Abstract
More than 57% of US adults take dietary supplements, with the most common being daily multivitamins. Daily multivitamins are typically formulated in a pill or tablet form; however, new options, including powder form that can be mixed with water, are being utilized to increase bioavailability. While limited data are available, the theory is that multivitamin tablets must be adequately dissolved before entering the small intestine for assimilation, while powders come pre-dissolved before consumption, which theoretically ensures enhanced bioavailability. Our aim was to investigate the bioaccessibility and bioavailability of minerals (magnesium (Mg), zinc (Zn), calcium (Ca), and potassium (K)) using a novel foundational nutrition supplement called AG1 compared to a multivitamin tablet. AG1 contains vitamins and minerals comparable to multivitamin tablets but also includes prebiotics, probiotics, and phytonutrients. We employed the adapted Simulator of Human Intestinal Microbial Ecosystem (SHIME®) model to assess the bioaccessibility and bioavailability of this study’s products using a simulated stomach and small intestine physiological environment equipped with a dialysis membrane (methylcellulose) to emulate absorption. Luminal contents were collected at the end of the stomach, duodenum, and 1-, 2-, and 3 h after small intestine absorption simulation (dialysis) to assess bioaccessibility. The dialysis solution was measured at 1-, 2-, and 3 h to assess bioavailability. A significantly higher (p < 0.05) percentage of the total amount of all minerals given at the baseline was present at the end of the stomach and duodenum portion for the powder form vs. the tablet. There was a significantly higher % maximal concentration (Cmax), bioavailability, and bioaccessibility for Mg, Ca, and Zn for AG1 vs. the tablet. These preclinical data demonstrate that a greater proportion of minerals in AG1 enter the small intestine, have a higher Cmax, and several are more bioaccessible and bioavailable than a tablet multivitamin in vitro.
1. Introduction
Americans consume diets that are low in several micronutrients [1], likely due to the suboptimal diet quality consumed in the United States (US) [2]. Many adults in the US consume dietary supplements to fill these micronutrient shortfalls [3], with cross-sectional data showing that more than 57% of adults have taken dietary supplements in the last 30 days [4]. Multivitamin and mineral supplements (MVMs) are the most commonly consumed dietary supplements for US adults aged 20 and over [4]. These MVMs are traditionally consumed in tablet form, but contemporary MVMs are now formulated as powders, with a primary reason being potential improvements in bioavailability.
There is some clinical evidence suggesting that powder supplements may be more bioavailable than tablets [5], which is likely driven by higher dissolution rates, leading to increased amounts of soluble minerals entering the small intestine for absorption. Tablet and capsule MVMs often have disintegration times greater than 20 min [6], potentially leading to reductions in the total soluble amounts of vitamins and minerals liberated from the tablet matrix upon entry into the duodenum. Powders are completely disintegrated when they enter the stomach, bypassing the longer disintegration times required for tablets. Clinical data suggest that powder formulations of single-ingredient supplements and pharmaceuticals have increased absorption compared to tablets [5,7,8]. However, only one study has assessed MVMs as a powder compared to a tablet, revealing the superior absorption of vitamin B12 and variable results for other minerals [9]. Current MVMs on the market are intentionally formulated as powders, not just crushed tablet MVMs or supplements like the previously discussed studies, and more research is needed to understand the differences in bioavailability and bioaccessibility compared to traditional tablet MVMs.
AG1®, a novel foundational nutrition supplement in a powder form, provides minerals, vitamins, probiotics, prebiotics, and phytonutrients. Our aim was to investigate the bioaccessibility and bioavailability of minerals [magnesium (Mg), zinc (Zn), calcium (Ca), and potassium (K)] in AG1 compared to a tablet multivitamin using the adapted Simulator of Human Intestinal Microbial Ecosystem (SHIME®) in vitro model. We hypothesized that the minerals in the powder MVM would be more bioaccessible and bioavailable than the chemically similar MVM tablet in this in vitro model.
