Characterisation and Quantification of Phenolic Compounds in Honeys from Sierra Nevada (Granada) †
Abstract
:1. Introduction
2. Methodology
2.1. Extraction of Phenolic Compounds
2.2. Analysis of Phenolic Compounds
2.3. Characterisation of Phenolic Compounds
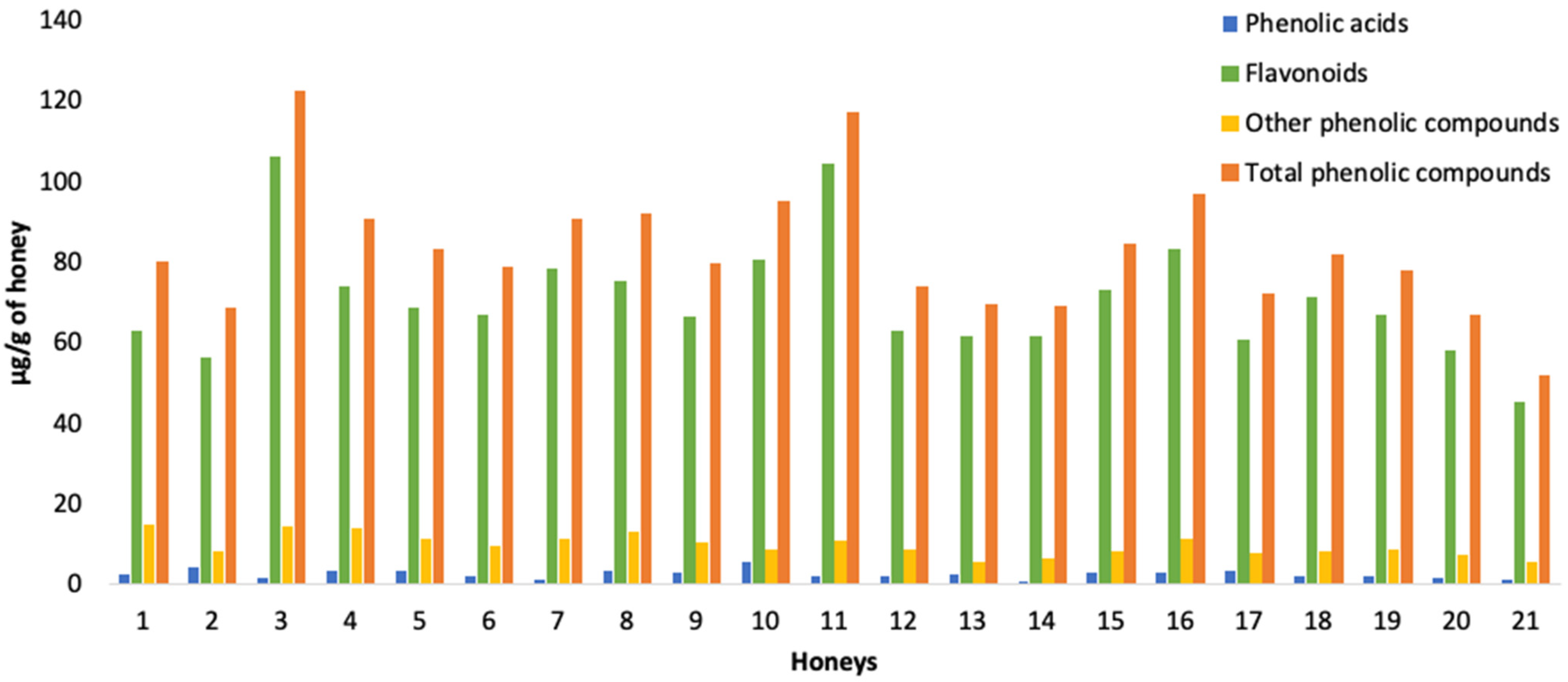
3. Results and Discussion
| [M-H]− | RT | Molecular Formula | Proposed Compound | Fragments | Reference |
|---|---|---|---|---|---|
| 135.0433 | 2.189 | C8H8O2 | Phenylacetic acid | 117 | [18] |
| 135.0438 | 2.01 | C8H8O2 | Vinylcatechol | 134, 133, 105 | [19] |
| 137.0222 | 4.014 | C7H6O3 | Hydroxybenzoic acid | 93 | [20] |
| 153.0208 | 4.852 | C7H6O4 | Protocatechuic acid | 109, 137 | [21] |
| 163.0386 | 3.225 | C9H8O3 | Cumaric acid (Isomer 1) | 145, 119 | [20] |
| 163.0396 | 3.341 | C9H8O3 | Cumaric acid (Isomer 2) | 119, 117 | [20] |
| 165.0547 | 2.458 | C9H10O3 | 4-hydroxicinnamic acid | 161, 133, 132, 122 | [22] |
| 165.0558 | 3.448 | C9H10O3 | L-(-)-phenylactic acid | 147 | [23] |
| 167.0337 | 2.065 | C8H8O4 | Homogentisic acid | 134, 137, 131, 117, 108 | [24] |
| 177.0181 | 1.769 | C9H6O4 | Esculatin | 145, 125, 120, 144 | [25] |
| 193.0495 | 2.095 | C10H10O4 | Coniferic/ferulic acid | 133 | [26] |
| 195.0659 | 1.995 | C10H12O4 | 4-methoxyphenylactic acid | 133, 177, 149 | [27] |
| 197.0442 | 2.16 | C9H10O5 | Siringic acid (Isomer 1) | 106 | [20] |
| 197.0455 | 2.262 | C9H10O5 | Siringic acid (Isomer 2) | 121, 123 | [20] |
| 211.0599 | 4.875 | C10H12O5 | Methylsyringate | 181 | [28] |
| 221.0804 | 3.45 | C12H14O4 | 3-hydroxy-1-(2-methoxyphenyl)penta-1,4-dione (Isomer 1) | 133 | [29] |
| 221.0811 | 3.605 | C12H14O4 | 3-hydroxy-1-(2-methoxyphenyl)penta-1,4-dione (Isomer 2) | 133 | [29] |
| 223.0609 | 3.228 | C11H12O5 | Sinapic acid | 144, 116, 142, 160 | [21] |
| 223.0968 | 6.851 | C12H16O4 | Vanillin 1,2-butylene glycol | 151, 136, 108 | [30] |
| 253.0497 | 12.928 | C15H10O4 | Chrysin | 209, 143, 145, 119,195 | [31] |
| 255.0659 | 12.128 | C15H12O4 | Pinocembrin | 171, 133, 213, 134, 169 | [32] |
| 269.0439 | 10.498 | C15H10O5 | Apigenin | 117,149, 201, 145, 183, 107 | [33] |
| 269.0441 | 9.803 | C15H10O5 | Baicalein | 129, 143, 151 | [25] |
| 269.0445 | 13.681 | C15H10O5 | Galangin | 211, 239, 195,167, 151 | [34] |
| 271.0600 | 7.979 | C15H12O5 | Pinobanksin | 253, 197,225, 209, 125 | [35] |
| 271.0603 | 8.406 | C15H12O5 | Naringenin | 253,197, 161,125, 225 | [20] |
| 283.0597 | 11.218 | C16H12O5 | Prunetin | 211, 238, 167, 165 | [36] |
| 283.0604 | 13.878 | C16H12O5 | Biochanin A | 268, 211, 239, 269, 195 | [37] |
| 283.0605 | 13.469 | C16H12O5 | Genkwanin | 134, 175, 168, 148, 159 | [38] |
| 283.0969 | 13.161 | C17H16O4 | Phenylethyl caffeate | 135, 133, 161, 134 | [34] |
| 285.0381 | 10.787 | C15H10O6 | Kaempferol (Isomer 1) | 151, 184, 245, 255, 273 | [39] |
| 285.0392 | 6.831 | C15H10O6 | Luteolin (Isomer 1) | 151, 257 | [40] |
