Photoinactivation of Staphylococcus carnosus on Surfaces by Irradiation with Blue and Violet Light †
Abstract
:1. Introduction
2. Material and Methods
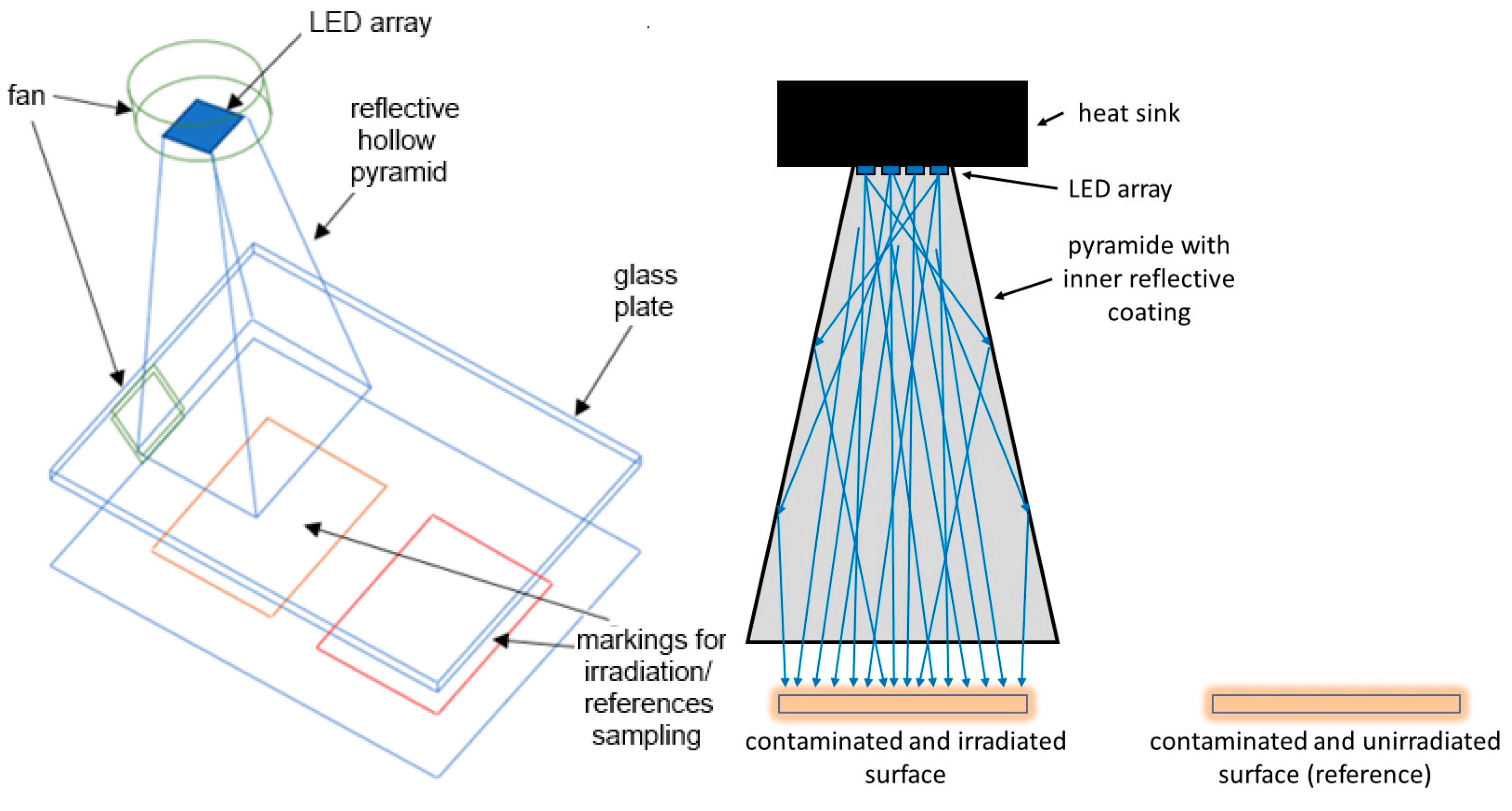
2.1. Irradiation Setup
2.2. Microbiological Procedure
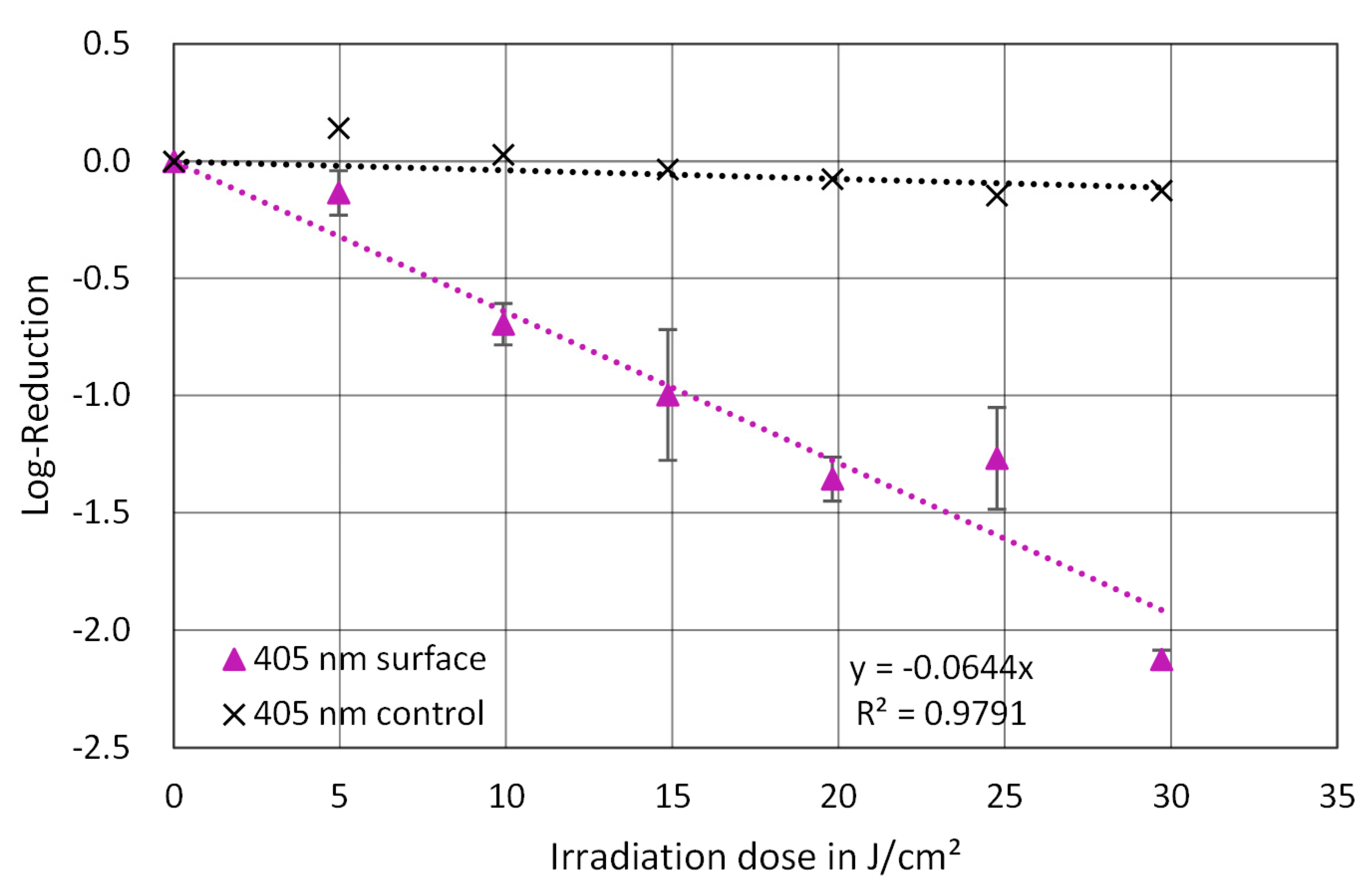
3. Results
4. Discussion
5. Conclusions
- Violet LEDs are applied together with white LEDs to achieve a sufficient color rendering index and the required illumination, which has already been attempted in other investigations [21]. This would ensure a continuous irradiation and a sufficient reduction of pathogens.
- Violet LEDs are installed in room lighting, but are only switched on temporarily, when neither working persons or, in the case of hospitals, patients are present in the affected area. This means that the irradiance can be adjusted as required within the limits of the technical possibilities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geffers, C.; Zuschneid, I.; Sohr, D.; Rüden, H.; Gastmeier, P. Erreger nosokomialer Infektionen auf Intensivstationen: Daten des Krankenhaus-Infektions-Surveillance-Systems (KISS) aus 274 Intensivstationen. AINS 2004, 39, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ruscher, C. Empfehlungen zur Prävention und Kontrolle von Methicillin-resistenten Staphylococcus aureus-Stämmen (MRSA) in medizinischen und pflegerischen Einrichtungen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014, 57, 695–732. [Google Scholar] [CrossRef]
- Christiansen, B.; Dettenkofer, M.; Becker, E.M.; Eikmann, T.; Exner, M.; Heeg, P.; Kramer, A.; Ruf, B.; Schwebke, I. Anforderungen an die Hygiene bei der Reinigung und Desinfektion von Flächen. Empfehlung der Kommission für Krankenhaushygiene und Infektionsprävention beim Robert Koch-Institut (RKI). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2004, 47, 51–61. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mikulich, A.V.; Tretyakova, A.I.; Leusenka, I.A.; Plavskaya, L.G.; Kazyuchits, O.A.; Dobysh, I.I.; Krasnenkova, T.P. