Abstract
Chia oil is a source of α-linolenic (omega-3) fatty acid, which is known to promote human health but is highly prone to oxidation. Amylose (a polymer of α-1,4 D-glucose units) can molecularly encapsulate hydrophobic molecules, forming inclusion complexes that could potentially allow the incorporation of sensitive bioactive substances into functional foods. The evaluation of their oxidative stability is relevant to understand their behavior as delivery systems, but monitoring this parameter under real storage conditions requires long periods. In the present work, the oxidative stability of amylose-hydrolyzed chia oil inclusion complexes at 25 °C was estimated from the extrapolation of the exponential dependence of the Rancimat induction times determined at different temperatures (70–98 °C). The complexes were formed with high amylose corn starch and enzymatically hydrolyzed chia oil (10% or 20% hydrolysate/starch), with and without crystallization, using the KOH/HCl method followed by freeze-drying. The spectra of attenuated total reflectance Fourier-transform infrared spectroscopy revealed typical bands that confirmed the effective retention of chia oil fatty acids by the starch structure. The scanning electron micrographs showed that these samples were formed by irregular and porous solid particles. The induction time at 25 °C of crystallized complexes decreased with an increasing hydrolysate content, while the opposite was observed in non-crystallized complexes, as those formed with 20% hydrolysate were the ones that showed the highest stability. Although these findings should be confirmed under real storage conditions, the Rancimat results could be considered as a preliminary quick prediction of the behavior of inclusion complexes as carriers of omega-3 fatty acids.
1. Introduction
Omega-3 fatty acids are known for their multiple benefits, such as decreasing the risk of cardiovascular and inflammatory diseases and eye health promotion. Chia (Salvia hispanica L.) seed oil is a vegetable-rich source of α-linolenic acid (omega-3, ~63%). However, it is highly susceptible to oxidative deterioration, which restricts its incorporation into food products.
During the last few years, there have been carried out several research studies aimed at developing delivery systems capable of protecting sensitive bioactive compounds against deteriorating conditions (T, O2, pH, light). Among them, amylose inclusion complexes (ICs) have demonstrated suitable functional properties as potential carriers of several molecules. They are formed by amylose, the linear component of starch, which can self-assemble into a single left-handed helix, hosting the gust molecule (ligand) within its inner hydrophobic cavity [1]. In previous work, it was shown that chia oil fatty acids successfully formed inclusion complexes under different times and temperatures of crystallization [2]. Later research demonstrated that the accelerated oxidative stability of these crystallized structures depended on the initial ligand concentration [3], but information about their protective capacity under real storage conditions is still lacking. However, the evaluation of this parameter requires long periods of analysis. In this sense, the present work aimed to study the stability of crystallized and non-crystallized inclusion complexes (10% and 20% w/w ligand/starch) at 25 °C by using the Rancimat method. Moreover, the non-crystallized ICs were characterized by attenuated total reflectance Fourier-transform infrared spectroscopy and scanning electron microscopy. The results of this research would contribute to understanding the behavior of inclusion complexes as potential vehicles of omega-3 from chia oil.
2. Materials and Methods
2.1. Materials
Ingredion Inc. (Westchester, IL, USA) provided high amylose corn starch. Commercial chia oil obtained by cold pressing was purchased from Solazteca SDA S.A. (Buenos Aires, Argentina). The lipase from Candida rugosa (type VII, >700 units/mg) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All reagents used were of analytical grade.
2.2. Formation of Inclusion Complexes
Before the complex formation, the chia oil was hydrolyzed by following the procedure previously described [2]. The Candida rugosa lipase was incorporated into a chia oil-in-water emulsion (20% w/w), and the reaction was carried out for 5 h under continuous stirring at 37 °C and pH = 7. The hydrolysate obtained was separated by acidification with HCl 2N to pH < 2 and centrifugation (4000× g, 20 min). The non-crystallized inclusion complexes formed with a 10% and 20% w/w hydrolysate/starch (H/S) ratio (NC10 and NC20) were obtained according to the alkaline methodology previously described [3], but without heating the complexes at 90 °C. Additionally, for the oxidative stability test, inclusion complexes crystallized for 2 h at 90 °C with 10% and 20% w/w H/S (C10 and C20) were also formed, according to a previous protocol [3]. The ICs were freeze-dried, ground with a mortar, and sieved.
