Abstract
The production of silage is carried out in cylindrical bales covered with polyethylene foils. In this study, a novel approach was tested towards obtaining an innovative composition of these films. In the first stage of the experiment, different additives, including microcellulose and nanosilver particles, were analyzed. The second stage was aimed at testing the applicability of recycled polyethylene as a film component. The forage value after ensiling was assessed during storage. In order to evaluate the microbial forage quality, the abundance of lactic acid bacteria was determined and compared with the number of aerobic bacteria, yeasts, and molds. The foil properties were also analyzed with the appropriate chemical and microbiological methods. The results showed no significant differences (p < 0.05) between the standard commercial films and tested formulae. In the second stage, obtained results suggested that the film with the addition of nanosilver may be successfully used in agriculture.
1. Introduction
Conservation of forage as silage is one of the preservation methods that provide a very good source of energy, vitamins, and nutrients for livestock [1]. Ensiling has become popular in Europe in the 21st century, exceeding hay production and allowing better conservation of fodder for prolonged storage time [1,2,3,4]. For silage production, almost every type of crop plant can be used [1].
Lactic acid bacteria (LAB) are the group of microorganisms responsible for the ensiling process. Within this group, the strains belonging to Lactobacillus, Lactococcus, Enterococcus, Leuconostoc, Pediococcus, Streptococcus, and Weissella genera can be found, representing both homo- and heterofermentative groups [2,3]. The natural microbiota of forage plants initiate the fermentation process, then species succession occurs, leading to changes in the ensiled material. The final product quality is dependent on these strains as well as on their metabolism products [2].
In Europe, almost half of the plastic-based materials used in agriculture are allocated to silage production and bale formation [4]. The most important features of good wrapping film are thickness, extensibility, UV and damage resistance, high durability, and air and water impermeability [5]. The growth of undesirable microorganisms, such as aerobic bacteria, yeasts, and molds, can also be a great problem leading to silage quality loss [2]. Therefore, the addition of various components has been considered for polyethylene (PE) film modification to enable mechanical strength improvement, enhance oxygen barrier properties, and ensure anaerobic LAB growth promotion together with the inhibition of the unfavorable microbiota growth.
The main aim of the presented research was to monitor the microbiota population dynamics during the storage of silage bales wrapped with different types of film containing innovative variant additives to improve their quality.
2. Materials and Methods
2.1. Plant Material, Bale Preparation Method, and Tested Films
The experiment was carried out at the Pedigree Breeding Center in Osiek (Nidek, Małopolskie voivodeship; 49°54′ N, 19°19′ E). The material allocated for ensiling was a mixture of grasses (80–85%) and legumes (10–15%) consisting of Italian ryegrass, perennial ryegrass, red fescue, rough bluegrass, and white clover, as well as other dicotyledon species (up to 5%). The cylindrical bales were formed from material mowed and dried to about 50% [6]. Bales were wrapped with the tested films and stored on the concrete basement site, fenced, and covered with the safety net.
In the first year of the experiment, the analyses were performed after 5 (marked as t1), 11 (t2), and 17 (t3) months of storage. The following year, considering the results from the first stage, all the procedures were repeated after 4 (t1) and 10 (t2) months of storage.
In the study, innovative multi-layer films produced by the company ERG Bieruń Folie were used. The recipes were developed by the Central Mining Institute in Katowice. The nanosilver particles were incorporated into the EVA (vinyl ethylene and vinyl acetate copolymer) polymer matrix at 400 ppm quantities and used to improve the antimicrobial properties of the film. Three types of microcellulose of different particle sizes were tested as components responsible for better biodegradation properties. Microcellulose-containing biocomposites were produced upon introduction of microcellulose particles (at 15% mass content) to the metallocene linear low-density polyethylene (mLLDPE). For the first experimental stage, the following film formulations were used: (P1) with the nanosilver in the external layer; (P2) with the nanosilver in the external layer and microcellulose type 1 in the middle layer; (P3) with the nanosilver in the external layer and microcellulose type 2 in the middle layer; (P4) microcellulose type 1 in the middle layer; (P5) microcellulose type 2 in the middle layer; (P6) microcellulose type 3 in the middle layer; (P7) control film. During the second stage, the possibility of use of the recycled film was tested, so the experimental combinations of formulae were as follows: (PR1) with the nanosilver in the external layer, and the middle layer made of the recycled PE; (PR2) with the nanosilver in the external layer, and the middle layer made of standard PE; (PR0) control film. As a control, a commercially-available film produced by ERG Bieruń Folie was used.