2. Methods Section
2.1. Test Products
Before the study started, the amounts of all minerals and vitamins in AG1 were quantified by the manufacturer to formulate chemically similar tablets with the same amount of minerals. The types of minerals in the tablet and AG1 powder were zinc citrate, magnesium glycinate, dipotassium phosphate, and calcium (calcium citrate, calcium carbonate, and calcium phosphate). The recommended dose of AG1 is 12 g per serving. A dose of 6 g/bioreactor was chosen to circumvent potential physical complications that could impact the biological and mechanical factors of the SHIME® model.
2.2. Determination of Initial Soluble Fraction and Subsequent Mineral Analysis
The manufacturer provided the absolute amounts of each mineral for the powder and tablet. The inductively coupled plasma optical emission spectroscopy (ICP-OES) methodology was employed to measure the minerals prior to their entry into the SHIME model. Briefly, the average weight of the test dose (i.e., 1 tablet) was calculated by weighing six individual tablets. The six tablets were crushed into a powder, and the exact average weight of one dose was collected and added to 80 mL of MilliQ water. Both products (in a powder form) were individually homogenized in the water suspension, and 5 mL samples were used for the mineral analysis. The samples underwent a deconstruction process with acid to liberate the minerals from the matrices. Soluble fractions of Zn and Ca were significantly higher in AG1 powder, Mg was higher in the tablet, and no differences were observed for K.
Subsequently, the tablet was not crushed when it entered the SHIME model. The same deconstruction process and ICP-OES were used to assess the soluble fractions of the powder and tablet throughout the model. These baseline testing results were used as a theoretical maximal soluble fraction and to calculate the % soluble fraction to determine the theoretical bioavailability (assessed only during the dialysis phase) and bioaccessibility (assessed throughout the whole model). Bioaccessibility was defined as the soluble fraction still inside the dialysis membrane (the estimation of the unabsorbed luminal fraction). Bioavailability was defined as the soluble fraction that diffused across the dialysis membrane (the estimation of the absorbed fraction able to enter the intestinal periphery).
2.3. Test Gastrointestinal Tract System
We employed the SHIME model adapted from [10] utilizing one reactor that simulated the upper gastrointestinal tract (i.e., stomach and small intestine). Fasted conditions were simulated and maintained by adding a specific gastric suspension to the reactor over time, followed by specific enzyme and bile acid solutions to mimic the human small intestine. Prespecified incubation times and pH have been established to emulate the in vivo conditions for the upper gastrointestinal tract [11].
The sample was incubated at 37 °C for 45 min with a constant pH of 2.0 and continuous mixing via stirring. Pepsin and phosphatidylcholine (1000 U/mL and 0.02 mM, respectively) were added [12]. The background medium recommended by the consensus method was used [11]. Chyme was collected at the end of the gastric phase (stomach end) to measure the bioaccessible fractions.
The contents from the gastric phase were mixed via stirring, and the pH was automatically increased from 2.0 to 6.5 in the duodenal phase. Luminal contents were sampled at the end of the duodenal (duodenum end) phase (following 27 min of mixing) to measure the bioaccessible fraction at the duodenum end. Following the duodenal phase, a simulated absorptive phase was completed using a dialysis method mimicking the ilium and jejunum for 3 h with a constant pH of 7.0 at 37 °C. A methylcellulose membrane was used for the dialysis phase with a cut-off of 14 kDa. The entire luminal content was transferred into the methylcellulose membrane and immersed in dialysis fluid. The dialysis solution was refreshed every hour. A raw animal pancreatic extract (pancreatin) containing all the relevant enzymes in a specific ratio was used during the small intestine phase. Trypsin activity was measured (TAME assay) to normalize for a specific activity (1.12 TAME U/mL) [12]. The activity of trypsin and chymotrypsin was predefined at 3.1 U/mL and 0.76 BTEE U/mL, respectively [12] Based on Riethorst et al., 2016, the bile salt (derived from bovine bile) concentration was reduced by a factor of 3 with a general amount of 3.33 mM bovine bile extract being supplemented [12]. The luminal content and dialysis solution were measured at 1-, 2-, and 3 h during the dialysis phase to assess the bioavailable and bioaccessible fractions.