| 285.0394 | 13.682 | C15H10O6 | Kaempferol (Isomer 2) | 269, 268, 211, 239 | [39] |
| 285.0396 | 8.691 | C15H10O6 | Luteolin (Isomer 2) | 255, 133, 283, 151 | [40] |
| 285.0404 | 7.646 | C15H10O6 | Kaempferol (Isomer 3) | 255, 227, 211, 284 | [39] |
| 285.0408 | 9.135 | C15H10O6 | Luteolin (Isomer 3) | 241, 133 | [40] |
| 285.0772 | 12.291 | C16H14O5 | 5-O-Methylnaringenin | 188, 191, 255, 243, 158 | [20] |
| 287.0555 | 5.52 | C15H12O6 | Eriodictyol | 161, 269, 251 | [41] |
| 299.0545 | 11.479 | C16H12O6 | Kaempferide (Isomer 1) | 284, 227, 256, 165, 269 | [42] |
| 299.0547 | 11.155 | C16H12O6 | Kaempferide (Isomer 2) | 284 | [42] |
| 301.0322 | 4.236 | C15H10O7 | Quercetin | 175, 183, 201, 225, 245 | [42] |
| 301.0339 | 4.077 | C15H10O7 | Quercetin | 255, 273, 213, 151 | [42] |
| 301.0354 | 9.343 | C15H10O7 | Morin | 273, 151, 257, 178, 255 | [34] |
| 301.0696 | 9.9400 | C16H14O6 | Hesperetin (Isomer 1) | 164 | [43] |
| 301.0713 | 5.3190 | C16H14O6 | Hesperetin (Isomer 2) | 151, 177 | [43] |
| 301.0717 | 5.4440 | C16H14O6 | Hesperetin (Isomer 3) | 177, 286 | [43] |
| 301.2008 | 8.5460 | C15H10O7 | Tricetin | 255, 151 | [44] |
| 315.0487 | 10.875 | C16H12O7 | 3-Methylquercetin/Isorhamnetin (Isomer 1) | 241, 242, 270, 313, 300 | [39] |
| 315.0500 | 10.157 | C16H12O7 | 3-Methylquercetin/Isorhamnetin (Isomer 2) | 300 | [39] |
| 315.0506 | 9.136 | C16H12O7 | 3-Methylquercetin/Isorhamnetin (Isomer 3) | 241, 242, 270, 271, 300, 313 | [39] |
| 329.0650 | 9.824 | C17H14O7 | Quercetin dimethyl ether (Isomer 1) | 314 | [45] |
| 329.0664 | 11.956 | C17H14O7 | Quercetin dimethyl ether (Isomer 2) | 314 | [45] |
| 329.1744 | 15.415 | C20H26O4 | Carnosol (Isomer 1) | 285 | [35] |
| 329.1745 | 15.068 | C20H26O4 | Carnosol (Isomer 2) | 285 | [35] |
| 329.1753 | 15.708 | C20H26O4 | Carnosol (Isomer 3) | 285 | [35] |
| 329.1758 | 14.856 | C20H26O4 | Carnosol (Isomer 4) | 285 | [35] |
| 431.0974 | 7.651 | C21H20O10 | Kaempferol-rharmnoside | 285, 255, 227 | [46] |
| 461.1065 | 9.049 | C22H22O11 | 8-Methoxykaempferol 7-rhamnopyranoside | 287, 299, 315, 259, 139 | [46] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- Baloš, M.M.Ž.; Popov, N.S.; Radulović, J.Z.P.; Stojanov, I.M.; Jakšić, S.M. Sugar profile of different floral origin honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Palma-Morales, M.; Huertas, J.R.; Rodríguez-Pérez, C. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, N.; Chong, P.J.; Mohd Tom, N.N.; Saiful Anuar, N.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing Relationship between Vitamins, Total Phenolic and Total Flavonoid Content and Antioxidant Activities in Various Honey Types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef] [PubMed]
- Arráez-Román, D.; Gómez-Caravaca, A.M.; Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Identification of phenolic compounds in rosemary honey using solid-phase extraction by capillary electrophoresis–electrospray ionization-mass spectrometry. J. Pharm. Biomed. Anal. 2006, 41, 1648–1656. [Google Scholar] [CrossRef] [PubMed]
