Exploring Peruvian Cocoa Populations and Their Influence on Rat Metabolism †
Abstract
:1. Introduction
2. Material and Methods
2.1. Cocoa Population Characterization
2.2. In Vivo Study
2.2.1. Diets and Animals
2.2.2. Oral Glucose Tolerance Test
2.2.3. Sample Collection and Processing
2.3. Statistical Analysis
3. Results and Discussion
3.1. Peruvian Cocoa Composition
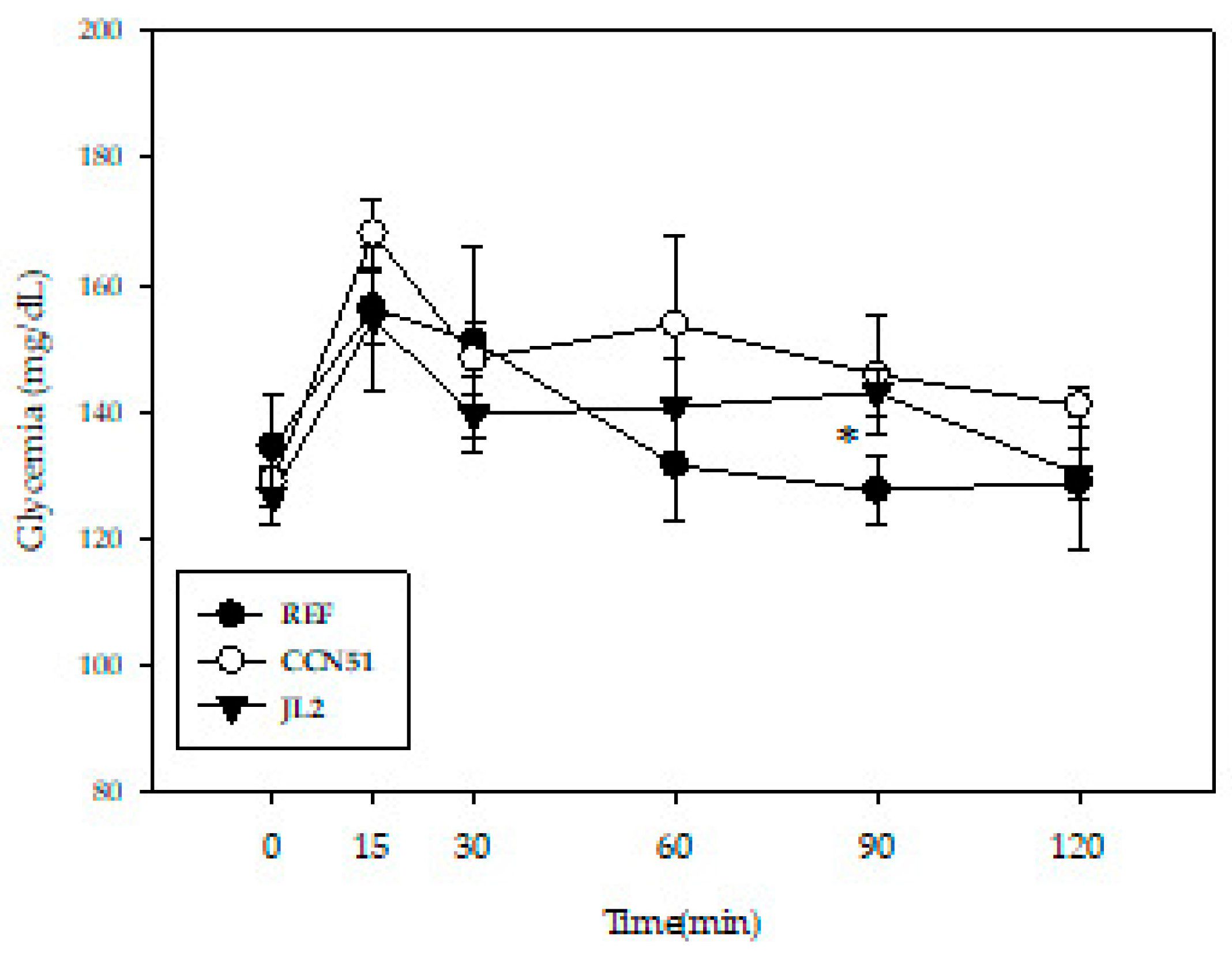
3.2. Effect of Peruvian Cocoa Populations on Glucose Tolerance Test
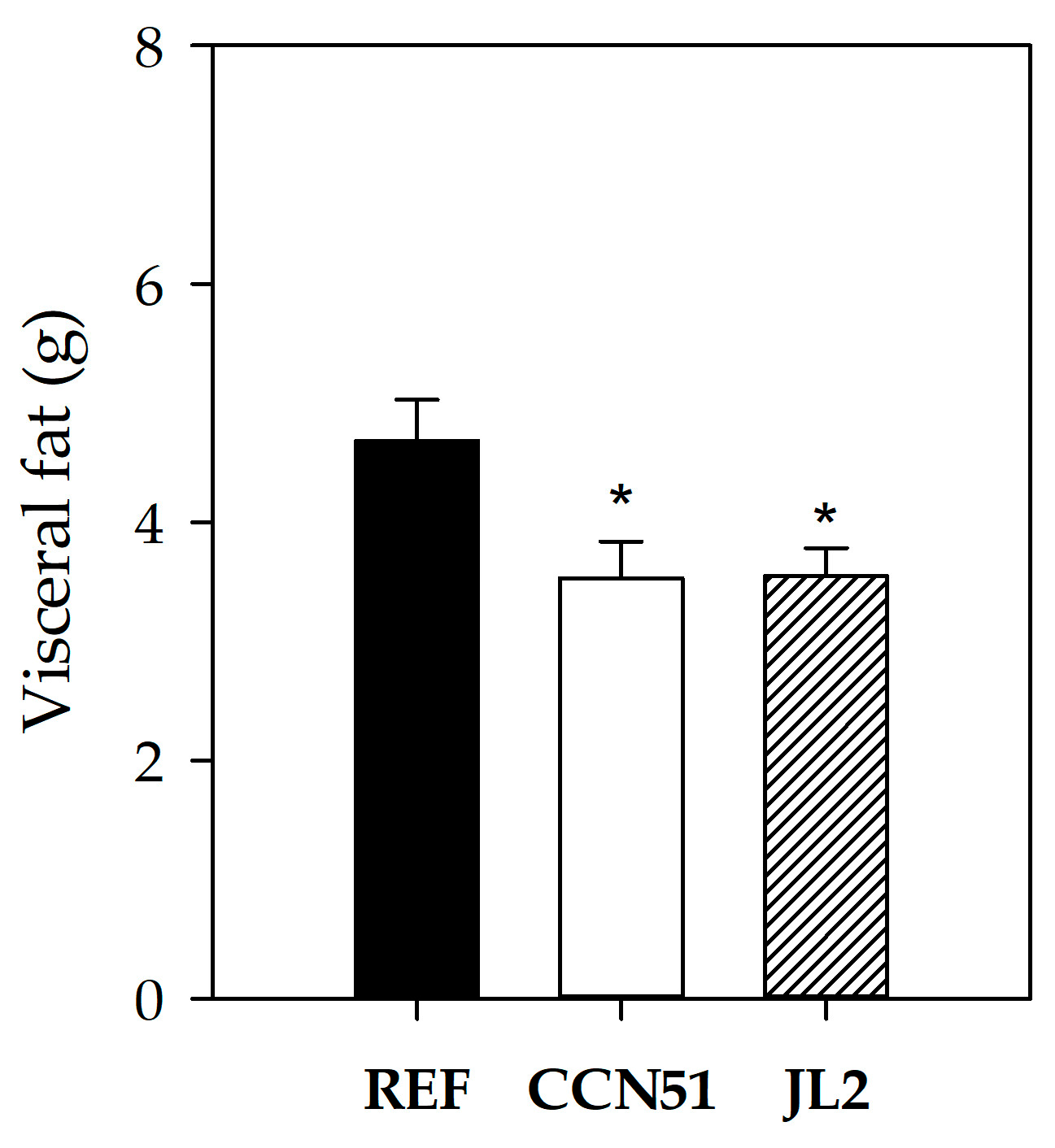
3.3. Effect of Peruvian Cocoa Populations on Body and Organ Weight
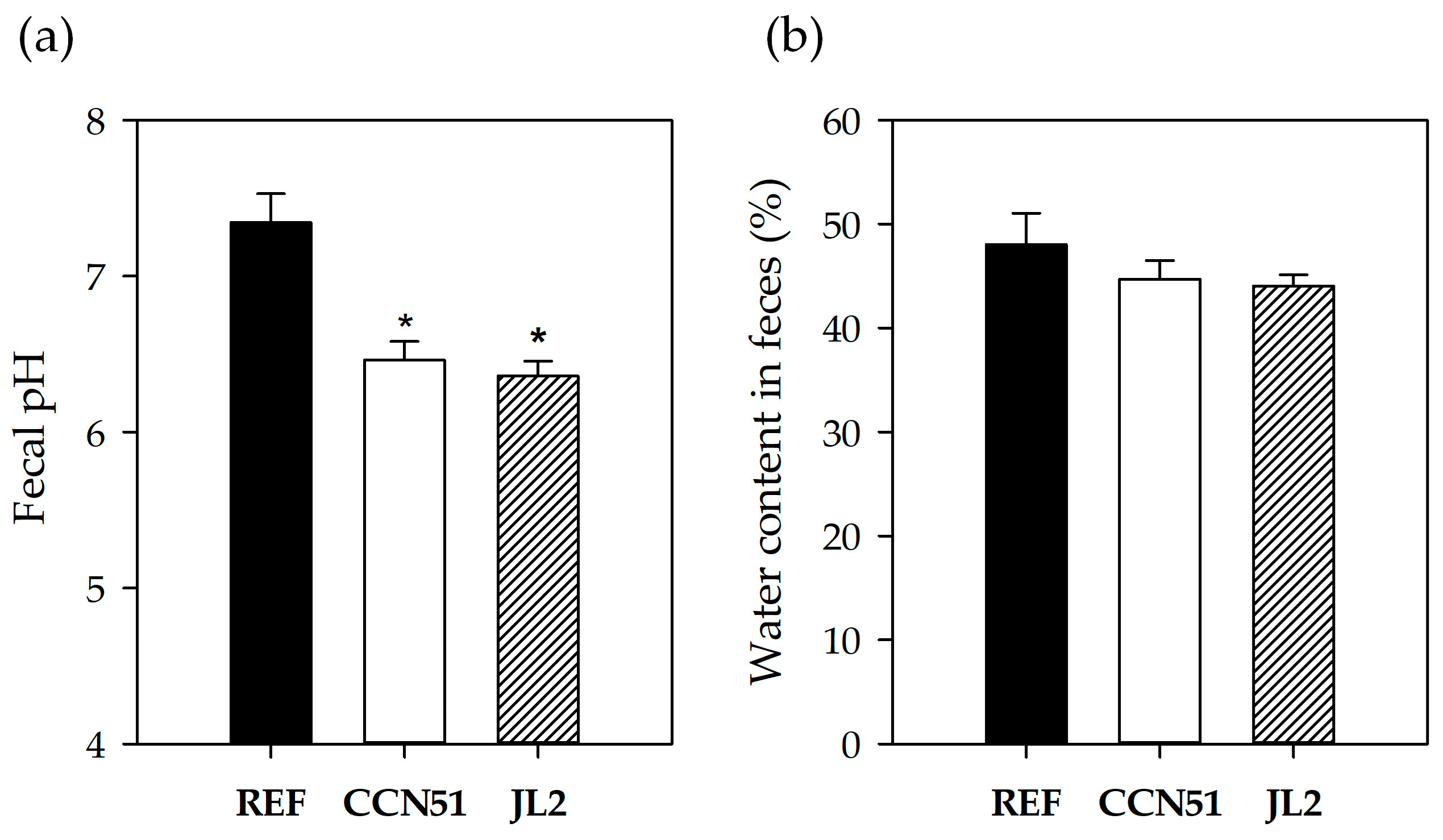
3.4. Fecal Variables
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramos, W.; López, T.; Revilla, L.; More, L.; Huamaní, M.; Pozo, M. Results of the Epidemiological Surveillance of Diabtes Mellitus in Hospitals in Peru, 2012. Rev. Peru. Med. Exp. Salud Pública 2014, 34, 680–687. [Google Scholar]
- Ahman, N.; Amr, A. The Effect of Defatted Cocoa Powder on Cholesterol-Induced Changes of Serum Lipids in Rats. Nutr. Hosp. 2022, 39, 537–546. [Google Scholar]
