Impact of Different Raw Materials on Changes in Volatile Compounds during Moromi Fermentation †
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Sample Preparation
2.3. Equipment
2.4. SPME Extraction of Volatile Compounds
2.5. GC–MS Analysis of Volatile Compounds
2.6. Identification and Semi-Quantification of Volatile Compounds
2.6.1. Identification
2.6.2. Semi-Quantification
3. Results and Discussion
3.1. Volatile Compound Identification of 24 Soy Sauces
3.1.1. Alcohols
3.1.2. Phenols
3.1.3. Acids
3.1.4. Esters, Furan(one)s and Pyrazines
3.1.5. Aldehydes and Ketones
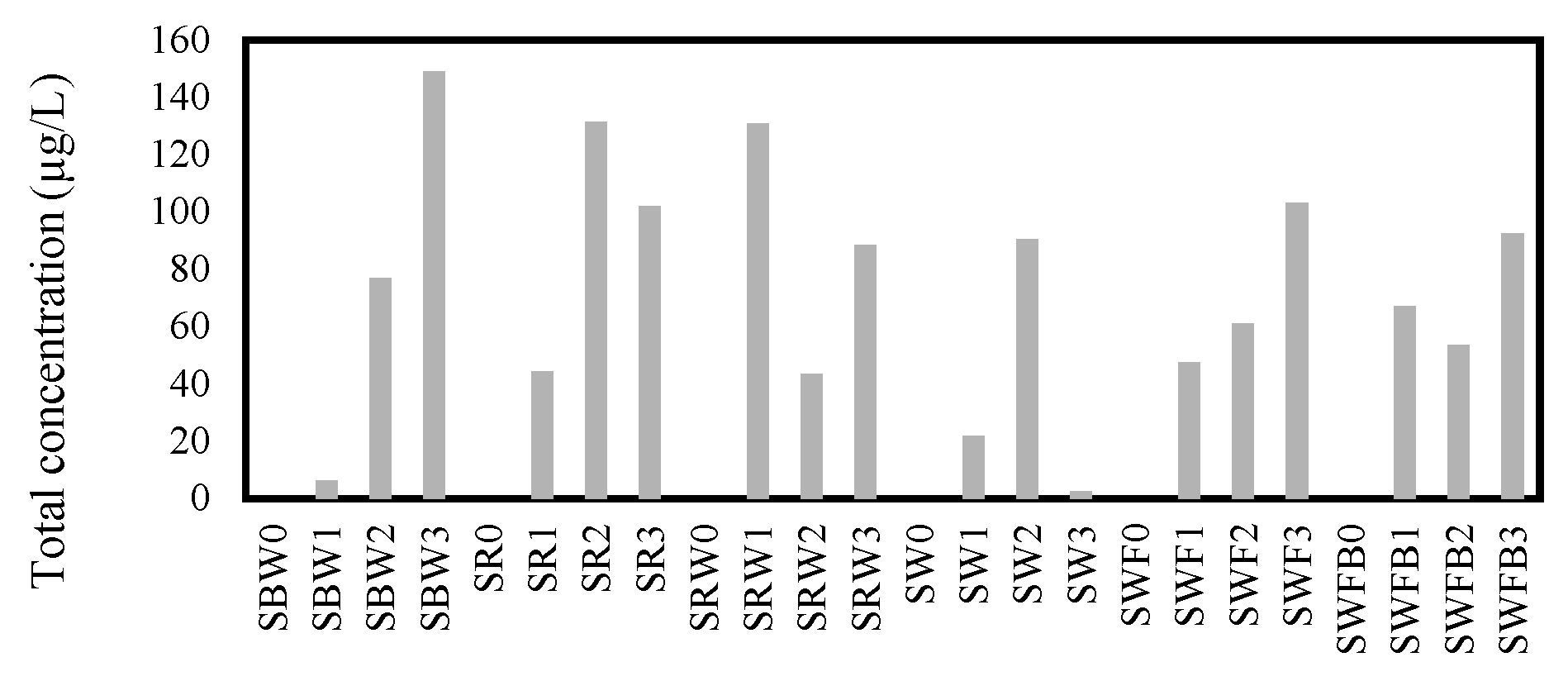
3.1.6. All Volatile Compounds Included
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theary, C.; Panagides, D.; Laillou, A.; Vonthanak, S.; Kanarath, C.; Chhorvann, C.; Sambath, P.; Sowath, S.; Moench-Pfanner, R. Fish sauce, soy sauce, and vegetable oil fortification in Cambodia: Where do we stand to date? Food Nutr. Bull. 2013, 34 (Suppl. 2), S62–S71. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.E. The Chinese in Cambodia. By William E. Willmott. [Vancouver: University of British Columbia, 1967. 132 pp.]. China Q. 1967, 31, 173. [Google Scholar] [CrossRef]
- Liu, K.S. Food Use of Whole Soybeans. Soybeans: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2008; pp. 441–481. [Google Scholar]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and Sensory Characteristics of Soy Sauce: A Review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Peng, D.; Zhang, W.; Duan, M.; Ruan, Z.; Huang, S.; Zhou, S.; Fang, Q. Effect of aroma-producing yeasts in high-salt liquid-state fermentation soy sauce and the biosynthesis pathways of the dominant esters. Food Chem. 2021, 344, 128681. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu CDe Huang, J.; Zhou, R.Q.; Liao, X.P. Analysis of volatile compounds in Chinese soy sauces moromi cultured by different fermentation processes. Food Sci. Biotechnol. 2013, 22, 605–612. [Google Scholar] [CrossRef]
- Fukushima, D. Fermented vegetable (soybean) protein and related foods of Japan and China. J. Am. Oil Chem. Soc. 1979, 56, 357–362. [Google Scholar] [CrossRef]
- Gao, X.L.; Cui, C.; Zhao, H.F.; Zhao, M.M.; Yang, L.; Ren, J.Y. Changes in volatile aroma compounds of traditional chinese-type soy sauce during moromi fermentation and heat treatment. Food Sci. Biotechnol. 2010, 19, 889–898. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Eichelsheim, C.; Jacobs, D.M.; Mumm, R.; Hall, R.D. Stir bar sorptive extraction of aroma compounds in soy sauce: Revealing the chemical diversity. Food Res. Int. 2021, 144, 110348. [Google Scholar] [CrossRef]
