of incubation at 28 °C.
2.5. Molecular Identification of Act 02
The molecular identification of Act 02 using 16S rRNA gene barcoding and taxonomic affiliation was determined based on sequence similarity with referenced strains from GenBank database. A phylogenetic tree was constructed using the Maximum Likelihood method on MEGA 11 software [10].
2.6. Seed Bio-Priming Assays
Wheat seeds (Triticum durum) were disinfected with sodium hypochlorite (2% NaClO for 5 min) and 90% ethanol (for 5 min), rinsed three times with sterile distilled water and air-dried under a laminar flow hood. Actinobacteria spore suspensions at various concentrations were prepared, consisting of the following treatments: A: 6 × 105, B: 2.5 × 106, C: 6.4 × 106, D: 7.2 × 106, E: 9 × 106, F: 3.4 × 107 (spores mL−1), and sterile distilled water as control. Seeds were germinated in the dark and then transferred to pots and incubated at 25 °C/16 h of light per day for two weeks. The germination rates and seedling heights of the co-inoculated group were calculated and statistically compared with the control group.
2.7. Statistical Analysis
All experimental data consisted of the mean of three replicates ± standard deviation (SD). One-way analysis of variance (ANOVA), followed by a Tukey post hoc test, comparing mean values at a 5% significance level (p < 0.05), was used to determine the statistical significance of differences between groups.
3. Results
3.1. In Vitro Antagonism of Actinobacteria Isolates
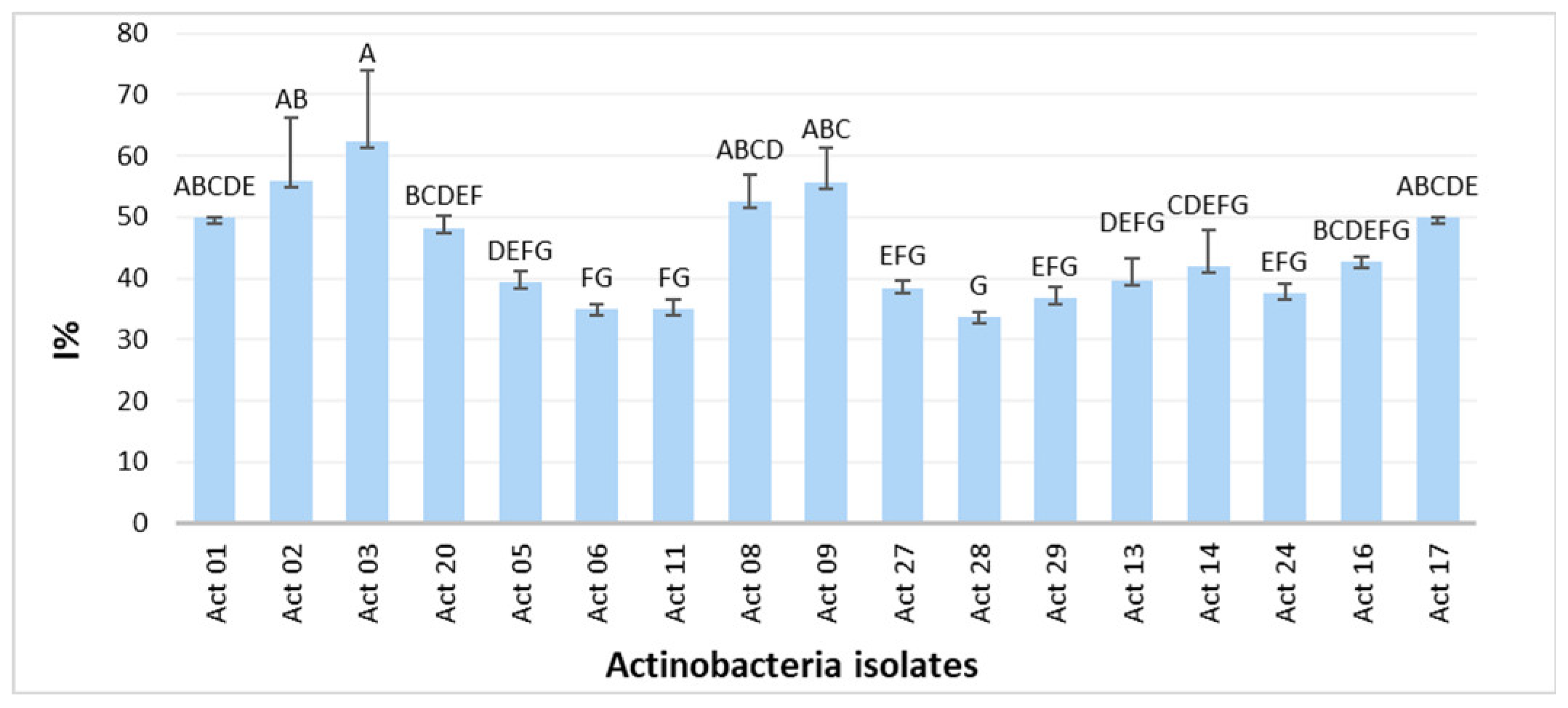
Figure 1 displays the in vitro antagonism of Actinobacteria against F. culmorum.
