Abstract
Background and Aims: Obesity is a public health problem. The usual treatment is a reduction in calorie intake and an increase in energy expenditure, but not all individuals respond equally to these treatments. Epigenetics could be a factor that contributes to this heterogeneity. The aim of this research was to determine the association between DNA methylation at baseline and the percentage of BMI loss (%BMIL) after two dietary interventions in order to design a prediction model to evaluate %BMIL based on methylation data. Methods and Results: Spanish participants with overweight or obesity (n = 306) were randomly assigned to two lifestyle interventions with hypocaloric diets: one moderately high in protein (MHP) and the other low in fat (LF) during 4 months (Obekit study). DNA methylation was analyzed in white blood cells using the Infinium MethylationEPIC array. After identifying those methylation sites associated with %BMIL, (p < 0.05 and SD > 0.1), two weighted methylation sub-scores were constructed for each diet: 15 CpGs were used for MHP diet and 11 CpGs for LF diet. Afterwards, a total methylation score was obtained by subtracting the previous sub-scores. These data were used to design a prediction model for %BMIL through a linear mixed effect model in which the interaction between diet and total score. Conclusion: Overall, DNA methylation predicted %BMIL of two hypocaloric diets after 4 months and was able to determine which type of diet is the most appropriate for each individual. These results confirm that epigenetic biomarkers may be further used for precision nutrition and the design of personalized dietary strategies against obesity.
1. Introduction
DNA methylation is considered a key part of the pathogenesis and clinical manifestations of obesity [1]. It has been previously shown that methylation can influence the development of obesity as well as the response to dietary weight loss treatments [2,3]. This is due to the different complex metabolic mechanisms in which methylation may be involved [4]. It has also been suggested that methylation in genes related to hunger and satiety mechanisms contributes to the development of obesity [5]. This is why the use of precision nutrition is being increasingly considered [6]. Given this perspective, the aim of this research was to determine the association between basal DNA methylation and the percentage of BMI loss after a dietary intervention in order to design a model that, based on a methylation score, predicts the percentage loss of BMI with two different types of diets.
2. Materials and Methods
2.1. Study Population
The population selected in this research was from the Obekit study in which 314 Spanish individuals with overweight and obesity were initially recruited. The study lasted from October 2015 to February 2017 and was carried out in the metabolic unit of the nutrition research center of the University of Navarra. Major exclusion criteria were type 1 diabetes mellitus, pregnant or breastfeeding women, cardiovascular disease, cancer, eating and cognitive disorders. Of the 314 subjects initially recruited, 8 did not meet the inclusion criteria. The intervention began with 306 participants, who were randomly assigned to two types of hypocaloric diet, 146 participants with a moderately high-protein diet (MHP) and 160 participants with a low-fat diet (LF). The intervention lasted 4 months. All research procedures were carried out following the ethical principles of the Declaration of Helsinki of 2013 [7]. The study protocol was approved by the Research Ethics Committee of the University of Navarra (ref.132/2015). All participants gave their written informed consent before their inclusion in the study.
2.2. Nutritional Intervention
The diets used for the study had a 30% calorie restriction. The individual energy requirements of each participant were estimated at the beginning, calculating their energy expenditure at rest and during physical activity, to prescribe hypocaloric diets in a random manner. The macronutrient distribution for the moderately high protein (MHP) diet was 40% carbohydrate, 30% protein, 30% fat and for the low fat (LF) diet it was 60% carbohydrate, 18% protein, and 22% fat. Both the LF and MHP diets were designed on the basis of a food exchange system. Participants received detailed information from trained dietitians on portion sizes, dietary patterns/eating schedules, and food preparation techniques.
2.3. DNA Isolation and Bisulfite Conversion
Blood samples taken at the beginning of the study were centrifuged at 4 °C for 15 min to obtain plasma and isolate the buffy coat fraction. DNA extraction was performed with the “MasterPure” DNA purification kit” for blood version II (Epicentre Biotechnologies, Madison, WI, USA), and it was quantified with a spectrophotometer (Nanodrop, Thermo Scientific, Wilmington, DE, USA) and stored at −80 °C. In the second step, 500 ng of DNA was treated with sodium bisulfite using the EZ-96 DNA methylation kit (Zymo Research Corporation, Irvine, CA, USA) to convert unmethylated cytosine residues to uracil, while methylated cytokines remain unchanged.
2.4. Array Analysis
The levels of methylated DNA were evaluated using the “Infinium MethylationEPIC BeadChip” kit (Illumina, San Diego, CA, USA), which includes 850,000 methylation sites. Samples were scanned with an “Illumina HiScanSQ” system and image intensities were extracted with “GenomeStudio v1.9” methylation software (Illumina, CA, USA). The within-array quantile subset normalization (SWAN) method was used to improve the results obtained from the platform, thereby reducing technical variation within and between arrays. The ComBat method was used to adjust for batch effects and remove technical variation. In addition, DNA methylation was corrected for cellular composition (granulocytes, monocytes, B cells, CD8+ cytotoxic cells, CD4+ T helper cells, and natural killer cells) using Houseman’s algorithm [8].