- Lee, B.Q.; Khor, S.M. 3-Chloropropane-1,2-diol (3-MCPD) in Soy Sauce: A Review on the Formation, Reduction, and Detection of This Potential Carcinogen. Compr. Rev. Food Sci. Food Saf. 2015, 14, 48–66. [Google Scholar] [CrossRef]
- Yamana, T.; Taniguchi, M.; Nakahara, T.; Ito, Y.; Okochi, N.; Putri, S.P.; Fukusaki, E. Component Profiling of Soy-Sauce-Like Seasoning Produced from Different Raw Materials. Metabolites 2020, 10, 137. [Google Scholar] [CrossRef]
- Luo, J.; Ding, L.; Chen, X.; Wan, Y. Desalination of soy sauce by nanofiltration. Sep. Purif. Technol. 2009, 66, 429–437. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Y.; Tao, W.; Wang, L.; Wu, S. Rapid determination of volatile flavor compounds in soy sauce using head space solid-phase microextraction and gas chromatography-mass spectrometry. Chin. J. Chromatogr. (Se Pu) 2008, 26, 285–291. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, C.; Zhao, H.; Gao, X.; Zhao, M.; Sun, W. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int. J. Food Sci. Technol. 2013, 48, 609–619. [Google Scholar] [CrossRef]
- Kesen, S.; Amanpour, A.; Sarhir, S.T.; Sevindik, O.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of aroma-active compounds in seed extract of black cumin (Nigella sativa L.) by aroma extract dilution analysis. Foods 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Buyukkurt, O. Characterization of aroma compounds of cold-pressed avocado oil using solid-phase microextraction techniques with gas chromatography–mass spectrometry. J. Raw Mater. Process. Foods 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, H.; Zhao, M.; Cui, C.; Ren, J. Comparative Study on Volatile Flavor Compounds of Traditional Chinese-type Soy Sauces Prepared with Soybean and Defatted Soy Meal. Food Sci. Biotechnol. 2009, 18, 1447–1458. [Google Scholar]
- Diez-Simon, C.; Mumm, R.; Hall, R.D. Mass spectrometry-based metabolomics of volatiles as a new tool for understanding aroma and flavour chemistry in processed food products. Metabolomics 2019, 15, 1–20. [Google Scholar] [CrossRef]
- Sun, S.Y.; Jiang, W.G.; Zhao, Y.P. Profile of volatile compounds in 12 Chinese soy sauces produced by a high-salt-diluted state fermentation. J. Inst. Brew. 2010, 116, 316–328. [Google Scholar] [CrossRef]
- Luh, B.S. Industrial production of soy sauce. J. Ind. Microbiol. 1995, 14, 467–471. [Google Scholar] [CrossRef]
- Van Der Sluis, C.; Tramper, J.; Wijffels, R.H. Enhancing and accelerating flavour formation by salt-tolerant yeasts in Japanese soy-sauce processes. Trends Food Sci. Technol. 2001, 12, 322–327. [Google Scholar] [CrossRef]