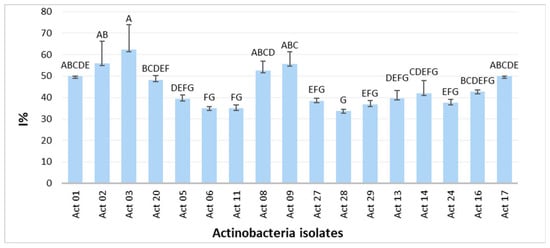
Figure 1.
Growth inhibitory rate I% of Fusarium culmorum. One-way ANOVA (p < 0.05) was used to compare means. Each assay was performed three times with three biological replicates, and values represent their means ± standard deviation (SD). Values sharing a letter are not statistically different.
3.2. Plant-Growth-Promoting Activities
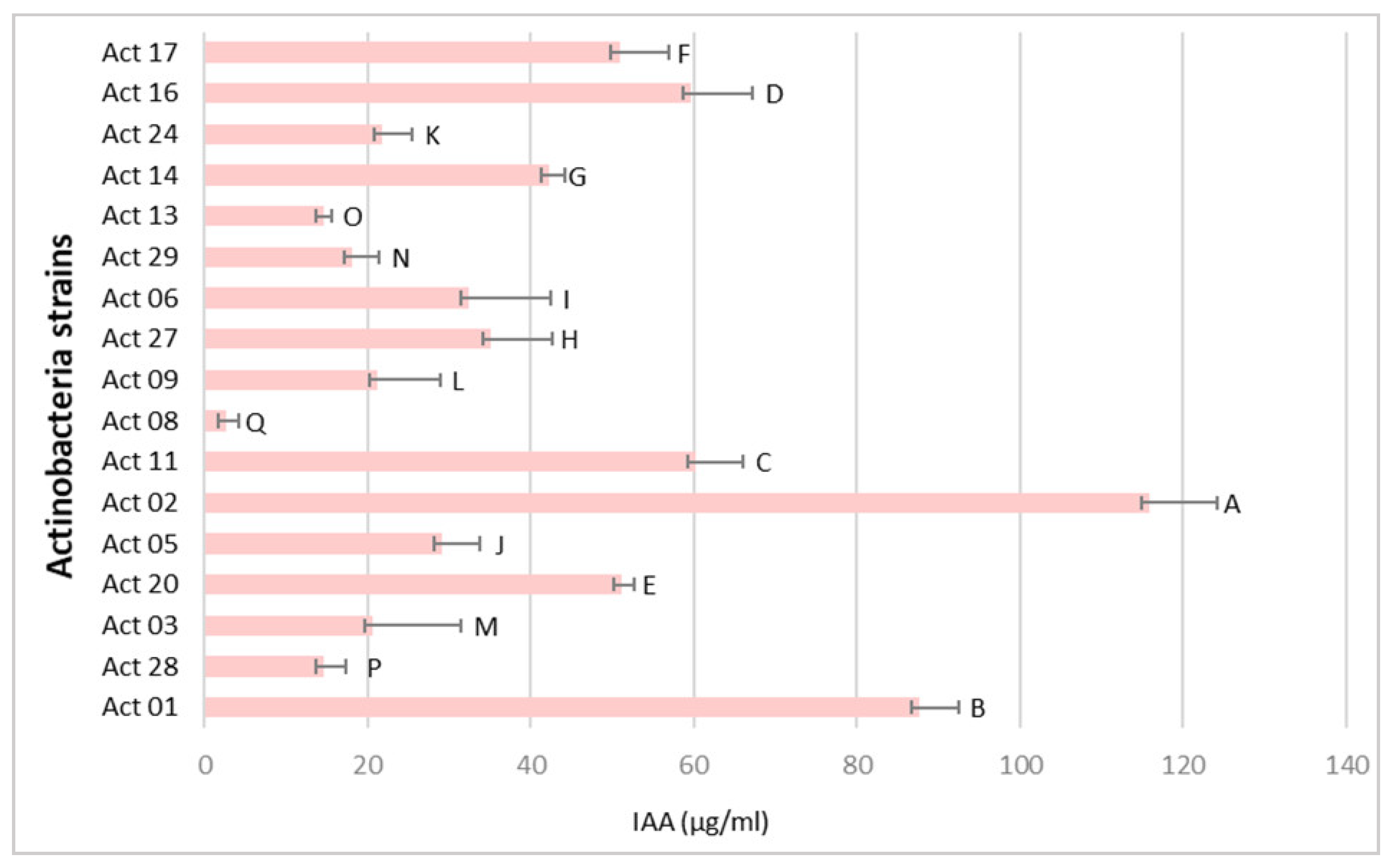
The IAA production from Actinobacteria isolates is represented in Figure 2.
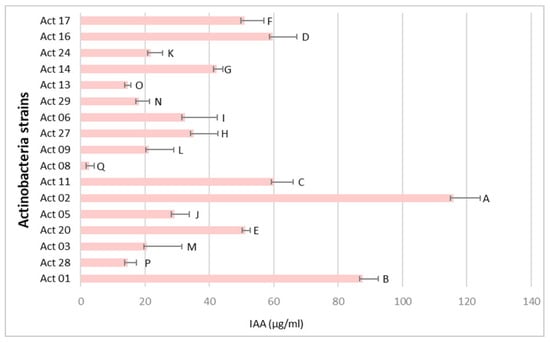
Figure 2.
IAA production from Actinobacteria strains (µg mL−1). One-way ANOVA (p < 0.05) was used to compare means. Each assay was performed three times with three biological replicates, and values represent their means ± standard deviation (SD). Values sharing a letter are not statistically different.
The PGP traits of the collection of rhizospheric Actinobacteria, including HCN, NH3, phosphate solubilisation and enzyme production, are displayed in Table 1.

Table 1.
Enzymatic profile, HCN and ammonia production and P solubilisation of a collection of Actinobacteria isolates.
3.3. Molecular Identification of Act 02
The phylogenetic and taxonomic analysis of the isolate ACT 02 is exhibited in Figure 3.

Figure 3.
Phylogenetic tree based on NJ method of 16S rRNA gene sequences of Streptomyces lividans Act 02 (framed in red) and related strains.