2.2. Microbiological and Biochemical Analyses
The microbial cell population density in silage was monitored with a modified Koch surface-plating method of silage aqueous suspensions. The methods used to determine the total number of aerobic bacteria, LAB, molds, and yeasts, were described in Supel et al. [7]. In addition to the above, a degree of surface colonization of wrapping film with microorganisms was estimated after plating suspensions prepared by slow rinsing of the film surface with sterile water onto microbiological media [7].
The DM content was established with the standard drying method, and pH was measured potentiometrically [8]. Total sugars were estimated with the method of Bertrand, while the total protein level was calculated from total nitrogen content measured with the Kjeldahl method [8]. Ammonia nitrogen was measured with the Convay method [9], and the crude fiber content was determined as a leftover after hydrolysis [8]. Organic acids (lactic, acetic, butyric, and propionic) were determined with the gas chromatography method, as described in Radkowski [9].
3. Results and Discussion
The process of intense fermentation in silages lasts up to 2 months. After this time, the material is fully ensiled, and the stability phase begins under which only small changes in the environmental conditions may occur [10].
3.1. Addition of Nanosilver or Microcellulose to the Wrapping Film
Earlier, it was shown that in the stability phase, the number of LAB observed in silage decreased compared to the fermentation phase [2]. The results of our experiment indicate that the number of LAB in most of the studied cases still tends to increase during storage (Figure 1), although a significant increase in the population of unfavorable microorganisms was also observed, except for variants P1 and P2 (Figure 2). Avila [2] claims that if the number of yeasts and molds is higher than 105 cfu·g−1, an aerobic silage deterioration may occur. This phenomenon was observed for P3, P4, and P5 silages after 11 months of storage, and it may suggest that the ensiled material should be fed-out before a year of storage. The best-quality silage should contain a relatively high number of LAB combined with a low number of unfavorable strains [3,11]. Assuming the above, formula P6 seems to be the best one.
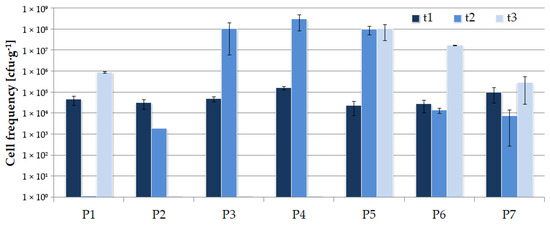
Figure 1.
Lactic acid bacteria cell frequency in silage during the first stage of the experiment.
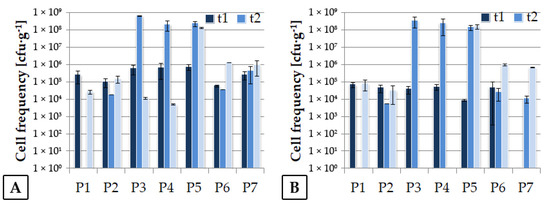
Figure 2.
Unfavorable strains cell frequency in silage during the first stage of the experiment. (A) Aerobic bacteria, (B) Molds and yeasts.
Upon evaluation of the film surface microbial colonization, it was assumed that low frequency of molds was the most important expected goal together with the presence of LAB, which suggests that components added to the films were not toxic for this group of microorganisms. In this respect, the analyses of film colonization revealed that the best results were obtained for the variant P1, P2, P6, and the control sample (data not shown). Great variability of microbial frequency was observed, possibly as a result of changeable weather conditions.
Chemical analyses showed that there were no significant differences between silages wrapped with either the standard film or the innovative ones (Table 1). It is known that upon ensiling, due to the fermentation, dry mass decreases [2]. In the present study, the DM of forage was established to be 52% and its decrease within the first months of ensiling was significant for all the variants except for P2 and P4. Later, a small DM increase could be observed for most combinations. High sugar content in forage (14.75%) ensures proper conditions for LAB metabolic activity. For good-quality silages produced from meadow plants, the chemical characteristics should be as follows: crude fiber, 20–26%; total protein, up to 18% [3,6,9]. Slightly higher crude fiber content may be an effect of forage composition. The acidity of analyzed silages after 5 months of ensiling was between pH 4,4 and 4,7 (data not shown). A slight tendency to decrease could be observed over time, which seems to be in line with the results obtained by other authors [3,6,9,10].

Table 1.
Dry mass changes during the storage [%] and stored silage parameters (the average value, changes during the storage were not statistically significant at p < 0.05) [% of DM].