2.4. Statistics
All statistics were performed using GraphPad Prism (version 10.0.0 for Windows, GraphPad Software, Boston, MA, USA). Due to the variation in the total mineral amounts added to the SHIME model, all values are presented as a percentage unless otherwise stated. Two-way ANOVA with repeated measures was employed to evaluate changes in the % bioaccessible and % bioavailable for Mg, Zn, Ca, and K. The variables included in the analysis included supplement form, time, and the interaction between the two variables. The data were matched to account for the same reactor being used across various time points. A multiple comparisons test with a Sidak correction was used to evaluate the differences between the form of the supplement at each time point. Unpaired parametric t-tests were used to evaluate the differences in the % maximal concentration (Cmax) for Mg, Zn, Ca, and K. Welch’s correction was applied to each test because due to the variability in the disintegration of a powder versus tablet being assumed and standard deviation was predicted to not be equal between the two supplement forms.
3. Results
Bioaccessible fractions for all minerals at the end of the stomach and duodenum were higher for AG1 powder vs. the tablet (p < 0.05).
The bioaccessibility results for all minerals are presented in Figure 1. A significant effect (p < 0.0001) of time, form, and a time × form interaction was observed for the bioaccessible fraction of Mg with higher values for AG1 powder vs. the tablet. No significant effect of time or the time × form interaction was observed for the bioaccessible fraction of Zn, but there was a significant effect of form (p = 0.002) with higher values for powder. A significant effect (p < 0.0001) of time, form, and time × form interaction was observed for the bioaccessible fraction of Ca with higher values for powder. A significant effect (p < 0.001) of time and time × form interaction was observed for the bioaccessible fraction of K, but no significant effect was seen for form. Higher K was observed at 3 h for the tablet vs. powder.
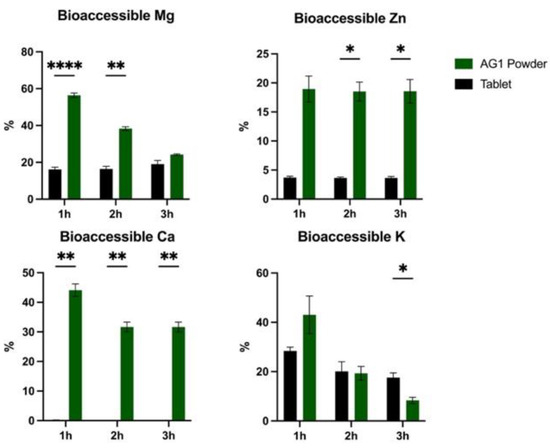
Figure 1.
Bioaccessibility of all minerals for AG1 powder vs. the tablet. All data are presented as percentages unless otherwise stated. Statistical analysis included two-way repeated measures ANOVA with post hoc testing (Sidak correction). Data are presented as the mean and standard error of the mean. * p < 0.05, ** p < 0.01, and **** p < 0.0001. Calcium, Ca; Potassium, K; Magnesium, Mg; and Zinc, Zn.
The bioavailability results for all minerals are presented in Figure 2. There was a significant effect (p < 0.05) of time, form, and time × form interaction for the bioavailable fraction of Mg with higher values for powder. A significant effect (p < 0.05) of time, form, and the time × form interaction was observed for the bioavailable fraction of Zn with higher values for powder vs. the tablet. Similarly, a significant effect (p < 0.0001) of the time, form, and time × form interaction for the bioavailable fraction of Ca was observed with higher values for powder compared to the tablet. Lastly, there was a significant effect (p < 0.01) of the time and time × form interaction for the bioavailable fraction of K with no independent effects of form and no significant differences at any time point.