2.3. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR) of Non-Crystallized Complexes
The ATR-FTIR spectra of non-crystallized complexes were obtained using an ATR-FTIR Thermo Nicolet iS10 spectrometer (Thermo Scientific, Waltham, MA, USA). The operating conditions were a 500–4000 cm−1 wavenumber range, with 4 cm−1 spectral resolution. The results were recorded by co-adding 16 scans and analyzed using OMNIC software (version 8.3, Thermo Scientific, MA, USA).
2.4. Scanning Electron Microscopy (SEM) of Non-Crystallized Complexes
The morphology of the NC10 and NC20 samples was studied using a scanning electron microscope model MA10 (Carl Zeiss SMT Ltd., Cambridge, UK). The powdered samples were adhered to a slide covered with a thin Au/Pd film and examined at 5 kV under high vacuum conditions.
2.5. Oxidative Stability Using the Rancimat Method
The induction times (tind) of both crystallized and non-crystallized complexes were determined at 70, 80, 90, and 98 °C under 20 L air/h using a Rancimat 743 (Metrohm AG, Herisau, Switzerland) containing 0.5 g of inclusion complexes. Data were analyzed using the 743 Rancimat v1.1 software (Metrohm AG, Herisau, Switzerland). The obtained results from tind at the different temperatures were fitted to an exponential mathematical model (Equation (1)) using the OriginPro 9.0 software (OriginLab Corporation, Northampton, MA, USA). To determine the tind 25 °C, a frequently used room storage temperature, an extrapolation to 25 °C was performed:
where tind is the induction time; A and B are the coefficients of the exponential formula; and T is the temperature (°C).
3. Results and Discussion
3.1. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy (ATR-FTIR)
The ATR-FTIR spectra of the non-crystallized inclusion complexes formed with 10% and 20% w/w hydrolysate/starch ratios are shown in Figure 1. As can be seen, both samples showed bands at 2852 and 2924 cm−1, which originated from the symmetric and asymmetric vibrations of the -C-H bonds of the CH2 groups of the alkyl chains of fatty acids, respectively [4]. In addition, they also displayed a band at ~1707 cm−1, corresponding to the stretching vibration of the carbonyl (-C=O) of the acid group of fatty acids [4]. The presence of these typical bands in the IC spectra confirms the effective retention of chia fatty acids by both complexes [5], which could be present both in the internal cavity of the amylose helix and/or physically trapped in the interstitial spaces of adjacent helices [6].

Figure 1.
ATR-FTIR spectra of non-crystallized inclusion complexes formed with 10% and 20% w/w hydrolysate/starch. H/S: hydrolysate/starch ratio.
Although the hydrolyzed chia oil used as a ligand had a high percentage of polyunsaturated fatty acids (PUFAs) [3], the characteristic band at 3010 cm−1 originating from the =C-H vibration of the cis double bonds was not observed in the spectra of the ICs (Figure 1). This may be due to the retention of unsaturated fatty acids into the starch structure, which may shield their IR absorption [7].
3.2. Scanning Electron Microscopy (SEM)
The scanning electron micrographs of the ICs under study are shown in Figure 2. SEM images revealed that they are formed by solid particles of variable sizes (<500 µm). In all cases, an irregular shape and a porous surface (Figure 2) were observed, in agreement with typical micrographs of amylose–fatty acid inclusion complexes formed by the alkaline method and freeze-dried [3,8]. The obtained micrographs are similar to those of the crystallized amylose–chia oil fatty acid ICs [3], and no morphological differences were observed between the non-crystallized complexes formed with 10% or 20% H/S, indicating that neither the omission of the heating at 90 °C during the formation process nor the increase in the H/S ratio modified the morphological properties of the ICs at the microscopic level.
