Abstract
Salmonellosis, which occurs most frequently (85%) as a result of consuming contaminated food, is brought on by salmonellae, which are bacteria that can infect both humans and animals. The aim of this study was to investigate the probiotic properties of three Bacillus spp. strains isolated from contaminated soil in North Macedonia and their antimicrobial activity. Additionally, their ability to survive in the presence of bile salts and at low pH and their susceptibility to antibiotics were examined. This research indicates that new Bacillus strains’ probiotic qualities are also promising and exhibit strong inhibition activity against Salmonella enterica ATCC 10708.
1. Introduction
Probiotics can be described as live microbial or cultured product feed supplements that have beneficial effects on the host through their production of inhibitory agents, vying for chemicals and adhesion sites, enhancing the microbial stability, and altering and boosting the immune system [1]. Probiotic supplements made from bacterial spore formers are utilized in prescription drugs, dietary supplements for mankind, and animal feeds. They are interesting as food additives because of their heat-resistant properties and capacity to cross the gastrointestinal barrier, and this role has now become advanced. Although sometimes thought of as soil organisms, Bacilli should actually be viewed as commensals in the gut [2]. A viable substitute for conventional antibiotic therapies is the use of probiotics to control illnesses and prevent the increase of antibiotic resistance. Additionally, by regulating the metabolic pathways that lead to the manufacture of hazardous compounds, probiotics can increase the immune system function, activate endogenous enzymes, create antimicrobial substances, and enhance the production of endogenous enzymes. Probiotics can create antimicrobial compounds that limit the growth of infections and the development of toxins [3]. Probiotics that produce spores have grown in popularity recently. Some Bacilli are utilized as probiotics in poultry and are Gram-positive spore-forming bacteria. Studies have demonstrated that Bacillus spores can germinate in the digestive system of chickens [4].
Salmonellosis, which occurs most frequently (85%) as a result of consuming contaminated food, is brought on by salmonellae, which are bacteria that can infect both humans and animals [5]. By assisting with the absorption of some critical nutrients, probiotics have the potential to exert growth-promoting effects by competitively excluding pathogens and boosting feed conversion rates. Salmonella remains active and can spread on meat and other livestock products that were not properly cooked or kept, making contaminated foods the primary mechanism of transmission. Salmonella can survive for years without showing any overt clinical symptoms in both humans and animals, and in certain situations, these individuals and animals can develop a chronic infection. Furthermore, an unprecedented increase in Salmonella that is resistant to antibacterial medications has been caused by overusing medicines in humans as well as animals [6]. As a result, researchers are currently considering alternative methods of preventing and treating Salmonella infections. The aim of this study was to investigate the probiotic properties of three Bacillus spp. strains isolated from contaminated soil in North Macedonia and their antimicrobial activity against Salmonella enterica ATCC 10708 using the agar well diffusion method. For identification, isolates were characterized morphologically and physiologically. Additionally, their ability to survive in the presence of bile salts, at low pH, and in high osmotic concentrations of NaCl and their susceptibility to antibiotics were examined. Each tested strain demonstrated antagonistic activity against Salmonella enterica ATCC 10708. The three different Bacillus strains were all resilient to an acidic environment (pH 3.0) and a high osmotic pressure (NaCl at 6.5%). This research indicates that new Bacillus strains’ probiotic qualities are also promising and exhibit strong inhibition activity against Salmonella enterica ATCC 10708.
2. Materials and Methods
2.1. Antimicrobial Activity
The determination of the antimicrobial activity of the isolates against the test microorganisms was conducted using the agar well diffusion method. The test bacteria Salmonella enterica ATCC 10708 used in this analysis is included in the microorganism collection of the Microbiology Laboratory at the Faculty of Natural Sciences and Mathematics, Skopje, North Macedonia. The test bacteria was inoculated in nutrient broth (NB) and incubated at 37 °C for 24 h. The test microorganism was inoculated onto each sterile nutrient agar (NA) Petri dish using a sterile swab, and then wells with a diameter of 6 mm were placed on the nutrient medium. Into these wells, cultures of Bacillus spp. were added which were allowed to pre-incubate at 37 °C for 24 h. The plates thus inoculated were incubated at 37 °C for 24 h, and then the diameter of the zone of inhibition was measured for each isolate.