- Ramos, S.; Martín, M.A.; Goya, L. Effects of Cocoa Antioxidants in Type 2 Diabetes Mellitus. Antioxidants 2017, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Laveriano-Santos, E.P.; Arancibia-Riveros, C.; Tresserra-Rimbau, A.; Castro-Barquero, S.; Ruiz-León, A.M.; Estruch, R.; Casas, R.; Bodega, P.; de Miguel, M.; de Cos-Gandoy, A.; et al. Flavonoid Intake From Cocoa-Based Products and Adiposity Parameters in Adolescents in Spain. Front. Nutr. 2022, 9, 931171. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Cocoa Polyphenols and Fiber Modify Colonic Gene Expression in Rats. Eur. J. Nutr. 2017, 56, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Ismail, A.; Esa, N.M.; Pei, C.P.; Kersten, S. Hepatic Genome-Wide Expression of Lipid Metabolism in Diet-Induced Obesity Rats Treated with Cocoa Polyphenols. J. Funct. Foods 2015, 17, 969–978. [Google Scholar] [CrossRef]
- Coronado-Cáceres, L.J.; Rabadán-Chávez, G.; Quevedo-Corona, L.; Hernández-Ledesma, B.; Garcia, A.M.; Mojica, L.; Lugo-Cervantes, E. Anti-Obesity Effect of Cocoa Proteins (Theobroma cacao L.) Variety “Criollo” and the Expression of Genes Related to the Dysfunction of White Adipose Tissue in High-Fat Diet-Induced Obese Rats. J. Funct. Foods 2019, 62, 103519. [Google Scholar] [CrossRef]