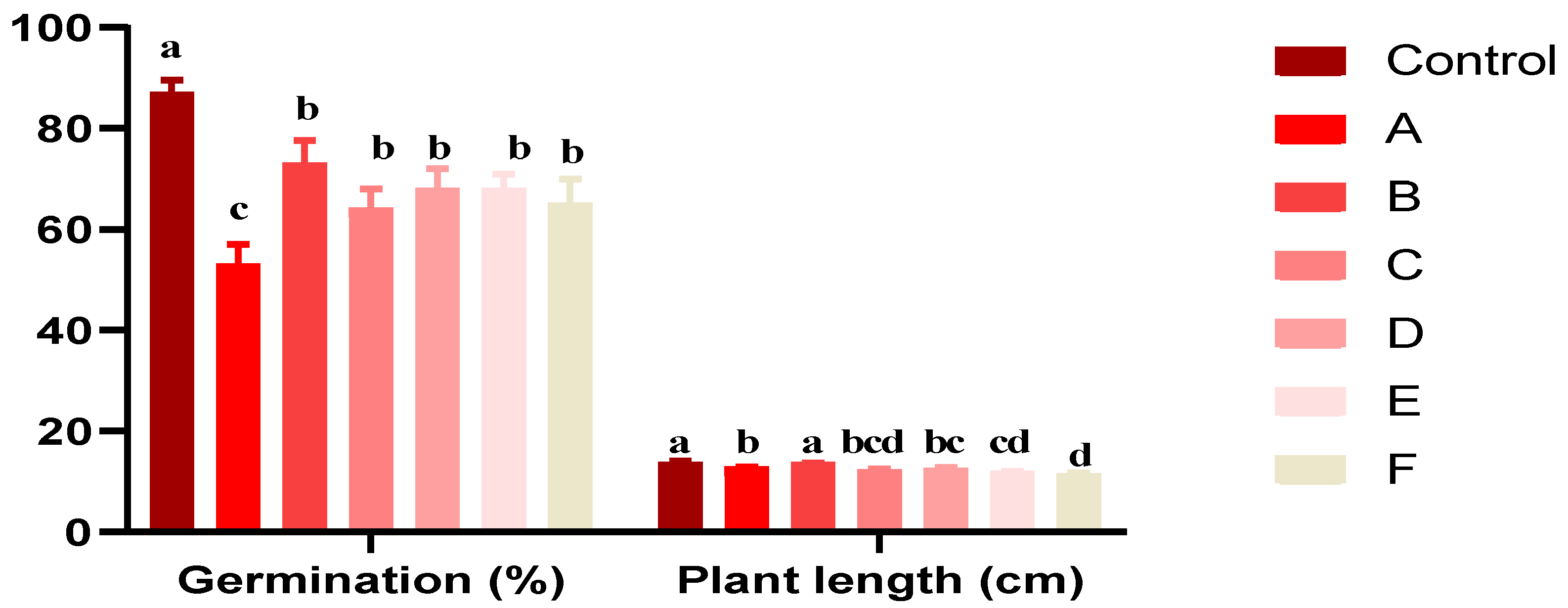
Figure 4 depicts the germination percentages of Triticum durum under various Act 02 spore concentrations (A, B, C, D, E, F) and a control group.
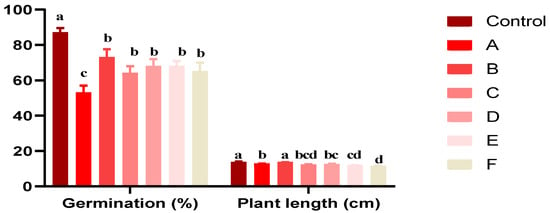
Figure 4.
Effect of various concentrations of Act 02 on germination rates and Triticum durum plant lengths after 15 dpi. Results are reported as mean ± SD (n = 3). Data with different letters were significantly different (Tukey test, p < 0.05).