2.2. Bile Salt Tolerance
To determine bile salt tolerance, Gilliland et al.’s approach [7] was modified in certain ways. Nutrient broth (NB) containing 0%, 0.3%, 0.5%, 1%, 1.5% and 2% of bile salts was inoculated with each probiotic strain and incubated for 24 and 48 h at 37 °C. Growth in the control (no bile salts) and test cultures was evaluated at 24 and 48 h by measuring the turbidity (FAU).
2.3. Antibiotic Resistance
By inoculating the isolates of choice on nutrient agar and then applying antibiotic discs with sterile tweezers, it was possible to test the isolates’ resistance to various antibiotics for antibiotic sensitivity using the Kirby–Bauer methodology. A zone of inhibition was observed after incubation.
2.4. Evaluation of Resistance in Intestinal Tract Conditions: Low pH and Sodium Chloride
The Nguyen et al. [8] approach was used to evaluate the tested isolates’ resistance to acidic pH values. Testing was conducted on isolates to see how sensitive they are to a nutrient broth containing 6.5% NaCl. The tubes were incubated at 37 °C for the tests on pH and concentrations of NaCl. At the time points evaluated, each sample was streaked on NA for the presence or absence of growth to confirm the livability of the strains, and the turbidity of the tubes was assessed.
3. Results
3.1. Antimicrobial Activity
All isolates were found to have inhibitory activity against Salmonella enterica ATCC 10708. The isolate B114 showed the greatest inhibitory effect on the test bacteria, with a zone of inhibition of 15.18 mm, while the isolates B37 and B62 showed zones of 14.22 mm and 14.83 mm (Figure 1).
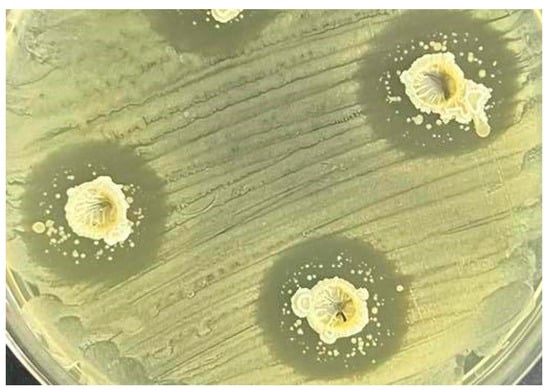
Figure 1.
Antibacterial activity (zones of inhibition) of Bacillus spp. from soil against Salmonella enterica ATCC 10708.
3.2. Bile Salt Tolerance
Evaluating bile salt tolerance of the vegetative cells of our selected strains, we found that all strains were able to grow when cultured at 0%, 0.3%, 0.5%, 1%, 1.5% and 2% of bile salts at 24 and 48 h of incubation (Figure 2).
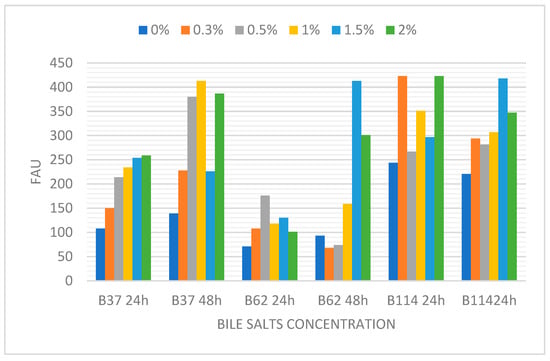
Figure 2.
Growth of the isolates B37, B62 and B114 at different % of bile salts at 24 and 48 h of incubation.
3.3. Antibiotic Resistance
The antibiotic resistance and susceptibility of the three Bacillus isolates to twenty-four antibiotics were analyzed. The isolate B37 was sensitive to all test antibiotics, while the isolate B62 was resistant to cefotaxime. The isolate B114 was resistant to ampicillin, cefotaxime, penicillin G and cephalexine. The diameter of the zones of inhibition is presented in Table 1.

Table 1.
Antibiotic sensitivity test for Bacillus spp. isolates.
3.4. Evaluation of Resistance in Intestinal Tract Conditions: Low pH and Sodium Chloride
Growth was seen on the control plates inoculated with the isolates as well as on the plates with medium and with a pH value of 3, indicating that the isolates have the capacity to grow at pH 3, i.e., tolerance to low pH values after the incubation period of 24 h. This quality is crucial in potential probiotics, since the bacterium needs to be tolerant to acidic pH in order to thrive both in the stomach and on food. All strains were also able to tolerate high osmotic concentrations of NaCl.