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B 2018, 183, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.Y.; Roser, D.; Corkish, R.; Ashbolt, N.J.; Stuetz, R. Point-of-use water disinfection using ultraviolet and visible light-emitting diodes. Sci. Total Environ. 2016, 553, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.L.; Krinsky, N.I.; Giovanazzi, S.M.; Peak, M.J. Superoxide anion is generated from cellular metabolites by solar radiation and its components. J. Free Radic. Biol. Med. 1985, 1, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2017, 364, fnw270. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Wenzel, U.; Meurle, T.; Spellerberg, B.; Hönes, K. Photoinactivation results of Enterococcus moraviensis with blue and violet light suggest the involvement of an unconsidered photosensitizer. Biochem. Biophys. Res. Commun. 2020, 533, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Hönes, K.; Bauer, R.; Meurle, T.; Spellerberg, B.; Hessling, M. Inactivation Effect of Violet and Blue Light on ESKAPE Pathogens and Closely Related Non-pathogenic Bacterial Species—A Promising Tool Against Antibiotic-Sensitive and Antibiotic-Resistant Microorganisms. Front. Microbiol. 2020, 11, 612367. [Google Scholar] [CrossRef]
- Hessling, M.; Haag, R.; Sicks, B. Review of microbial touchscreen contamination for the determination of reasonable ultraviolet disinfection doses. GMS Hyg. Infect. Control 2021, 16, Doc30. [Google Scholar] [CrossRef] [PubMed]
- Hönes, K.; Bauer, R.; Spellerberg, B.; Hessling, M. Microbial Photoinactivation by Visible Light Results in Limited Loss of Membrane Integrity. Antibiotics 2021, 10, 341. [Google Scholar] [CrossRef]
- Deutsche Sammlung von Mikroorganismen und Zellkulturen. M92: Trypticase Soy Yeast Extract Medium. Available online: https://www.dsmz.de/microorganisms/medium/pdf/DSMZ_Medium92.pdf (accessed on 21 September 2023).