4. Discussion
The role of Actinobacteria biological agents in protecting plants from a variety of soil-borne diseases and to engage in hostile environments has been reported [11]. In this study, 17 out of 32 isolates presented Streptomyces-like morphological and cultural characteristics and considerable inhibition rates (I%) of Fusarium culmorum growth (Figure 1) with statistically significant differences between the isolates. Actinobacteria’s biocontrol activity is correlated with host defence induction, lysis mechanisms and antibiosis [12]. Interest in integrating Actinobacteria strains in plant protection from phytopathogens is growing and promising candidates for discovering novel secondary metabolites for biocontrol application on crops have been found [2]. Moreover, Actinobacteria can promote plant growth via nutrient solubilization, nitrogen fixation and phytohormone synthesis [13]. Significant levels of IAA have been generated by rhizospheric Streptomyces strains [2,14]. Streptomyces griseorubens BC10 produced up to 128.44 µg mL−1, according to Boubekri et al. [15]. Our recent study reported the production of IAA from 28 Actinobacteria isolated from wheat rhizosphere in the region of Tiffeche, Algeria, with Streptomyces rubrogriseus AW22 exhibiting the highest IAA yields (24 μg mL−1) [2]. According to Figure 2, Act 02 exhibited the highest IAA yield compared with those obtained by Alloun et al. [2]. Ammonia and HCN were discovered to be produced by these isolates. Actinobacteria’s antagonistic potential may be linked to HCN generation and diffusible antifungal metabolites, as there was no direct contact between the target fungal pathogen and Actinobacteria [2].
Actinobacteria isolates had distinct enzymatic profiles from one isolate to another. These enzymes actively contribute to the organic matter degradation and the cycling of nutrients in soil, which are both necessary for the survival of strains under different growth conditions [16]. The isolate Act 02 was found to solubilize phosphate and tolerated high concentrations of NaCl 1–8% (optimal at 1–3%). The capacity of Actinobacteria to solubilize phosphate was reportedly less studied than the other PGPR features [17].
The isolate Act 02 presents 99.04% similarity with S. lividans using 16S rRNA gene barcoding, and the National Center for Biotechnology Information (NCBI) received its sequence submission for GenBank. Its phylogenetic tree is displayed in Figure 3.
This study investigated the effects of Actinobacteria strain Act 02 spore concentrations on Triticum durum germination and growth (Figure 4). The germination rate in the control group was the greatest at 87%, whereas in treatments with decreased spore concentrations (A and F), the germination rate was lower, potentially inhibiting germination. The effects of treatments (B, C, D, and E) with different spore concentrations on germination were variables; however, they were not statistically significant.
The length of the plants in these treatments was just marginally less than the control. Plant growth was unaffected significantly by Treatment B. Further statistical analysis is necessary in light of these findings, highlighting the complex link between Actinobacteria strain Act 02 spore concentrations and durum wheat germination and growth.
5. Conclusions
This study suggests Actinobacteria strains as a potential biocontrol agent against Fusarium spp. and plant-growth-promoting tools, offering an alternative to conventional pesticides and seed treatments for wheat seed emergence. However, further research is needed for sustainable cropping systems based on improved soil biodiversity. This report suggests enhancing soil and plant microorganisms for biocontrol, particularly Fusarium spp., by developing cost-effective fungicides and fertilizers for Algeria’s durum wheat cropping systems.
Author Contributions