The concentration of lactic acid in the tested samples varied between 2.1–9.3% of DM (Figure 3). It is notable that a very important parameter of silage quality is the lactic-to-acetic acid ratio, and lactic acid should contribute more than 70% [1,6]. The results of the present study show that only for 2 cases, variants P1 and P3, after 11 months of storage, the abovementioned ratio was lower than 72%. A decrease in total acids content over time was observed for most combinations, which may be an indicator of yeast and acetic acid bacteria activities in silage [2,10].
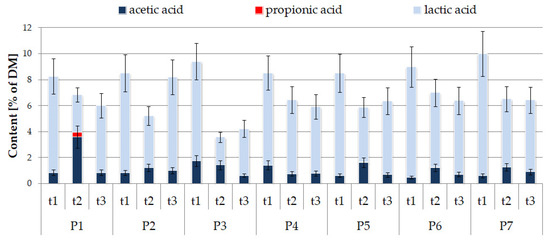
Figure 3.
Changes in the content of organic acids in silages during storage.
The first stage of the experiment showed that all the tested additives were not toxic to lactic acid bacteria and ensured proper conditions for the ensiling process. The resultant silage qualities were high and not affected significantly by storage time. The formula with the addition of microcellulose type 3 in the internal layer yielded the best results of microbial analyses compared to the control sample. Good results were also obtained for formulae with the addition of nanosilver.
It should be noted that the relatively expensive novel additives may add to the total cost of the resultant product; however, it is difficult to predict the final expenses at the current stage of testing, which was carried out on a semi-technical scale. Future large-scale production of the innovative films is expected to lower the total cost. Moreover, the cost calculations should also include the additional product value resulting from the increased eco-friendliness of the new materials.
3.2. The Use of Recycled PE for Film Preparation
The number of LAB in the silage (Figure 4) was moderate, ranging between 104 and 106 cfu·g−1 DM, and did not change significantly during storage for the tested samples PR1 and PR2. Except for PR0 after 4 months of storage, for all the tested silages, the number of molds and yeasts was kept below 106 cfu·g−1, which suggests that the material was well preserved and anaerobic conditions were maintained properly [2].
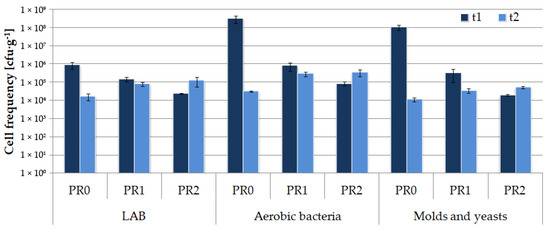
Figure 4.
Microbial population structure changes in silage during bale storage in the second stage of the experiment.
A relatively low frequency of strains was detected on the film’s internal surface (Figure 5A). For the external film layer colonization, greater variability in the microbial frequency was observed (Figure 5B), possibly resulting from more changeable weather conditions.
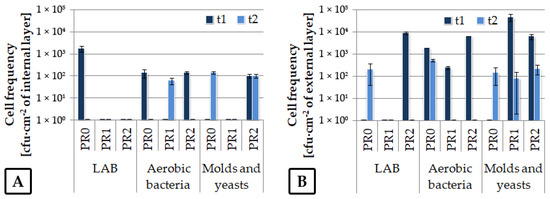
Figure 5.
Microbial colonization rate on the internal (A) and external (B) film surfaces obtained upon bale storage during the second experimental stage.
In general, the silage quality obtained in the second stage of the experiment was very good. pH values (Table 2) were similar to the optimal ones as suggested in literature [3,6,9,10] and slightly higher for the tested samples than for the control (PR0). A significant decrease in pH during storage was observed only for the sample PR1.

Table 2.
Dry mass content, pH changes, and lactic acid content in silage during storage.
The ratio of lactic acid content in total acids was over 77% after 4 months of storage and then decreased after 10 months (Table 2). For sample PR1 after 10 months of storage, high levels of both acetic and lactic acid were observed, whereas also butyric (0.05%) and propionic (0.02%) acids were detected (not shown). Note that butyric acid should not be present in properly conserved silage [10].
No significant differences in crude fiber content were observed between samples (Figure 6). In turn, a decrease in sugar content in samples PR1 and PR2 may indicate active microbial metabolism. A high level of ammonia nitrogen and the elevation of total protein content was observed in sample PR1 after 10 months of storage. This change is possibly linked to the degradation of plant tissues [5,6,10] and may additionally result from the disruption of the covering film structure.