- Wanakhachornkrai, P.; Lertsiri, S. Comparison of determination method for volatile compounds in Thai soy sauce. Food Chem. 2003, 83, 619–629. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Meng, Q.; Imamura, M.; Katayama, H.; Obata, A.; Sugawara, E. Key compounds contributing to the fruity aroma characterization in Japanese raw soy sauce. Biosci. Biotechnol. Biochem. 2017, 81, 1984–1989. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Lee, S.M.; Seo, B.C.; Kim, Y.S. Volatile compounds in fermented and acid-hydrolyzed soy sauces. J. Food Sci. 2006, 71, 146–156. [Google Scholar] [CrossRef]
- Harada, R.; Yuzuki, M.; Ito, K.; Shiga, K.; Bamba, T.; Fukusaki, E. Microbe participation in aroma production during soy sauce fermentation. J. Biosci. Bioeng. 2018, 125, 688–694. [Google Scholar] [CrossRef]
- Dahlen, T.; Hauck, T.; Wein, M.; Schwab, W. From D-Fructose-l, 6-Diphosphate Metabolism by Zygosaccharomyces rouxii. J. Biosci. Bioeng. 2001, 91, 352–358. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Zhang, Y. Characterization of pyrazines in some Chinese liquors and their approximate concentrations. J. Agric. Food Chem. 2007, 55, 9956–9962. [Google Scholar] [CrossRef]
- Han, E.; Ahn, H.; Lee, S.; Lee, K.G. Analysis of volatile compounds of black bean, mung bean, and soybean extracts prepared with distillation under reduced pressure–continuous liquid–liquid extraction and hot water extraction. Chem. Biol. Technol. Agric. 2022, 9, 1–12. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Aldehydes, Ketones, and Related Compounds. In Understanding Wine Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 79–87. [Google Scholar]








| RT (a) | Compounds (b) | RIE (c) | RIL (d) | Odor Descriptors | Mean Concentration ± SD (µg/L) | |||
|---|---|---|---|---|---|---|---|---|
| SRW0 | SRW1 | SRW2 | SRW3 | |||||
| Acids | ||||||||
| 1.2867 | Acetic acid | <700 | 610 | Acidic | n.d. | 96.29 ± 4.89 | 38.46 ± 0.05 | 84.41 ± 7.44 |
| 5.0229 | Butanoic acid, 3-methyl- | 844 | 850 | Rancid | n.d. | 22.19 ± 2.35 | 4.96 ± 0.2 | 3.82 ± 0.17 |
| 5.439 | Butanoic acid, 2-methyl- | 856 | 861 | Cheesy | n.d. | 12.19 ± 0.75 | n.d. | n.d. |
| Octanoic acid | 1180 | Cheesy | n.d. | n.d. | n.d. | n.d. | ||
| Alcohols | ||||||||
| 1.485 | 1-Propanol, 2-methyl- | <700 | 624 | Wine | 0.51 ± 0.5 | n.d. | 9.62 ± 0.37 | 22.96 ± 0.74 |
| 1-Butanol | 659 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| 1-Butanol, 2-methyl- | 739 | Malty | n.d. | n.d. | n.d. | n.d. | ||
