Assay Development for Phagocytosis Activity Evaluation †
Abstract
:1. Introduction
2. Materials and Methods
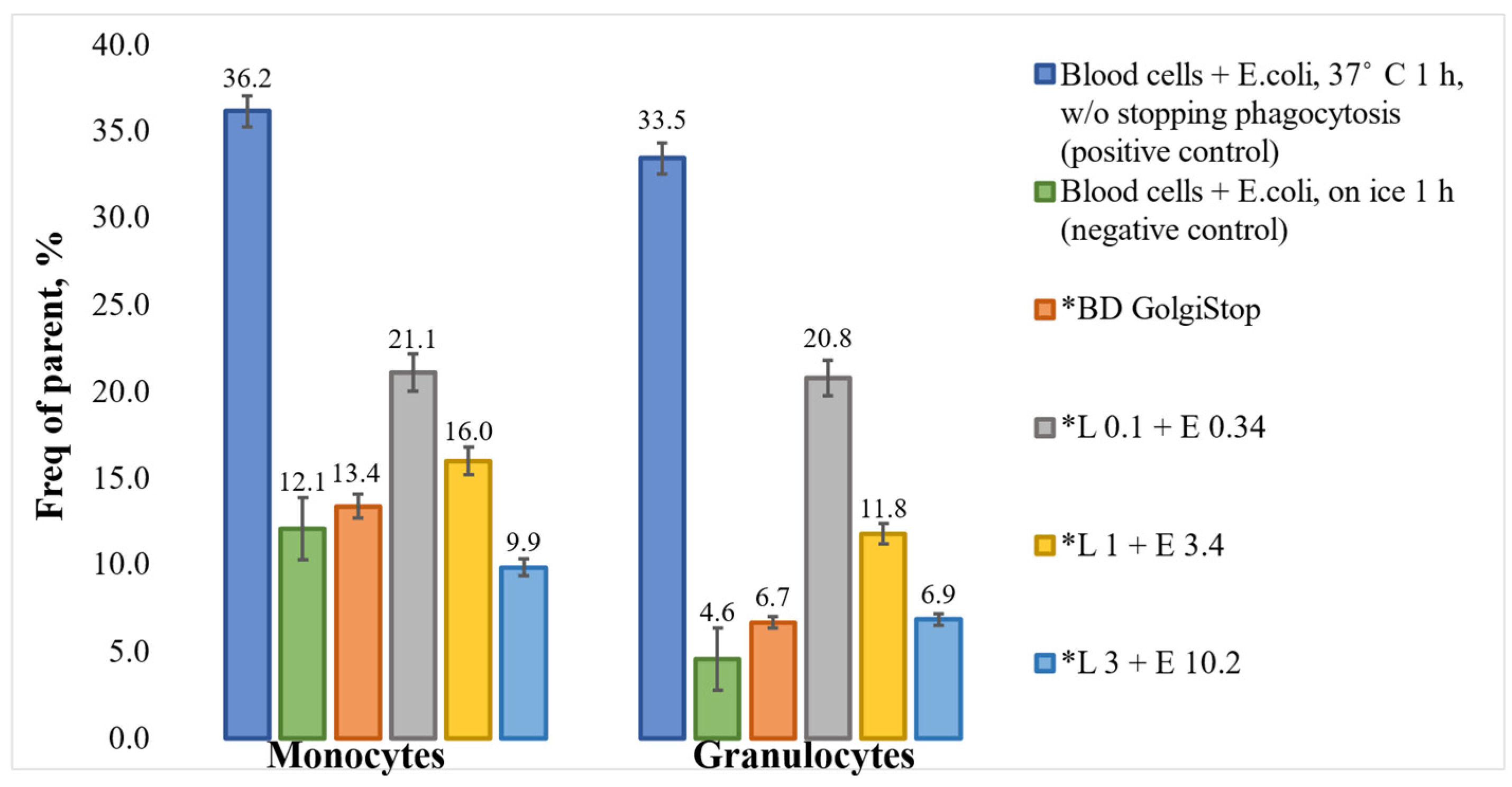
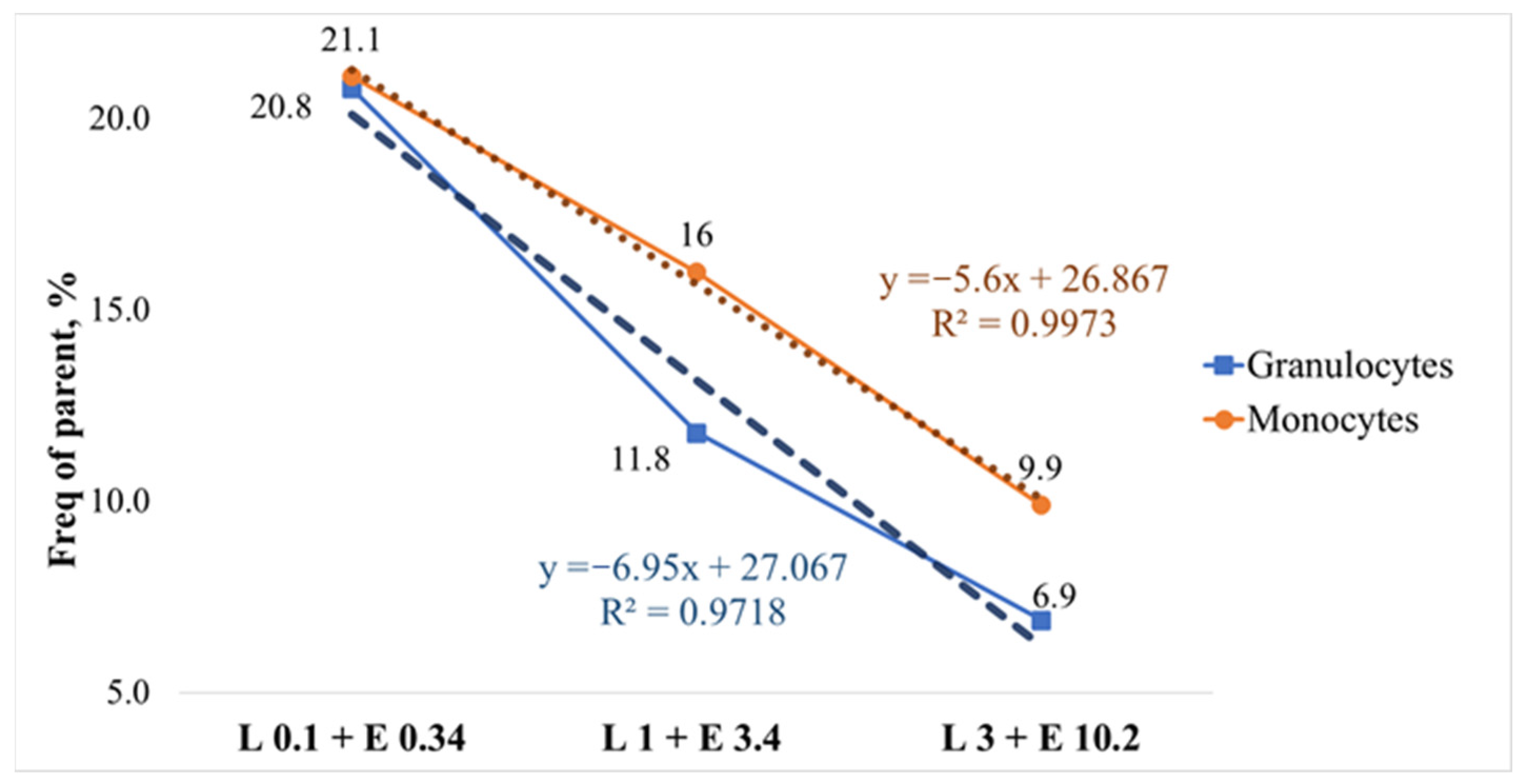
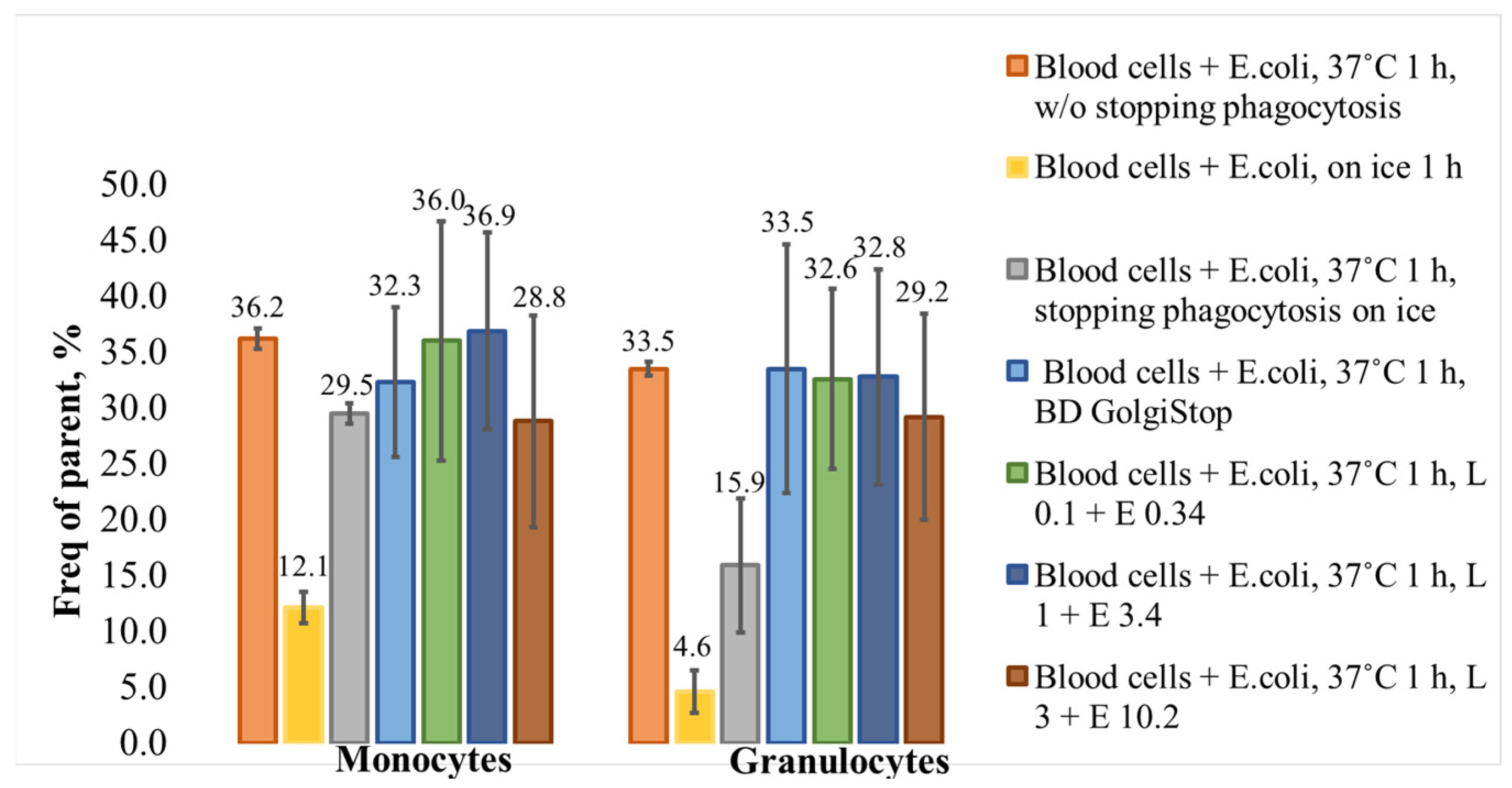
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azuma, Y.; Wang, P.L.; Shinohara, M.; Ohura, K. Differentiation by in vitro treatment of lidocaine–epinephrine and prilocaine–felypressine in neutrophils. Immunol. Lett. 2001, 77, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Seyda, N.; Seveau, V.; Manca, F.; Biarnes-Pelicot, M.; Valignat, M.P.; Bajenoff, M.; Theodoly, O. Human