2.5. Design of the BMI Percentage Loss Prediction Model Based on MHP and LF Diet Methylation Data
- Selection of CpG sites for prediction model obtained in the “Illumina” methylation array;
- Weighted sub-score and total CpG site methylation score for prediction model;
- Design of a linear mixed effect model for the prediction of percentage loss of BMI in which the interaction between diet and total Score is analyzed;
- Information on the CpG sites obtained from the “Illumina” methylation array of each diet to characterize the CpG sites.
2.6. Statistical Analysis for the Prediction Model
Standard deviation of the CpG sites obtained in the Illumina array was performed, and then a Spearman measurement analysis was performed with the CpG sites correlating their methylation with the percentage of BMI loss and with these CpG sites the “furnival-Wilson” algorithm was applied. “by leaps and bounds” (vselect in stata) [9] was used to obtain the best combinations in the multiple linear regression of the percentage of BMI loss with the methylation sites for the MHP diet and the LF diet. With the values of the beta coefficients obtained in the linear regressions, the weighted methylation sub-scores for each diet and the total methylation score were constructed. This total methylation score was used for the design of the BMI percent loss prediction model, which was performed using a linear mixed-effects model. The prediction model was drawn applying marginals. A Z test was used to evaluate the distribution of subjects in the prediction model. Statistical analyses were performed using Stata MP 14 (StataCorp LLC, College Station, TX, USA; http://www.stata.com, accessed on 1 February 2023).
3. Results
3.1. Selection of CpG Sites for the Prediction Model
Of the 850,000 CpG sites obtained in the Illumina, those that had a standard deviation > 0.1 were selected, obtaining 1233 CpG sites. With these CpG sites, a Spearman correlation was performed between the methylation of the 1233 selected CpG sites and the percentage of BMI loss for each of the diets after 4 months of intervention, selecting those CpG sites that presented a significant correlation with p < 0.05. Thus, 34 CpG sites for the MHP diet and 20 CpG sites for the LF diet were obtained. To better predict the percentage of BMI loss with each of the diets, the “furnival-Wilson leaps and bounds” algorithm (“vselect” in stata) [9] was used. The algorithm recognized 19 CpG sites for the MHP diet and 14 CpG sites for the LF diet. Then, the CpG sites that presented a p < 0.19 in the last regression model were selected and those that presented multicollinearity were eliminated, obtaining 15 CpG sites for the MHP diet and 11 CpG sites for the LF diet, which were included. In the next analytical step, calculation of the sub-scores was conducted.
3.2. Design of Weighted Sub-Scores That Contain the CpG Sites of Each Diet and the Calculation of the Total Methylation Score for the Prediction Model
Weighted sub-scores were made for each diet, using the sum of the previously selected CpG sites and multiplying them by the beta coefficients obtained in each of the multiple linear regressions of the MHP diet and the LF diet (Table 1). To obtain a total score for each individual that would allow to be included as a term for the interaction with the diet variable, the MHP diet sub-score was subtracted from the LF diet sub-score as shown in Table 1.

Table 1.
Design of weighted sub-scores for diet MHP, LF and total score.
A linear mixed effect model was used to predict, based on the total methylation score of each individual, which diet will be the best for them based on the greatest percentage loss of BMI. Therefore, a linear mixed effect model was designed with the percentage of BMI loss as the dependent variable and total score, diet (MHP/LF) and the interaction term between the total score and the diet as a fixed effect. Moreover, the ids of the participants were included as a random effect. The model was adjusted for sex and age. Table 2 shows the independent variables, the beta coefficient with the standard error and the p value. As it can be seen in the table, the model is not affected when adjusting for sex and age, since none of these variables showed statistical significance.

Table 2.
Linear mixed effect model for the prediction of the percentage loss of BMI.
3.3. Representation of the Prediction Model
Based on the linear mixed effect model, a “diet x total score” interaction graph was created showing the marginal percentage loss of BMI for each diet. As shown in Figure 1, where the “X” axis represents the total methylation score and the “Y” axis shows the percentage of BMI loss, the percentage of BMI loss with each diet is estimated (MHP in blue and LF in red) according to the methylation score presented by the subjects before starting the intervention. Thus, for methylation score values between −10 and −1, the error bars that predict the percentage of BMI loss for each diet overlap, indicating that both diets are effective in losing similar BMI values. It should be noted that between −13 and −10, the overlap is less. Conversely, when an individual has a score that is between 2 and 11, the error bars separate, suggesting that the methylation score is effective in predicting the type of diet that is the best for weight loss in that individual.