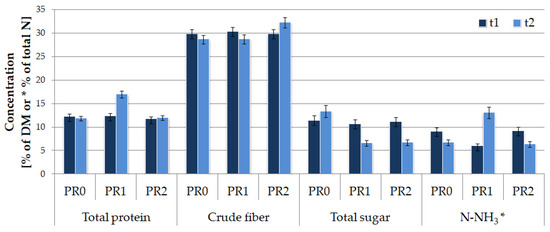
Figure 6.
Silage parameters in the second stage of the experiment.
4. Conclusions
Our study proves that novel, multilayered films with additives such as microcellulose and nanosilver may be used in the production of high-quality silages. For all the tested film variants, the silage quality did not change significantly during 17 months of storage. However, the risk of growth intensification of unfavorable strains increased after 11 months of storage, and therefore it is recommended to limit the bale storage time to less than 1 year.
The results obtained for silage generated in bales wrapped with the film containing nanosilver in the external layer were similar or, in some cases, even better than for the control sample (standard film). These observations suggest that nanosilver did not negatively affect the strength and air impermeability of the film, thus providing favorable conditions for fermentation. Therefore, it may be successfully used in agriculture as an antimicrobial agent supplemented with wrapping films.
Supplementary Materials
The presentation file is available online at https://www.mdpi.com/article/10.3390/IECAG2021-09731/s1.
Author Contributions
Conceptualization: P.K. (Piotr Kacorzyk) and M.K.; methodology: P.S., P.K. (Paweł Kaszycki) and P.K. (Piotr Kacorzyk); formal analysis: P.S. and P.K. (Piotr Kacorzyk); investigation and data curation: P.S., P.K. (Paweł Kaszycki) and P.K. (Piotr Kacorzyk); writing—original draft preparation: P.S.; writing—review and editing: P.S., P.K. (Paweł Kaszycki) and P.K. (Piotr Kacorzyk); visualization: P.S.; supervision: M.K.; project administration: P.K. (Piotr Kacorzyk); funding acquisition: P.K. (Piotr Kacorzyk) and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work was carried out as part of the research project PBS 3/B9/30/2015 financed by the Polish National Research and Development Centre, the Applied Research Program.
Data Availability Statement
All the data supporting the conclusions of this article will be made available by the authors, without undue reservation. Further inquiries can be made to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wilkinson, J.M.; Rinne, M. Highlights of progress in silage conservation and future perspectives. Grass Forage Sci. 2018, 73, 40–52. [Google Scholar] [CrossRef]
- Avila, C.L.S.; Carvalho, B.F. Silage fermentation—Updates focusing on the performance of micro-organisms. J. Appl. Microbiol. 2020, 128, 966–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microbiol. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowicz, M.; Kaszkowiak, J.; Dorszewski, P.; Szterk, P. Przydatność folii oksybiodegradowalnej do przechowywania kiszonek (in Polish). Logistyka 2015, 4, 1869–1876. [Google Scholar]
- Borreani, G.; Piano, S.; Tabacco, E. Aerobic stability of maize silage stored under plastic films with different oxygen permeability. J. Sci. Food Agric. 2014, 94, 2684–2690. [Google Scholar] [CrossRef] [PubMed]
- Micek, P. Analizy chemiczne obnażają błędy podczas kiszenia traw. Top Agrar Polska. Top Bydło 2007, 4, 10–13. (In Polish) [Google Scholar]
- Supel, P.; Kaszycki, P.; Kasperczyk, M.; Kacorzyk, P. Dynamics of microbiota changes of silage environment resulting from the use of new generation films (in Polish). Przem. Chem. 2018, 97, 1333–1339. [Google Scholar] [CrossRef]
- Bathelt, P. Jakość sianokiszonki w zależności od użytej folii. Master’s Thesis, University of Agriculture, Krakow, Poland, 2017. (In Polish). [Google Scholar]
- Radkowski, A.; Radkowska, I. Ocena jakości i wartości pokarmowej kiszonek z runi łąkowej wybranych gospodarstw Polski południowo-wschodniej. Wiadomości Zootechniczne 2014, 1, 32–37. (In Polish) [Google Scholar]
- Kung, L.J.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, O.C.M.; Arriola, K.G.; Daniel, J.L.P.; Adesogan, A.T. Effects of 8 chemical and bacterial additives on the quality of corn silage. J. Dairy Sci. 2013, 96, 5836–5843. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).