- Massot-Cladera, M.; Costabile, A.; Childs, C.E.; Yaqoob, P.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Prebiotic Effects of Cocoa Fibre on Rats. J. Funct. Foods 2015, 19, 341–352. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Ali, S.; Mancin, L.; Davinelli, S.; Paoli, A.; Scapagnini, G. Cocoa Polyphenols and Gut Microbiota Interplay: Bioavailability, Prebiotic Effect and Impact on Human Health. Nutrients 2020, 12, 1908. [Google Scholar] [CrossRef] [PubMed]
- Sanz, Y.; Rastmanesh, R.; Agostonic, C. Understanding the Role of Gut Microbes and Probiotics in Obesity: How Far Are We? Pharmacol. Res. 2013, 69, 144–155. [Google Scholar] [CrossRef] [PubMed]
| CCN51 | JL2 | VRAE99 | |
|---|---|---|---|
| Total phenolics (mg GA equivalents/g) | 62.31 ± 7.50 a | 106.62 ± 6.55 b | 85.47 ± 1.12 ab |
| Total flavonoid (mg catechin equivalents/g) | 18.95 ± 0.08 a | 19.050 ± 0.71 a | 15.70 ± 0.08 b |
| Catechin (mg/g) | 0.41 ± 0.067 a | 0.63 ± 0.004 b | 0.44 ± 0.001 a |
| Epicatechin (mg/g) | 0.99 ± 0.001 a | 0.91 ± 0.008 b | 0.94 ± 0.002 c |