- Ouchemoukh, S.; Amessis-Ouchemoukh, N.; Gómez-Romero, M.; Aboud, F.; Giuseppe, A.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Characterisation of phenolic compounds in Algerian honeys by RP-HPLC coupled to electrospray time-of-flight mass spectrometry. LWT-Food Sci. Technol. 2017, 85, 460–469. [Google Scholar] [CrossRef]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Hanieh, H.; Hairul Islam, V.I.; Saravanan, S.; Chellappandian, M.; Ragul, K.; Durga, A.; Venugopal, K.; Senthilkumar, V.; Senthilkumar, P.; Thirugnanasambantham, K. Pinocembrin, a novel histidine decarboxylase inhibitor with anti-allergic potential in in vitro. Eur. J. Pharmacol. 2017, 814, 178–186. [Google Scholar] [CrossRef]
- Alday, E.; Valencia, D.; Carreño, A.L.; Picerno, P.; Piccinelli, A.L.; Rastrelli, L.; Robles-Zepeda, R.; Hernandez, J.; Velazquez, C. Apoptotic induction by pinobanksin and some of its ester derivatives from Sonoran propolis in a B-cell lymphoma cell line. Chem. Biol. Interact. 2015, 242, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Ting, P.; Srinuanchai, W.; Suttisansanee, U.; Tuntipopipat, S.; Charoenkiatkul, S.; Praengam, K.; Chantong, B.; Temviriyanukul, P.; Nuchuchua, O. Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods 2021, 10, 1912. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Song, Q.; Yang, J.; Yu, S.; Zhao, J.; Yu, G. Carnosol inhibits Hedgehog signaling pathway in both LNCaP and DU145 prostate cancer cell lines. Cell. Mol. Biol. 2017, 63, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.-C.; Lin, Y.-C.; Huang, Y.-F.; Hsieh, C.-P.; Wu, C.-C.; Chang, I.-L.; Chen, C.-L.; Cheng, C.-H.; Chen, H.-Y. Carnosol-induced ROS inhibits cell viability of human osteosarcoma by apoptosis and autophagy. Am. J. Chin. Med. 2017, 45, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, Y.; Yang, Y.; Ding, Y.; Sun, X. Carnosol suppresses microglia cell inflammation and apoptosis through PI3K/AKT/mTOR signaling pathway. Immunopharmacol. Immunotoxicol. 2022, 44, 656–662. [Google Scholar] [CrossRef]
- Thapa, R.; Afzal, O.; Alfawaz Altamimi, A.S.; Goyal, A.; Almalki, W.H.; Alzarea, S.I.; Kazmi, I.; Jakhmola, V.; Singh, S.K.; Dua, K.; et al. Galangin as an inflammatory response modulator: An updated overview and therapeutic potential. Chem. Biol. Interact. 2023, 378, 110482. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Information, N.C.f.B. PubChem Compound Summary for CID 999, Phenylacetic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Phenylacetic-Acid (accessed on 25 October 2023).
- Kalkan Yıldırım, H.; Canbay, E.; Öztürk, Ş.; Aldemir, O.; Sözmen, E.Y. Biotransformation of propolis phenols by L. plantarum as a strategy for reduction of allergens. Food Sci. Biotechnol. 2018, 27, 1727–1733. [Google Scholar] [CrossRef]