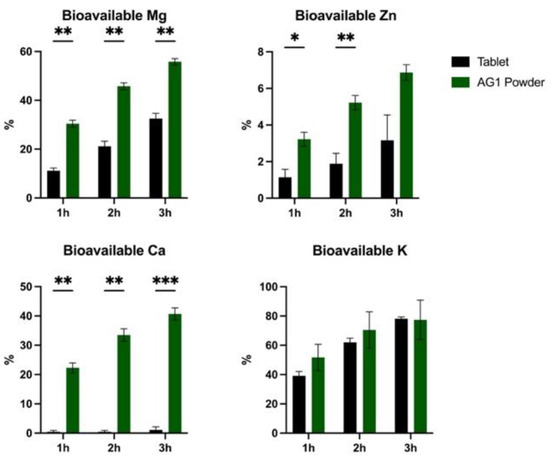
Figure 2.
The bioavailability of minerals for AG1 powder vs. the tablet. Data are presented as percentages unless otherwise stated. Statistical analysis included two-way repeated measures ANOVA with post hoc testing (Sidak correction). Data are shown as the mean and standard error of the mean. * p < 0.05, ** p < 0.01, and *** p < 0.001. Calcium, Ca; Potassium, K; Magnesium, Mg; and Zinc, Zn.
Cmax results are presented in Figure 3. Soluble % Cmax was significantly higher (p = 0.002) for AG1 powder vs. the tablet. Soluble % Cmax was significantly higher (p = 0.03) for AG1 powder vs. the tablet. The soluble % Cmax for Ca was significantly higher (p < 0.0001) for AG1 powder vs. the tablet. There were no differences in % Cmax for K.
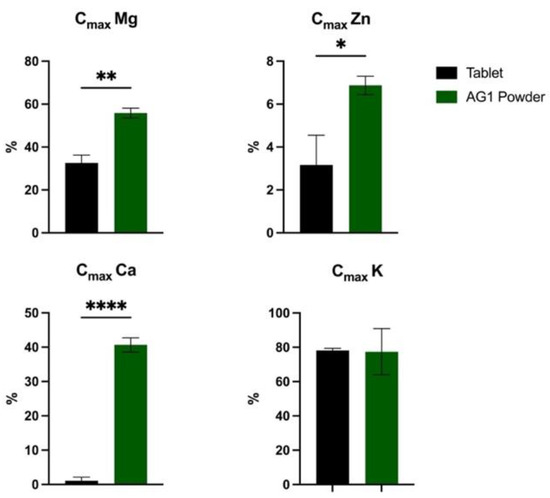
Figure 3.
Cmax of minerals for AG1 powder vs. the tablet. Data are presented as percentages unless otherwise stated. Statistical analysis included an unpaired parametric t-test (Welch’s correction). Data are shown as the mean and standard error of the mean. * p < 0.05, ** p < 0.01, and **** p < 0.0001. Calcium, Ca; maximum concentration, Cmax; Potassium, K; Magnesium, Mg; and Zinc, Zn.
4. Discussion
The aim of this study was to use an in vitro model simulating the human upper gastrointestinal tract to evaluate the bioavailability and bioaccessibility of several minerals from two MVM supplements with similar chemical inputs but different formulations (tablet vs. powder) with matching mineral contents. AG1 demonstrated superior bioaccessibility for all minerals at the end of the stomach and duodenum compared to the tablet. Following the dialysis phase, the bioaccessibility and bioavailability of Zn, Ca, and Mg were significantly higher for the powder vs. the tablet. Lastly, higher Cmax values were observed for Ca, Mg, and Zn for the powder compared to the tablet.
