Rat Strain-Specific Differences in Alcohol Intake Following Patterned Feeding of a Palatable Diet †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
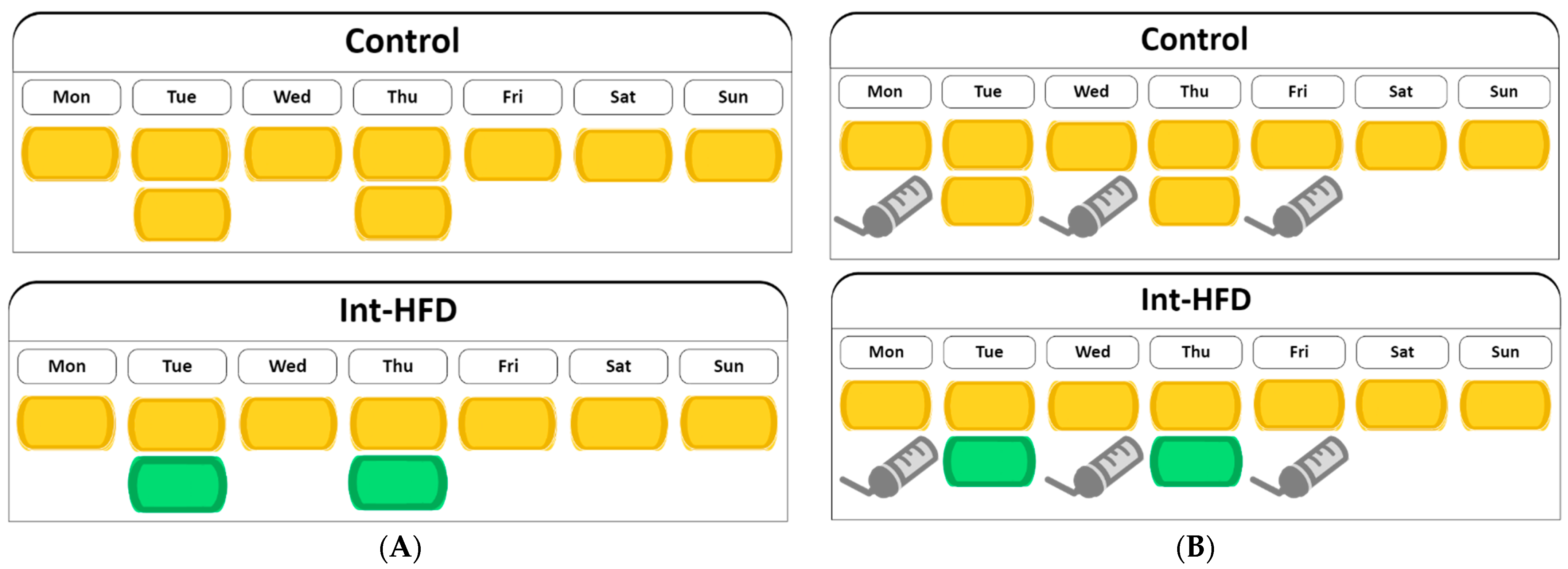
2.2. Diet and Alcohol
2.3. Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Alcohol Related Disease Impact (ARDI) Application Website. 2022. Available online: www.cdc.gov/ARDI (accessed on 6 October 2023).
- Westman, J.; Wahlbeck, K.; Laursen, T.M.; Gissler, M.; Nordentoft, M.; Hällgren, J.; Arffman, M.; Ösby, U. Mortality and life expectancy of people with alcohol use disorder in Denmark, Finland and Sweden. Acta Psychiatr. Scand. 2015, 131, 297–306. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Sacks, J.J.; Gonzales, K.R.; Bouchery, E.E.; Tomedi, L.E.; Brewer, R.D. 2010 National and State Costs of Excessive Alcohol Consumption. Am. J. Prev. Med. 2015, 49, e73–e79. [Google Scholar] [CrossRef] [PubMed]
- Glória, L.; Cravo, M.; Camilo, M.E.; Resende, M.; Cardoso, J.N.; Oliveira, A.G.; Leitão, C.N.; Mira, F.C. Nutritional deficiencies in chronic alcoholics: Relation to dietary intake and alcohol consumption. Am. J. Gastroenterol. 1997, 92, 485–489. [Google Scholar] [PubMed]
- Green, P.H. Alcohol, nutrition and malabsorption. Clin. Gastroenterol. 1983, 12, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Maillot, F.; Farad, S.; Lamisse, F. Alcool et nutrition [Alcohol and nutrition]. Pathol.-Biol. 2001, 49, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Relationships between nutrition, alcohol use, and liver disease. Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2003, 27, 220–231. [Google Scholar]
- Vedder, L.C.; Hall, J.M.; Jabrouin, K.R.; Savage, L.M. Interactions between chronic ethanol consumption and thiamine deficiency on neural plasticity, spatial memory, and cognitive flexibility. Alcohol. Clin. Exp. Res. 2015, 39, 2143–2153. [Google Scholar] [CrossRef]
- Clergue-Duval, V.; Azuar, J.; Fonsart, J.; Delage, C.; Rollet, D.; Amami, J.; Frapsauce, A.; Gautron, M.A.; Hispard, E.; Bellivier, F.; et al. Ascorbic Acid Deficiency Prevalence and Associated Cognitive Impairment in Alcohol Detoxification Inpatients: A Pilot Study. Antioxidants 2021, 10, 1892. [Google Scholar] [CrossRef]
- Neupane, S.P.; Lien, L.; Hilberg, T.; Bramness, J.G. Vitamin D deficiency in alcohol-use disorders and its relationship to comorbid major depression: A cross-sectional study of inpatients in Nepal. Drug Alcohol Depend. 2013, 133, 480–485. [Google Scholar] [CrossRef]
- Gruchow, H.W.; Sobocinski, K.A.; Barboriak, J.J.; Scheller, J.G. Alcohol consumption, nutrient intake and relative body weight among US adults. Am. J. Clin. Nutr. 1985, 42, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Stickel, A.; Rohdemann, M.; Landes, T.; Engel, K.; Banas, R.; Heinz, A.; Müller, C.A. Changes in Nutrition-Related Behaviors in Alcohol-Dependent Patients After Outpatient Detoxification: The Role of Chocolate. Subst. Use Misuse 2016, 51, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.; Gordis, E.; Holt, J. Dietary choices and likelihood of abstinence among alcoholic patients in an outpatient clinic. Drug Alcohol Depend. 1983, 12, 355–362. [Google Scholar] [CrossRef]