W.A.: Conceptualization, Project administration, Formal analysis, Software, Methodology, Validation, Writing—original draft, Writing—review and editing. H.K.: Formal analysis, Software, Methodology, Writing—review and editing. N.K.C.: Writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We thank the Laboratory of Mycology, Biotechnology and Microbial Activity, Department of Applied Biology, Constantine 1 University of Brothers Mentourithe. We would also like to express our gratitude to the Biotechnology Research Center (CRBt Constantine), Algeria.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdelmoteleb, A.; Troncoso-Rojas, R.; Gonzalez-Soto, T.; González-Mendoza, D. Antifungical activity of autochthonous Bacillus subtilis isolated from prosopis juliflora against phytopathogenic fungi. Mycobiology 2017, 45, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Alloun, W.; Berkani, M.; Benaissa, A.; Shavandi, A.; Gares, M.; Danesh, C.; Lakhdari, D.; Ghfar, A.A.; Kacem Chaouche, N. Waste valorization as low-cost media engineering for auxin production from the newly isolated Streptomyces rubrogriseus AW22: Model development. Chemosphere 2023, 326, 138394. [Google Scholar] [CrossRef] [PubMed]
- Jamali, H.; Sharma, A.; Roohi; Srivastava, A.K. Biocontrol potential of Bacillus subtilis RH5 against sheath blight of rice caused by Rhizoctonia solani. J. Basic Microbiol. 2020, 60, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Farda, B.; Mattedi, A.; Djebaili, R.; Pace, L.; Del Gallo, M.; Pellegrini, M. Microbial Community Investigation of Wild Brambles with Root Nodulation from a Calcareous Nitrogen-Deficient Soil. Soil Syst. 2022, 6, 96. [Google Scholar] [CrossRef]
- Passari, A.K.; Mishra, V.K.; Gupta, V.K.; Yadav, M.K.; Saikia, R.; Singh, B.P. In vitro and in vivo plant growth promoting activities and DNA fingerprinting of antagonistic endophytic actinomycetes associates with medicinal plants. PLoS ONE 2015, 10, e0139468. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Karimi, E.; Dahaji, P.A.; Javid, M.G.; Dalvand, Y.; Askari, H. Plant growth promoting activity of an auxin and siderophore producing isolate of Streptomyces under saline soil conditions. World J. Microbiol. Biotechnol. 2012, 28, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Khenaka, K.; Canfora, L.; Benedetti, A.; Leulmi, N.; Boulahrouf, A. Effect of Capsicum annuum cultivated in sub-alkaline soil on bacterial community and activities of cultivable plant growth promoting bacteria under field conditions. Arch. Agron. Soil Sci. 2019, 65, 1417–1430. [Google Scholar] [CrossRef]
- Slama, H.B.; Cherif-Silini, H.; Bouket, A.C.; Qader, M.; Silini, A.; Yahiaoui, B.; Alenezi, F.N.; Luptakova, L.; Triki, M.A.; Vallat, A.; et al. Screening for fusarium antagonistic bacteria from contrasting niches designated the endophyte bacillus halotoleransas plant warden against fusarium. Front. Microbiol. 2019, 10, 3236. [Google Scholar] [CrossRef]
- Suárez-Moreno, Z.R.; Vinchira-Villarraga, D.M.; Vergara-Morales, D.I.; Castellanos, L.; Ramos, F.A.; Guarnaccia, C.; Degrassi, G.; Venturi, V.; Moreno-Sarmiento, N. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019, 10, 290. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ansari, W.A.; Krishna, R.; Zeyad, M.T.; Singh, S.; Yadav, A.K. Endophytic actinomycetes-mediated modulation of defense and systemic resistance confers host plant fitness under biotic stress conditions. In Microbial Versatility in Varied Environments: Microbes in Sensitive Environments; Springer: Singapore, 2020; pp. 167–180. [Google Scholar]
- Toumatia, O.; Compant, S.; Yekkour, A.; Goudjal, Y.; Sabaou, N.; Mathieu, F.; Sessitsch, A.; Zitouni, A. Biocontrol and plant growth promoting properties of Streptomyces mutabilis strain IA1 isolated from a Saharan soil on wheat seedlings and visualization of its niches of colonization. S. Afr. J. Bot. 2016, 105, 234–239. [Google Scholar] [CrossRef]
- Alloun, W.; Kecis, H.; Chaoua, S.; Cornu, B.; Djelid, H.; Gares, M.; Kacem Chaouche, N. Auxin originated from Actinobacteria participates in abiotic stress mitigation and sustainable crop production. Not. Sci. Biol. 2023, 15, 11602. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef] [PubMed]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The screening of potassium-and phosphate-solubilizing actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef] [PubMed]
- Gares, M.; Benaissa, A.; Hiligsmann, S.; Cherfia, R.; Flahaut, S.; Alloun, W.; Djelid, H.; Chaoua, S.; Kacem Chaouche, N. Box-Behnken design optimization of xylanase and cellulase production by Aspergillus fumigatus on Stipa tenacissima biomass. Mycologia 2023, 115, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Sudiana, I.M.; Putri, A.; Napitupulu, T.P.; Idris; Purnaningsih, I.; Kanti, A. Growth inhibition of Fusarium solani and F. Oxysporum by Streptomyces sasae TG01, and its ability to solubilize insoluble phosphate. Biodiversitas 2020, 21, 429–435. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).