The chyme and luminal contents at the end of the stomach and duodenum contained higher soluble fractions for all minerals for the powder vs. the tablet. This could be partially explained by the lower surface area of the tablet compared to the powder. The surface area can affect the ionization of the mineral by changing the amount of water that can directly access the salt [13,14,15]. By contrast, powders have a greater surface area relative to tablets, leading to increased rates of disintegration [16] and dissolution [17]. Increasing the rate of ionization may increase bioavailability through increased absorption. Microcrystalline cellulose is highly hygroscopic and is a commonly used excipient in MVM tablets to make them more compressible [18]. Hygroscopicity is related to solubility [19,20] and efficiency in disintegration rates [21]. Therefore, the increased composite hygroscopicity of a tablet results in decreased disintegration rates. This is likely driven by competition for water molecules, leading to reduced hydration and, thus, disintegration [22]. Together, increased competition for water and a reduced surface area resulted in decreased solubility for the mineral salts, which was observed in the luminal of the duodenum and stomach in this study.
The bioavailable fractions of Ca, Zn, and Mg were significantly higher for AG1 vs. the tablet, with no differences for K. The methylcellulose membrane used in the SHIME model simulates absorption via passive diffusion [23]. Therefore, the disintegration of the tablet, followed by the dissolution of the mineral salts, is crucial for the passing of ionized minerals across the membrane. As expected, due to the bioaccessibility at the end of the duodenum, the tablet failed to reach similar rates of dissolution compared to the powder. This can be seen when looking at the significantly higher Cmax values for Mg, Ca, and Zn. Future research using Caco-2 cells to account for other forms of transport is necessary to confirm the differences in Cmax values between the table and powder.
There was a significantly higher % Cmax, bioavailability, and bioaccessibility for Mg, Ca, and Zn for AG1 vs. the tablet. These differences are likely explained by differences in the additives and physical properties leading to differential dissolution and disintegration rates altering mineral salt solubility. The consideration of excipients and the physical form is important when formulating MVM supplements.
Author Contributions
Conceptualization, P.A.S., J.R.T., T.O.K., T.M.M. and R.E.; investigation, M.G., C.D. and M.M.; writing—original draft preparation, P.A.S., J.R.T. and T.O.K.; writing—review and editing, P.A.S., J.R.T., T.O.K., M.G., T.M.M. and R.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Athletic Greens (AG; Athletic Greens International, Carson City, NV 89701).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon reasonable request, the corresponding author will make data available; however, certain data may not be made available owing to privacy issues.
Conflicts of Interest
P.A.S. and J.R.T. have conducted sponsored research on nutritional supplements. P.A.S., J.R.T., T.O.K., T.M.M. and R.E. are employees of Athletic Greens (AG1) international. ProDigest BVBA has no conflict of interest. There is no other conflict of interest related to this report.
References
- USDA, Agricultural Research Service. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat in America, NHANES 2017-March 2020 Prepandemic. 2023. Available online: http://www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 1 September 2023).
- Shams-White, M.M.; Pannucci, T.E.; Lerman, J.L.; Herrick, K.A.; Zimmer, M.; Meyers Mathieu, K.; Stoody, E.E.; Reedy, J. Healthy Eating Index-2020: Review and Update Process to Reflect the Dietary Guidelines for Americans, 2020–2025. J. Acad. Nutr. Diet 2023, 123, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, A.; Blatman, J.; El-Dash, N.; Franco, J.C. Consumer Usage and Reasons for Using Dietary Supplements: Report of a Series of Surveys. J. Am. Coll. Nutr. 2014, 33, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Data Brief 399: Dietary Supplement Use among Adults: United States, 2017–2018; National Center for Health Statistics: Hyattsville, MD, USA, 2021. [Google Scholar]