- Braun, T.D.; Kunicki, Z.J.; Blevins, C.E.; Stein, M.D.; Marsh, E.; Feltus, S.; Miranda, R., Jr.; Thomas, J.G.; Abrantes, A.M. Prospective Associations between Attitudes toward Sweet Foods, Sugar Consumption, and Cravings for Alcohol and Sweets in Early Recovery from Alcohol Use Disorders. Alcohol. Treat. Q. 2021, 39, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Alcoholics Anonymous. Living Sober; Alcoholics Anonymous World Services, Inc.: New York, NY, USA, 2007; pp. 18–19. [Google Scholar]
- Shah, K.; Shaw, C.; Sirohi, S. Reduced alcohol drinking following patterned feeding: Role of palatability and acute contingent availability. Physiol. Behav. 2020, 224, 113020. [Google Scholar] [CrossRef] [PubMed]
- Villavasso, S.; Shaw, C.; Skripnikova, E.; Shah, K.; Davis, J.F.; Sirohi, S. Nutritional Contingency Reduces Alcohol Drinking by Altering Central Neurotransmitter Receptor Gene Expression in Rats. Nutrients 2019, 11, 2731. [Google Scholar] [CrossRef]
- Cook, J.B.; Hendrickson, L.M.; Garwood, G.M.; Toungate, K.M.; Nania, C.V.; Morikawa, H. Junk food diet-induced obesity increases D2 receptor autoinhibition in the ventral tegmental area and reduces ethanol drinking. PLoS ONE 2017, 12, e0183685. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Sugar and fat bingeing have notable differences in addictive-like behavior. J. Nutr. 2009, 139, 623–628. [Google Scholar] [CrossRef]
- Carnicella, S.; Ron, D.; Barak, S. Intermittent ethanol access schedule in rats as a preclinical model of alcohol abuse. Alcohol 2014, 48, 243–252. [Google Scholar] [CrossRef]
- Bell, R.L.; Hauser, S.R.; Liang, T.; Sari, Y.; Maldonado-Devincci, A.; Rodd, Z.A. Rat animal models for screening medications to treat alcohol use disorders. Neuropharmacology 2017, 122, 201–243. [Google Scholar] [CrossRef]
- Simms, J.A.; Steensland, P.; Medina, B.; Abernathy, K.E.; Chandler, L.J.; Wise, R.; Bartlett, S.E. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol. Clin. Exp. Res. 2008, 32, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, B.; Pham, S.; Houeye, J.M.; Rush, K.; Sirohi, S. Rat Strain-Specific Differences in Alcohol Intake Following Patterned Feeding of a Palatable Diet. Biol. Life Sci. Forum 2023, 29, 24. https://doi.org/10.3390/IECN2023-15821
White B, Pham S, Houeye JM, Rush K, Sirohi S. Rat Strain-Specific Differences in Alcohol Intake Following Patterned Feeding of a Palatable Diet. Biology and Life Sciences Forum. 2023; 29(1):24. https://doi.org/10.3390/IECN2023-15821
Chicago/Turabian StyleWhite, Brooke, Sabrina Pham, John Michael Houeye, Kaiyah Rush, and Sunil Sirohi. 2023. "Rat Strain-Specific Differences in Alcohol Intake Following Patterned Feeding of a Palatable Diet" Biology and Life Sciences Forum 29, no. 1: 24. https://doi.org/10.3390/IECN2023-15821
APA StyleWhite, B., Pham, S., Houeye, J. M., Rush, K., & Sirohi, S. (2023). Rat Strain-Specific Differences in Alcohol Intake Following Patterned Feeding of a Palatable Diet. Biology and Life Sciences Forum, 29(1), 24. https://doi.org/10.3390/IECN2023-15821