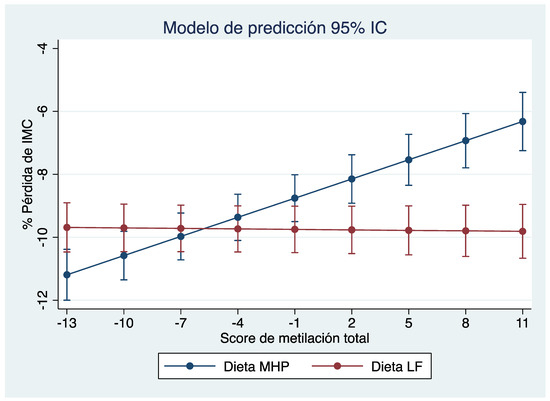
Figure 1.
Prediction model of percentage of BMI loss according to the total methylation score of each individual. “Y” axis, percentage loss of BMI; “X” axis, methylation score. According to the total score, it can be predicted with which diet the greatest percentage of BMI would be achieved for each individual.
The statistical differences between the predictions of the percentage loss of BMI with both diets for each individual were analyzed using the “Z” test that involves the standard errors of each estimation of the score. A significant p (<0.05) allows for prescribing the most appropriate diet for each individual. However, if the p is not significant (p > 0.05), the two diets have a similar effect, so they can be prescribed interchangeably.
With this test it was observed that, of the 201 participants, for 126 participants, it could not be predicted which diet would be better, but 75 participants can be advised that one diet as better than the other based on BMI loss. As shown in Figure 2, in which the “X” axis represents the total score and the “Y” axis shows the p value obtained in the Z test, the non-significant p values (>0.05) are between the total methylation score values of −10 and −2; this is the population for which it is not possible to predict which diet is better (n = 126). While the individuals (n = 75) who have a score between −13 and −11 or between −1 and 11, a type of diet for losing weight can be recommended to them according to their baseline methylation score value (p < 0.05).
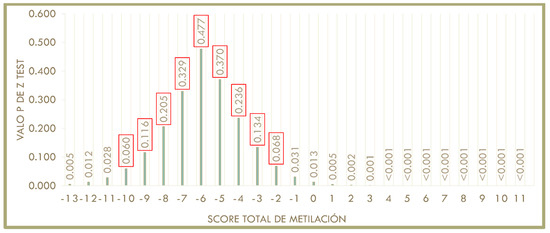
Figure 2.
Representation of the distribution of the P value of the Z test with respect to the total methylation score. “X” axis shows the total score. “Y” axis shows P value of Z test. A “P” value <0.05 was considered significant. From −10 to −1 of the methylation score a non-significant P is presented. Red boxes indicate p > 0.05 (non-significant) in the Z test.
4. Discussion
In this research, CpG sites whose baseline methylation was associated with BMI reduction after 4 months of dietary intervention in a population were identified, then used to construct a total methylation score that was finally included in a model along with the two types of diet. This model adequately predicts the percentage of BMI loss.
Currently, the usual management for overweight and obesity is carried out by calculating energy expenditure, establishing daily nutritional requirements and performing caloric restriction, in addition to giving recommendations for lifestyle changes specific to each individual [10,11]. The main limitation of this routine management is that it does not take into account the variability of each individual’s response to these interventions. Therefore, a prediction model was designed based on methylation data from the studied population that predicts the percentage of BMI loss with the MHP diet and the LF diet. With the aim of obtaining a methylation score that predicts the most appropriate diet for percentage of BMI loss to an intervention for the dietary management of obesity or overweight. The model that predicts the percentage of BMI loss was made with a total methylation score that was constructed considering the MHP diet methylation score in which 15 CpG sites were selected that were better associated with the reduction in BMI percentage and 11 CpG sites for LF diet. With this model, it is possible to predict which diet with the highest percentage of BMI would be lost for 75 participants corresponding to 37.3% of the total population of this study. This total methylation score could be used to predict which diet is more appropriate for each individual or if it can be chosen by dietary preference, since there are subjects who would lose a similar percentage of BMI with both diets. The potential of DNA methylation to predict BMI loss has been described before, as demonstrated in a study in which a prediction score was performed based on 83 CpG sites that found an association with BMI. The result they obtained was a prediction that represented 29% of the variation in BMI in the studied population [12]. The prediction model of the percentage of BMI loss has no similarity in CpG sites and genes related to these CpG sites for both diets. This may be due to the differences in the investigations, such as different methods and selection parameters of the CpG sites, sample size, population characteristics, and experimental protocols.
5. Conclusions
This research demonstrates that DNA methylation is an individual characteristic that can be used to have greater precision in the nutritional treatment of obesity. The model designed based on the methylation information through the linear mixed effect model allows for predicting the loss of BMI percentage. This model can be useful to determine which diet is more adequate for weight loss for each individual.