- Wang, H.; Bua, P.; Capodice, J. A Comparative Study of Calcium Absorption Following a Single Serving Administration of Calcium Carbonate Powder versus Calcium Citrate Tablets in Healthy Premenopausal Women. Food Nutr. Res. 2014, 58, 23229. [Google Scholar] [CrossRef] [PubMed]
- Löbenberg, R.; Steinke, W. Investigation of Vitamin and Mineral Tablets and Capsules on the Canadian Market. J. Pharm. Pharm. Sci. 2006, 9, 40–49. [Google Scholar] [PubMed]
- Sakurada, T.; Oishi, D.; Shibagaki, Y.; Yasuda, T.; Kimura, K. Efficacy of Oral Powder Compared with Chewable Tablets for Lanthanum Carbonate Administration in Hemodialysis Patients. Hemodial. Int. 2013, 17, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Bende, G.; Biswal, S.; Bhad, P.; Chen, Y.; Salunke, A.; Winter, S.; Wagner, R.; Sunkara, G. Relative Bioavailability of Diclofenac Potassium from Softgel Capsule versus Powder for Oral Solution and Immediate-Release Tablet Formulation. Clin. Pharmacol. Drug Dev. 2016, 5, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Wood, R.J. Plasma Changes in Micronutrients Following a Multivitamin and Mineral Supplement in Healthy Adults. J. Am. Coll. Nutr. 2003, 22, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-Step Multi-Chamber Reactor as a Simulation of the Human Intestinal Microbial Ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Rigby, N. InfoGest Consensus Method. In The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–22. [Google Scholar]
- Riethorst, D.; Mols, R.; Duchateau, G.; Tack, J.; Brouwers, J.; Augustijns, P. Characterization of Human Duodenal Fluids in Fasted and Fed State Conditions. J. Pharm. Sci. 2016, 105, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Wang, J.; Shi, G.; Chen, L. Influence of the Formula on the Properties of a Fast Dispersible Fruit Tablet Made from Mango, Chlorella, and Cactus Powder. Food Sci. Nutr. 2020, 8, 479–488. [Google Scholar] [CrossRef] [PubMed]
- CK-12 Foundation. 16.2: Rate of Dissolution. In Introductory, Conceptual, and GOB Chemistry; LibreTexts: Davis, CA, USA, 2023. [Google Scholar]
- Bergstrom, G. 2.4: Water Chemistry. In Cell and Molecular Biology (Bergstrom); LibreTexts: Davis, CA, USA, 2023. [Google Scholar]
- Molavi, F.; Hamishehkar, H.; Nokhodchi, A. Impact of Tablet Shape on Drug Dissolution Rate Through Immediate Released Tablets. Adv. Pharm. Bull. 2020, 10, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.R.; Areefulla, S.H.; Mallikarjun, V. Solubility and Dissolution Enhancement: An Overview. J. Pharm. Res. 2010, 3, 141–145. [Google Scholar]
- Yassin, S.; Goodwin, D.J.; Anderson, A.; Sibik, J.; Ian Wilson, D.; Gladden, L.F.; Axel Zeitler, J. The Disintegration Process in Microcrystalline Cellulose Based Tablets, Part 1: Influence of Temperature, Porosity and Superdisintegrants. J. Pharm. Sci. 2015, 104, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hong, J.; Luo, Q.; Xu, H.; Tan, H.; Wang, Q.; Tao, J.; Zhou, Y.; Peng, L.; He, Y.; et al. Hygroscopicity of Organic Compounds as a Function of Organic Functionality, Water Solubility, Molecular Weight and Oxidation Level. Atmos. Chem. Phys. 2022, 22, 3985–4004. [Google Scholar] [CrossRef]
- Maclean, N.; Khadra, I.; Mann, J.; Williams, H.; Abbott, A.; Mead, H.; Markl, D. Investigating the Role of Excipients on the Physical Stability of Directly Compressed Tablets. Int. J. Pharm. X 2022, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Wang, L.H.; Gordon, M.S.; Chowhan, Z.T. Effect of Formulation Solubility and Hygroscopicity on Disintegrant Efficiency in Tablets Prepared by Wet Granulation, in Terms of Dissolution. J. Pharm. Sci. 1991, 80, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Ekmekciyan, N.; Tuglu, T.; El-Saleh, F.; Muehlenfeld, C.; Stoyanov, E.; Quodbach, J. Competing for Water: A New Approach to Understand Disintegrant Performance. Int. J. Pharm. 2018, 548, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Blancquaert, L.; Vervaet, C.; Derave, W. Predicting and Testing Bioavailability of Magnesium Supplements. Nutrients 2019, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).