- Prestrelski, S.J.; Tedeschi, N.; Arakawa, T.; Carpenter, J.F. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys. J. 1993, 65, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Wood, B.R. The importance of hydration and DNA conformation in interpreting infrared spectra of cells and tissues. Chem. Soc. Rev. 2016, 45, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Bumah, V.V.; Aboualizadeh, E.; Masson-Meyers, D.S.; Eells, J.T.; Enwemeka, C.S.; Hirschmugl, C.J. Spectrally resolved infrared microscopy and chemometric tools to reveal the interaction between blue light (470 nm) and methicillin-resistant Staphylococcus aureus. J. Photochem. Photobiol. B 2017, 167, 150–157. [Google Scholar] [CrossRef] [PubMed]
- França, M.B.; Panek, A.D.; Eleutherio, E.C.A. Oxidative stress and its effects during dehydration. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Leprince, O.; Hendry, G.A.F.; McKersie, B.D. The mechanisms of desiccation tolerance in developing seeds. Seed Sci. Res. 1993, 3, 231–246. [Google Scholar] [CrossRef]
- Leprince, O.; Atherton, N.M.; Deltour, R.; Hendry, G. The Involvement of Respiration in Free Radical Processes during Loss of Desiccation Tolerance in Germinating Zea mays L. (An Electron Paramagnetic Resonance Study). Plant Physiol. 1994, 104, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- DIN EN 12464-1:2011-08; Licht und Beleuchtung- Beleuchtung von Arbeitsstätten—Teil 1: Arbeitsstätten in Innenräumen. Deutsche Fassung EN 12464-1:2011; Beuth Verlag GmbH: Berlin, Germany, 2011.
- Bundesanstalt für Arbeitsschutz und Arbeitsmedizin. Technische Regeln für Arbeitsstätten: Beleuchtung. Available online: https://www.baua.de/DE/Angebote/Rechtstexte-und-Technische-Regeln/Regelwerk/ASR/pdf/ASR-A3-4.pdf?__blob=publicationFile (accessed on 21 February 2022).
- Bühler, J.; Sommerfeld, F.; Meurle, T.; Hönes, K.; Hessling, M. Disinfection Properties of Conventional White LED Illumination and Their Potential Increase by Violet LEDs for Applications in Medical and Domestic Environments. Adv. Sci. Technol. Res. J. 2021, 15, 169–175. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommerfeld, F.; Osswald, P.; Weller, P.; Hessling, M. Photoinactivation of Staphylococcus carnosus on Surfaces by Irradiation with Blue and Violet Light. Biol. Life Sci. Forum 2024, 31, 2. https://doi.org/10.3390/ECM2023-16474
Sommerfeld F, Osswald P, Weller P, Hessling M. Photoinactivation of Staphylococcus carnosus on Surfaces by Irradiation with Blue and Violet Light. Biology and Life Sciences Forum. 2024; 31(1):2. https://doi.org/10.3390/ECM2023-16474
Chicago/Turabian StyleSommerfeld, Florian, Patricia Osswald, Pia Weller, and Martin Hessling. 2024. "Photoinactivation of Staphylococcus carnosus on Surfaces by Irradiation with Blue and Violet Light" Biology and Life Sciences Forum 31, no. 1: 2. https://doi.org/10.3390/ECM2023-16474
APA StyleSommerfeld, F., Osswald, P., Weller, P., & Hessling, M. (2024). Photoinactivation of Staphylococcus carnosus on Surfaces by Irradiation with Blue and Violet Light. Biology and Life Sciences Forum, 31(1), 2. https://doi.org/10.3390/ECM2023-16474