| 5.3255 | 2-Furanmethanol | 852 | 860 | Baked | n.d. | n.d. | 1.29 ± 0.09 | 2.52 ± 0.09 |
| 5.6829 | 1-Hexanol | 866 | 868 | Floral, green | 2.14 ± 0.1 | 6.68 ± 0.33 | 3.63 ± 0.09 | 20.55 ± 1.9 |
| 8.654 | 1-Heptanol | 971 | 970 | Fruity | n.d. | n.d. | n.d. | 1.32 ± 0.09 |
| 8.9007 | 1-Octen-3-ol | 979 | 980 | Mushroom | 73.12 ± 0.64 | 36.04 ± 1.05 | 5.78 ± 1.06 | n.d. |
| 9.3984 | 3-Octanol | 996 | 993 | Mushroom | 0.83 ± 0.05 | n.d. | n.d. | n.d. |
| 10.4324 | 1-Hexanol, 2-ethyl- | 1031 | 1030 | Floral | 2.76 ± 0.05 | 1.79 ± 0.07 | n.d. | n.d. |
| 10.5742 | Benzyl alcohol | 1036 | 1036 | Floral | n.d. | n.d. | 3.58 ± 0.7 | 20.65 ± 1.23 |
| 2-Octen-1-ol, (E)- | 1067 | Baked | n.d. | n.d. | n.d. | n.d. | ||
| 2-Octen-1-ol, (Z)- | 1068 | Floral | n.d. | n.d. | n.d. | n.d. | ||
| 1-Octanol | 1070 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| Phenylethyl alcohol | 1116 | Rosy, honey | n.d. | 13.12 ± 1.01 | 30.44 ± 2.7 | 103.07 ± 7.1 | ||
| Aldehydes | ||||||||
| 1.6868 | Butanal, 3-methyl- | <700 | 652 | Malty | n.d. | 9.15 ± 0.92 | 7.77 ± 1.62 | 7.85 ± 0.46 |
| 1.7881 | Butanal, 2-methyl- | <700 | 662 | Malty | n.d. | 6.04 ± 0.32 | 5.39 ± 1.35 | n.d. |
| 3.8675 | Hexanal | 789 | 801 | herbaceous | n.d. | n.d. | n.d. | 19.21 ± 1.53 |
| 4.7206 | 3-Furaldehyde | 827 | 831 | Almond-like | n.d. | n.d. | n.d. | n.d. |
| 7.7210 | Furfural | 828 | 829 | Sweet | n.d. | 0.69 ± 0.08 | n.d. | n.d. |
| Heptanal | 901 | Rancid | n.d. | n.d. | n.d. | n.d. | ||
| Methional | 907 | Mashed potato | n.d. | n.d. | n.d. | n.d. | ||
| 8.2653 | Benzaldehyde | 959 | 962 | Fruity | 2.42 ± 0.23 | 56.35 ± 1.26 | 114.53 ± 30.18 | 43.38 ± 6.4 |
| 10.8437 | Benzeneacetaldehyde | 1044 | 1045 | Honey-like | 0.15 ± 0.01 | 9.26 ± 0.53 | 11.55 ± 2.09 | 15.17 ± 1.11 |
| 11.2966 | 2-Octenal, (E)- | 1059 | 1060 | Fatty | 0.77 ± 0.07 | 0.72 ± 0.02 | n.d. | 5.87 ± 0.28 |
| 12.6861 | Nonanal | 1105 | 1104 | Fatty | n.d. | n.d. | 1.87 ± 0.18 | 6.9 ± 0.24 |
| 15.9031 | 2,4-Nonadienal, (E,E)- | 1215 | 1216 | Floral, fatty | n.d. | n.d. | n.d. | 3.21 ± 0.13 |
| Esters | ||||||||
| 1.3992 | Ethyl Acetate | <700 | 612 | Fruity | n.d. | 45.54 ± 3.17 | 97.79 ± 22.71 | 220.13 ± 13.74 |
| 2.4975 | Propanoic acid, ethyl ester | 705 | 705 | Fruity | n.d. | 0.2 ± 0.02 | n.d. | 1.07 ± 0.06 |
| Butanoic acid, ethyl ester | 802 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| Acetic acid, butyl ester | 812 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| 5.1531 | Butanoic acid, 2-methyl-, ethyl ester | 845 | 849 | Fruity | n.d. | 1.3 ± 0.79 | 2.06 ± 0.49 | 6.85 ± 0.68 |