These results have been the basis of an extended article published in Nutrients 2023, 15(24), 5023; https://doi.org/10.3390/nu15245023.
Author Contributions
Conceptualization, F.I.M. and J.A.M.; methodology, F.I.M. and J.I.R.-B.; formal analysis, J.I.R.-B. and S.G.-C.; investigation, N.C.G.-Á. and S.G.-C.; resources, F.I.M. and J.A.M.; data curation, J.I.R.-B.; writing—original draft preparation, N.C.G.-Á.; writing—review and editing, F.I.M. and S.G.-C.; supervision, F.I.M. and S.G.-C.; project administration, F.I.M. and S.G.-C.; funding acquisition, F.I.M. and J.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CIBEROBN (Grant number: CB12/03/30002), Government of Navarra (Obekit-PT024 and Microbiota-PI035 projects), the Spanish Ministerio de Ciencia, Innovación y Universidades (reference RTI2018-102205-B-I00.
Institutional Review Board Statement
The study was performed in line with the guidelines of the Declaration of Helsinki and received approval by the Ethics Committee of the University of Navarra (ref. 132/2015).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data will be available upon request by contacting the corresponding author.
Acknowledgments
The authors acknowledge the Obekit team (Miren Iosune Zubieta, Laura Olazarán, Salomé Pérez and Ana Lorente) for collaborating in the recruitment and follow-up of the volunteers, sampling and biochemical analyses.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Wu, F.Y.; Yin, R.X. Recent progress in epigenetics of obesity. Diabetol. Metab. Syndr. 2022, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Heianza, Y.; Li, X.; Shang, X.; Smith, S.R.; Bray, G.A.; Sacks, F.M.; Qi, L. Genetic, epigenetic and transcriptional variations at NFATC2IP locus with weight loss in response to diet interventions: The POUNDS Lost Trial. Diabetes Obes. Metab. 2018, 20, 2298–2303. [Google Scholar] [CrossRef] [PubMed]
- Samblas, M.; Milagro, F.I.; Martínez, A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics 2019, 14, 421–444. [Google Scholar] [CrossRef] [PubMed]
- Fuso, A.; Raia, T.; Orticello, M.; Lucarelli, M. The complex interplay between DNA methylation and miRNAs in gene expression regulation. Biochimie 2020, 173, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Czogała, W.; Strojny, W.; Schab, M.; Grabowska, A.; Miklusiak, K.; Kowalczyk, W.; Łazarczyk, A.; Tomasik, P.; Skoczeń, S. Fto and plag1 genes expression and fto methylation predict changes in circulating levels of adipokines and gastrointestinal peptides in children. Nutrients 2021, 13, 3585. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, K.M.; Ramos-Lopez, O.; Pérusse, L.; Kato, H.; Ordovas, J.M.; Martínez, J.A. Precision nutrition: A review of current approaches and future endeavors. Trends Food Sci. Technol. 2022, 128, 253–264. [Google Scholar] [CrossRef]
- World Medical Association declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Houseman, E.A.; Accomando, W.P.; Koestler, D.C.; Christensen, B.C.; Marsit, C.J.; Nelson, H.H.; Wiencke, J.K.; Kelsey, K.T. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinform. 2012, 13, 86. [Google Scholar] [CrossRef]
- Lindsey, C. Stat Softw Components. Boston College Department of Economics. VSELECT: Stata Module to Perform Linear Regression Variable Selection. Available online: https://ideas.repec.org/c/boc/bocode/s457808.html (accessed on 23 August 2023).
- Thibault, R.; Abbasoglu, O.; Ioannou, E.; Meija, L.; Ottens-Oussoren, K.; Pichard, C.; Rothenberg, E.; Rubin, D.; Siljamäki-Ojansuu, U.; Vaillant, M.F.; et al. ESPEN guideline on hospital nutrition. Clin. Nutr. 2021, 40, 5684–5709. [Google Scholar] [CrossRef] [PubMed]
- Pomar, M.D.; García, N.V.; Herrera, M.Á.; Barahona, M.J.; Bueno, M.; Caixàs, A.; Continente, A.C.; Ciudin, A.; Cordido, F.; de Hollanda, A.; et al. Abordaje clínico integral SEEN de la obesidad en la edad adulta. Endocrinol. Diabetes Nutr. (Engl. Ed). 2021, 68, 130–136. [Google Scholar]
- Do, W.L.; Sun, D.; Meeks, K.; Dugué, P.A.; Demerath, E.; Guan, W.; Li, S.; Chen, W.; Milne, R.; Adeyemo, A.; et al. Epigenome-wide meta-analysis of BMI in nine cohorts: Examining the utility of epigenetically predicted BMI. Am. J. Hum. Genet. 2023, 110, 273–283. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).