neutrophils swim and phagocytise bacteria. Biol. Cell 2021, 113, 28–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lysakova, E.; Shumeev, A.; Laktyushkin, V.; Chuvpilo, S.; Rybtsov, S. Assay Development for Phagocytosis Activity Evaluation. Biol. Life Sci. Forum 2024, 31, 15. https://doi.org/10.3390/ECM2023-16444
Lysakova E, Shumeev A, Laktyushkin V, Chuvpilo S, Rybtsov S. Assay Development for Phagocytosis Activity Evaluation. Biology and Life Sciences Forum. 2024; 31(1):15. https://doi.org/10.3390/ECM2023-16444
Chicago/Turabian StyleLysakova, Elena, Alexander Shumeev, Victor Laktyushkin, Sergey Chuvpilo, and Stanislav Rybtsov. 2024. "Assay Development for Phagocytosis Activity Evaluation" Biology and Life Sciences Forum 31, no. 1: 15. https://doi.org/10.3390/ECM2023-16444
APA StyleLysakova, E., Shumeev, A., Laktyushkin, V., Chuvpilo, S., & Rybtsov, S. (2024). Assay Development for Phagocytosis Activity Evaluation. Biology and Life Sciences Forum, 31(1), 15. https://doi.org/10.3390/ECM2023-16444