| Butanoic acid, 3-methyl-, ethyl ester | 853 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| 5.8818 | 1-Butanol, 3-methyl-, acetate | 873 | 876 | Banana-like | n.d. | n.d. | n.d. | 1.19 ± 0.18 |
| 1-Butanol, 2-methyl-, acetate | 879 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| Hexanoic acid, methyl ester | 925 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| 9.5227 | Hexanoic acid, ethyl ester | 999 | 999 | Fruity | n.d. | n.d. | n.d. | 34.9 ± 5.91 |
| Heptanoic acid, methyl ester | 1023 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| 12.401 | Benzoic acid, methyl ester | 1095 | 1094 | Floral, honey | n.d. | 2.13 ± 0.15 | 3.13 ± 0.76 | n.d. |
| 12.5231 | Heptanoic acid, ethyl ester | 1099 | 1098 | Fruity | n.d. | n.d. | n.d. | 7.1 ± 0.95 |
| Octanoic acid, methyl ester | 1126 | Fruity | n.d. | n.d. | n.d. | n.d. | ||
| 14.6641 | Benzoic acid, ethyl ester | 1172 | 1172 | Fruity, floral | n.d. | 1.76 ± 0.21 | 12.64 ± 2.98 | 31.8 ± 3.23 |
| Benzeneacetic acid, methyl ester | 1178 | Honey-like | n.d. | n.d. | n.d. | n.d. | ||
| 15.0129 | Butanedioic acid, diethyl ester | 1184 | 1181 | Fruity | n.d. | n.d. | n.d. | 3.87 ± 0.17 |
| 15.442 | Octanoic acid, ethyl ester | 1198 | 1196 | Fruity | n.d. | n.d. | 2.32 ± 0.65 | 22.29 ± 3.44 |
| 16.8066 | Benzeneacetic acid, ethyl ester | 1247 | 1247 | Floral | n.d. | 0.7 ± 0.02 | 5.5 ± 1.21 | 12.06 ± 0.7 |
| 17.1384 | Acetic acid, 2-phenylethyl ester | 1259 | 1258 | Honey-like | n.d. | n.d. | n.d. | 1.86 ± 0.09 |
| Decanoic acid, ethyl ester | 1396 | Wax-like | n.d. | n.d. | n.d. | n.d. | ||
| Dodecanoic acid, methyl ester | 1526 | Floral | n.d. | n.d. | n.d. | n.d. | ||
| Dodecanoic acid, ethyl ester | 1594 | Wax-like | n.d. | n.d. | n.d. | n.d. | ||
| 32.7043 | Hexadecanoic acid, methyl ester | 1928 | 1926 | Wax-like | 0.45 ± 0.08 | n.d. | n.d. | n.d. |
| 34.0109 | Hexadecanoic acid, ethyl ester | 1996 | 1993 | Wax-like | n.d. | n.d. | 4.7 ± 1.06 | 41.46 ± 6.26 |
| 37.1808 | 9-Octadecenoic acid, ethyl ester | 2169 | 2141 | Floral | n.d. | n.d. | n.d. | 21.16 ± 4.33 |
| Furan(one)s | ||||||||
| 2(3H)-Furanone, dihydro-3-methyl- | 953 | Creamy | n.d. | n.d. | n.d. | n.d. | ||
| 3(2H)-Furanone, 4-hydroxy-5-methyl- | 955 | Caramel-like | n.d. | n.d. | n.d. | n.d. | ||
| 9.2361 | Furan, 2-pentyl- | 990 | 993 | Green bean | n.d. | n.d. | n.d. | 11.72 ± 1.74 |
| 2(3H)-Furanone, 5-ethyldihydro- | 1056 | Caramel-like | n.d. | n.d. | n.d. | n.d. | ||
| 2(3H)-Furanone, dihydro-5-pentyl- | 1365 | Coconut-like | n.d. | n.d. | n.d. | n.d. | ||