| IC50 values of DPPH assay (µM) | 127.56 ± 2.58 a | 106.85 ± 7.19 b | 129.53 ± 0.75 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massot-Cladera, M.; García-Valdera, R.; Gálvez-González, D.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J.; Best, I.; Castell, M. Exploring Peruvian Cocoa Populations and Their Influence on Rat Metabolism. Biol. Life Sci. Forum 2023, 29, 8. https://doi.org/10.3390/IECN2023-15792
Massot-Cladera M, García-Valdera R, Gálvez-González D, Rodríguez-Lagunas MJ, Pérez-Cano FJ, Best I, Castell M. Exploring Peruvian Cocoa Populations and Their Influence on Rat Metabolism. Biology and Life Sciences Forum. 2023; 29(1):8. https://doi.org/10.3390/IECN2023-15792
Chicago/Turabian StyleMassot-Cladera, Malén, Raquel García-Valdera, Daniela Gálvez-González, María J. Rodríguez-Lagunas, Francisco J. Pérez-Cano, Ivan Best, and Margarida Castell. 2023. "Exploring Peruvian Cocoa Populations and Their Influence on Rat Metabolism" Biology and Life Sciences Forum 29, no. 1: 8. https://doi.org/10.3390/IECN2023-15792
APA StyleMassot-Cladera, M., García-Valdera, R., Gálvez-González, D., Rodríguez-Lagunas, M. J., Pérez-Cano, F. J., Best, I., & Castell, M. (2023). Exploring Peruvian Cocoa Populations and Their Influence on Rat Metabolism. Biology and Life Sciences Forum, 29(1), 8. https://doi.org/10.3390/IECN2023-15792