4. Discussion
Before entering the intestinal tract, probiotic bacteria must make it through the stomach, where the pH can drop as low as 1.5 to 2.0 [9], and remain alive for at least 4 h [10]. Probiotic microorganisms that exhibit resistance to a particular antibiotic may be administered along with antibiotic therapy [11]. Since conjugative plasmids frequently carry antibiotic resistance genes, they can spread to other bacteria [12] and potentially give rise to enteropathogenic bacteria that are resistant to antibiotics. Determining whether antibiotic-resistant genes are located on chromosomes or plasmids is therefore crucial [11]. In general, it has been thought that tolerance to bile salts is a need for bacterial colonization and metabolic activity in the host’s intestine. Depending on the person and the kind and quantity of food consumed, the small intestine’s bile salt content ranges from 0.2% to 0.3% on average and may reach 2% (w/v) [11,12]. However, bile levels in the colon fluctuate and are often low until a fatty meal is consumed. Bile secretion’s primary goal is to emulsify and dissolve ingested lipids. Bile salts can rupture the lipid barrier, enter the bacterial cell, denature proteins, chelate ions, and harm DNA, but they also have bactericidal effects [13].
5. Conclusions
Current probiotic products, which have been demonstrated to prevent gastrointestinal disorders in both humans and animals, contain bacterial spore formers, particularly those of the genus Bacillus. Numerous uses for these probiotic-based spores have been demonstrated, including the treatment of immunosuppressive disorders and diarrhea brought on by antibiotics. The findings of this investigation demonstrated the probiotic Bacillus spp. strains’ endurance of various physiological circumstances as well as Salmonella enterica suppression. Additionally, the techniques employed to screen isolates could be crucial in determining if Bacillus spp. are suitable for use as probiotics in both people and animals.
Author Contributions
S.K., D.K. and N.A.-P. contributed to the design and implementation of the research, to the analysis of the results and to the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare that no known conflicts of interest exist.
References
- Liu, C.H.; Chiu, S.W.; Cheng, W. Dietary administration of the probiotic, Bacillus subtilis E20, enhances the growth, innate immune responses, and disease resistance of the grouper, Epinephelus coioides. Fish Shellfish. Immunol. 2012, 33, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Cutting, S.M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Venkitanarayanan, K.; Johny, A.K. Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion mechanisms mediated by probiotics and prebiotics and their potential im-pact on human health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.T.; Lay, C.J.; Wang, C.L.; Koo, M. Characteristics of nontyphoidal Salmonella gastroenteritis in Taiwanese children: A 9-year pe-riod retrospective medical record review. J. Infect. Public Health 2017, 10, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Desin, T.S.; Koster, W.; Potter, A.A. Salmonella vaccines in poultry: Past, present and future. Exp. Rev. Vac. 2013, 12, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Gilliand, S.E.; Staley, T.E.; Bush, L.J. Importance of bile tolerance of Lactobacillus acidophilus used as a dietary adjunct. J. Dairy Sci. 1984, 67, 3045–3051. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Kang, J.H.; Lee, M.S. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int. J. Food Microbiol. 2007, 113, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Dunne, C.; O’Mahony, L. In vitro selection criteria for probiotic bacteria of human origin: Correlation with in vivo findings. Am. J. Clin. Nutr. 2001, 73, 386–392. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Bakari, D.; Tatsadjieu, N.L.; Mbawala, A.; Mbofung, C.M. Assessment of physiological properties of some lactic acid bacteria isolated from the intestine of chickens use as probiotics and antimicrobial agents against enteropathogenic bacteria. Innov. Rom. Food Biotechnol. 2011, 8, 33–40. [Google Scholar]
- Bennett, P.M. Plasmid encoded antibiotic resistance: Acquisition and transfer of antibiotic resistance genes in bacteria. Br. J. Pharmacol. 2008, 153, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.B.; Cota, I.; Ducret, A.; Aussel, L.; Casadesus, J. Adaptation and preadaptation of Salmonella enterica to bile. PLoS Genet. 2012, 8, 1–15. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).