- Machado, P.G.; Londero, D.S.; Farias, C.A.A.; Pudenzi, M.A.; Barcia, M.T.; Ballus, C.A. Guabijú (Myrcianthes pungens): A comprehensive evaluation of anthocyanins and free, esterified, glycosylated, and insoluble phenolic compounds in its peel, pulp, and seeds. Food Chem. 2024, 432, 137296. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Kumar, S.S.; Giridhar, P. Phytochemicals and UHPLC-QTOF-HRMS characterisation of bioactives of butterfly pea (Clitoria ternatea L.) seeds and their antioxidant potentials. Food Chem. 2024, 433, 137373. [Google Scholar] [CrossRef]
- Pinto, D.; López-Yerena, A.; Almeida, A.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Metabolomic insights into phenolics-rich chestnut shells extract as a nutraceutical ingredient—A comprehensive evaluation of its impacts on oxidative stress biomarkers by an in-vivo study. Food Res. Int. 2023, 170, 112963. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Sun, Y.; Xu, Z.; Chen, Y.; Li, L.; Lin, L.; Zhang, S.; Liao, Q.; Xie, Z. Rapid simultaneous determination of gut microbial phenylalanine, tyrosine, and tryptophan metabolites in rat serum, urine, and faeces using LC–MS/MS and its application to a type 2 diabetes mellitus study. Biomed. Chromatogr. 2021, 35, e4985. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.; Khalifa, S.M.; Ahmed, A.H.; Metwaly, A.M.; Sh Mohammed, H.; Taie, H.A.A. LC/HRESI-MS/MS screening, phytochemical characterization, and in vitro antioxidant and cytotoxic potential of Jatropha integerrima Jacq. extracts. Bioorg. Chem. 2023, 140, 106825. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elghiet, F.; Mohamed, S.A.; Yasin, N.A.E.; Temraz, A.; El-Tantawy, W.H.; Ahmed, S.F. The effect of Alnus incana (L.) Moench extracts in ameliorating iron overload-induced hepatotoxicity in male albino rats. Sci. Rep. 2023, 13, 7635. [Google Scholar] [CrossRef] [PubMed]
- Matkovits, A.; Nagy, K.; Fodor, M.; Jókai, Z. Analysis of polyphenolic components of Hungarian acacia (Robinia pseudoacacia) honey; method development, statistical evaluation. J. Food Compos. Anal. 2023, 120, 105336. [Google Scholar] [CrossRef]
- Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G.; Castro, J.M.; Tomás-Barberán, F.A.; Tornadijo, M.E.; Fresno-Baro, J.M. Bioactive Components and Antioxidant and Antibacterial Activities of Different Varieties of Honey: A Screening Prior to Clinical Application. J. Agric. Food Chem. 2019, 67, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Tuberoso, C.I.G.; Bifulco, E.; JerkoviĆ, I.; Caboni, P.; Cabras, P.; Floris, I. Methyl Syringate: A Chemical Marker of Asphodel (Asphodelus microcarpus Salzm. et Viv.) Monofloral Honey. J. Agric. Food Chem. 2009, 57, 3895–3900. [Google Scholar] [CrossRef]