| Ketones | ||||||||
| 2.3098 | Acetoin | 713 | 713 | Butter-like | 0.23 ± 0.09 | n.d. | 1.71 ± 0.42 | 2.67 ± 1.16 |
| 6.2811 | 2-Heptanone | 888 | 891 | Fruity | n.d. | 0.27 ± 0.01 | 1.02 ± 0.27 | 1.26 ± 0.07 |
| Butyrolactone | 916 | Creamy | n.d. | n.d. | n.d. | n.d. | ||
| 9.1202 | 3-Octanone | 987 | 986 | Pungent | 11.82 ± 0.71 | 6.23 ± 0.12 | 10.45 ± 1.64 | n.d. |
| 26.5063 | Benzophenone | 1634 | 1635 | Rose-like | n.d. | n.d. | 0.6 ± 0.19 | 1.27 ± 0.01 |
| Phenols | ||||||||
| Phenol, 2-methoxy- | 1090 | Smoky, burnt | n.d. | n.d. | n.d. | n.d. | ||
| 14.5765 | Phenol, 4-ethyl- | 1170 | 1168 | Spicy | n.d. | 3.63 ± 0.01 | 17.74 ± 3.81 | 12.87 ± 0.51 |
| 16.0725 | 4-Vinylphenol | 1222 | 1223 | Spicy | 3.46 ± 0.08 | 2.97 ± 0.28 | n.d. | n.d. |
| 17.7484 | Phenol, 4-ethyl-2-methoxy- | 1282 | 1282 | Spicy | n.d. | 28.24 ± 0.15 | 219.16 ± 49.03 | 202.59 ± 8.39 |
| 18.7017 | 2-Methoxy-4-vinylphenol | 1317 | 1316 | Spicy | 8.2 ± 0.06 | n.d. | n.d. | n.d. |
| 23.7694 | 2,4-Di-tert-butylphenol | 1516 | 1514 | Phenol | 1.24 ± 0.12 | 1.73 ± 0.33 | 4.35 ± 2.4 | n.d. |
| Pyrazines | ||||||||
| 4.5171 | Pyrazine, methyl- | 820 | 829 | Nutty | 0.58 ± 0.22 | 15.65 ± 0.89 | 4.74 ± 0.38 | 4.63 ± 0.37 |
| 6.9912 | Pyrazine, 2,5-dimethyl- | 915 | 917 | Roasted nut | n.d. | n.d. | n.d. | 0.91 ± 0.13 |
| Pyrazine, 2,6-dimethyl- | 917 | Roasted cocoa | n.d. | n.d. | n.d. | n.d. | ||
| 7.1351 | Pyrazine, 2,3-dimethyl- | 921 | 920 | Roasted nut | n.d. | 2.91 ± 0.29 | 1.36 ± 0.16 | n.d. |
| Pyrazine, 2-ethyl-3-methyl- | 1004 | Caramel-like | n.d. | n.d. | n.d. | n.d. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, L.; Te, C.; Chanto, M.T.; Tan, R. Impact of Different Raw Materials on Changes in Volatile Compounds during Moromi Fermentation. Biol. Life Sci. Forum 2023, 26, 103. https://doi.org/10.3390/Foods2023-14962
Ly L, Te C, Chanto MT, Tan R. Impact of Different Raw Materials on Changes in Volatile Compounds during Moromi Fermentation. Biology and Life Sciences Forum. 2023; 26(1):103. https://doi.org/10.3390/Foods2023-14962
Chicago/Turabian StyleLy, Luka, Chansehakpong Te, Monychot Tepy Chanto, and Reasmey Tan. 2023. "Impact of Different Raw Materials on Changes in Volatile Compounds during Moromi Fermentation" Biology and Life Sciences Forum 26, no. 1: 103. https://doi.org/10.3390/Foods2023-14962
APA StyleLy, L., Te, C., Chanto, M. T., & Tan, R. (2023). Impact of Different Raw Materials on Changes in Volatile Compounds during Moromi Fermentation. Biology and Life Sciences Forum, 26(1), 103. https://doi.org/10.3390/Foods2023-14962