- Mannina, L.; Sobolev, A.P.; Di Lorenzo, A.; Vista, S.; Tenore, G.C.; Daglia, M. Chemical Composition of Different Botanical Origin Honeys Produced by Sicilian Black Honeybees (Apis mellifera ssp. sicula). J. Agric. Food Chem. 2015, 63, 5864–5874. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Wang, Z.; Zhao, C.; Zhang, Y.; Wang, M. Danggui Shaoyao San: Chemical characterization and inhibition of oxidative stress and inflammation to treat CCl4-induced hepatic fibrosis. J. Ethnopharmacol. 2024, 318, 116870. [Google Scholar] [CrossRef]
- Information, N.C.f.B. PubChem Compound Summary for CID 5281607, Chrysin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Chrysin (accessed on 25 October 2023).
- Zhao, Y.-T.; Liu, Y.-R.; Yan, Y.-F.; Tang, Z.-S.; Duan, J.-A.; Yang, H.; Song, Z.-X.; You, X.-L.; Wang, M.-G. Fushenmu treatment ameliorates RyR2 with related metabolites in a zebrafish model of barium chloride induced arrhythmia. Chin. Med. 2023, 18, 103. [Google Scholar] [CrossRef]
- Pekacar, S.; Özüpek, B.; Akkol, E.K.; Taştan, H.; Ersan, H.; Orhan, D.D. Identification of bioactive components on antihemorrhoidal activity of Cistus laurifolius L. using RP-HPLC and LC-QTOF-MS. J. Ethnopharmacol. 2024, 319, 117122. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Hu, Z.; Jia, C.; Yang, M.; Li, D.; Xu, A.; Jiang, J.; Chen, Z.; Li, Y.; Li, S.; et al. Deciphering the mechanisms of Yinlan Tiaozhi capsule in treating hyperlipidemia by combining network pharmacology, molecular docking and experimental verification. Sci. Rep. 2023, 13, 6424. [Google Scholar] [CrossRef] [PubMed]
- Gašić, U.M.; Natić, M.M.; Mišić, D.M.; Lušić, D.V.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Lušić, D. Chemical markers for the authentication of unifloral Salvia officinalis L. honey. J. Food Compos. Anal. 2015, 44, 128–138. [Google Scholar] [CrossRef]
- Mahrous, R.S.R.; Fathy, H.; Ibrahim, R.S. Metabolic bioprofiling of different Glycyrrhiza glabra solvent fractions for the identification of anti-adenoviral compounds using LC-HRMS/MS and in-vitro cytopathic assay coupled with chemometry. BMC Complement. Med. Ther. 2023, 23, 259. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhang, G.; Wu, D.; Guo, L.; Huang, X.; Ning, F.; Liu, Y.; Luo, L. Identification of the botanical origins of honey based on nanoliter electrospray ionization mass spectrometry. Food Chem. 2023, 418, 135976. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Zhang, F.; Ren, M.; Chen, X.; Liu, C.; Li, G.; Gao, Q.; Qiao, L.; Jiang, Y.; Zhu, L.; et al. Eco-friendly and efficient extraction of polyphenols from Ligustrum robustum by deep eutectic solvent assisted ultrasound. Food Chem. 2023, 429, 136828. [Google Scholar] [CrossRef] [PubMed]
- Giovanini de Oliveira Sartori, A.; Martelli Chaib Saliba, A.S.; Sêneda Martarello, N.; Goldoni Lazarini, J.; Pedroso Gomes do Amaral, J.E.; Fernandes Pinto da Luz, C.; Alencar, S.M.d. Changes in phenolic profile and anti-inflammatory activity of Baccharis beebread during gastrointestinal digestion/intestinal permeability in vitro. Food Chem. 2024, 432, 137234. [Google Scholar] [CrossRef]
- Śliwka-Kaszyńska, M.; Anusiewicz, I.; Skurski, P. The Mechanism of a Retro-Diels–Alder Fragmentation of Luteolin: Theoretical Studies Supported by Electrospray Ionization Tandem Mass Spectrometry Results. Molecules 2022, 27, 1032. [Google Scholar] [CrossRef]
- Wang, Z.; Gmitter, F.G., Jr.; Grosser, J.W.; Wang, Y. Natural Sweeteners and Sweetness-Enhancing Compounds Identified in Citrus Using an Efficient Metabolomics-Based Screening Strategy. J. Agric. Food Chem. 2022, 70, 10593–10603. [Google Scholar] [CrossRef]
- Kibungu Kembelo, P.; Tuenter, E.; Vanhove, W.; Belesi Katula, H.; Van Damme, P.; Pieters, L. Phytochemical Profiling by UPLC-ESI-QTOF-MS of Kalaharia uncinata (Schinz) Moldenke, Widely Used in Traditional Medicine in DR Congo. Chem. Biodivers. 2023, 20, e202300826. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Zeng, J.; Cai, R.; Liang, Y.; Chen, C.; Chen, B.; Li, C. Database-aided UHPLC-Q-orbitrap MS/MS strategy putatively identifies 52 compounds from Wushicha Granule to propose anti-counterfeiting quality-markers for pharmacopoeia. Chin. Med. 2023, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Elessawy, F.M.; Wright, D.; Vandenberg, A.; El-Aneed, A.; Purves, R.W. Mass Spectrometry-Based Untargeted Metabolomics Reveals the Importance of Glycosylated Flavones in Patterned Lentil Seed Coats. J. Agric. Food Chem. 2023, 71, 3541–3549. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Quispe, C.; González, B. Phenolic Profiling of the South American “Baylahuen” Tea (Haplopappus spp., Asteraceae) by HPLC-DAD-ESI-MS. Molecules 2015, 20, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Amessis-Ouchemoukh, N.; Madani, K.; Segura-Carretero, A.; Fernández-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma-Morales, M.; Balzani, A.; Huertas, J.R.; Mercolini, L.; Rodríguez-Pérez, C. Characterisation and Quantification of Phenolic Compounds in Honeys from Sierra Nevada (Granada). Biol. Life Sci. Forum 2023, 26, 74. https://doi.org/10.3390/Foods2023-15513
Palma-Morales M, Balzani A, Huertas JR, Mercolini L, Rodríguez-Pérez C. Characterisation and Quantification of Phenolic Compounds in Honeys from Sierra Nevada (Granada). Biology and Life Sciences Forum. 2023; 26(1):74. https://doi.org/10.3390/Foods2023-15513
Chicago/Turabian StylePalma-Morales, Marta, Alessandro Balzani, Jesús R. Huertas, Laura Mercolini, and Celia Rodríguez-Pérez. 2023. "Characterisation and Quantification of Phenolic Compounds in Honeys from Sierra Nevada (Granada)" Biology and Life Sciences Forum 26, no. 1: 74. https://doi.org/10.3390/Foods2023-15513
APA StylePalma-Morales, M., Balzani, A., Huertas, J. R., Mercolini, L., & Rodríguez-Pérez, C. (2023). Characterisation and Quantification of Phenolic Compounds in Honeys from Sierra Nevada (Granada). Biology and Life Sciences Forum, 26(1), 74. https://doi.org/10.3390/Foods2023-15513