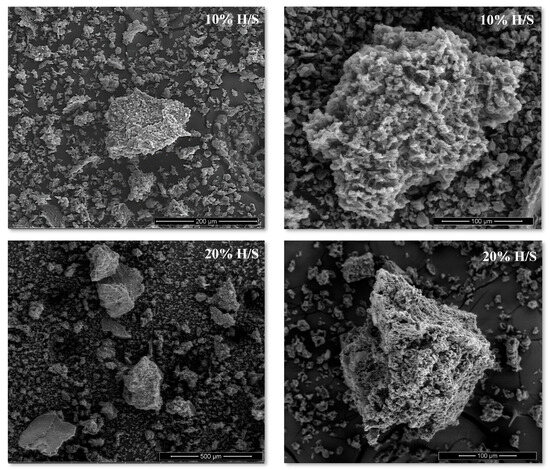
Figure 2.
Scanning electron micrographs of non-crystallized inclusion complexes formed with 10% and 20% w/w hydrolysate/starch. H/S: hydrolysate/starch ratio.
3.3. Oxidative Stability by Rancimat
The stability of inclusion complexes at 25 °C was estimated modelling the tind obtained by the accelerated Rancimat method at different temperatures. The results obtained are shown in Table 1. To assess the impact of formation temperature on the oxidative stability of complexes, the analysis also incorporated inclusion complexes (ICs) that were crystallized for 2 h at 90 °C [3]. This inclusion complements the study by enlarging the scope beyond non-crystallized ICs, which constitute the main focus of the present research. In the crystallized ICs, an increase in the H/S ratio from 10% to 20% led to a decrease in the calculated induction time at 25 °C (t25) (Table 1). This finding could be attributed to the higher percentage of PUFAs present in the C20 samples compared to C10 [3], which may be more accessible to oxygen. In contrast, the H/S increase resulted in a higher t25 in the non-crystallized ones. This is similar to the previous results of ATR-FTIR, which suggested that the higher initial hydrolysate concentration did not seem to increase the PUFA content of the non-crystallized complexes. The enhanced stability of NC20 in contrast to NC10 may stem from distinct interactions between amylose and fatty acids. Nevertheless, further investigation is required to fully elucidate these differences. Among all the ICs studied, NC20 was the one that showed the highest t25, indicating the most effective protective effect on chia oil fatty acids against oxidation. By not subjecting the formation process to heating at 90 °C, the degradation of polyunsaturated fatty acids (PUFAs) might have been avoided, potentially leading to prolonged stability of the ICs at 25 °C. However, it should be taken into account that the induction time at 25 °C obtained by Rancimat is a theoretical value that serves as a quick estimate of the oxidative stability. Therefore, these results should be validated under real storage conditions.

Table 1.
Parameters obtained from the exponential modeling of induction time vs. temperature and induction time extrapolated at 25 °C (t25) of crystallized and non-crystallized inclusion complexes formed with 10% and 20% w/w hydrolysate/starch.
4. Conclusions
According to the results of the present work, chia oil fatty acids were successfully retained in non-crystallized inclusion complexes, as verified by ATR-FTIR. These complexes were formed by irregular and porous solid particles, whose morphological properties did not vary with the different initial hydrolysate concentrations. The non-crystallized inclusion complexes formed with a 20% H/S ratio were those that showed the highest oxidative stability by the Rancimat method. Although further research is still needed, these preliminary results suggest their potential as effective carriers of chia oil essential fatty acids.
Author Contributions
Conceptualization, A.E.D.M., V.Y.I. and M.C.T.; methodology, A.E.D.M. and V.Y.I.; validation, A.E.D.M. and V.Y.I.; formal analysis, A.E.D.M. and V.Y.I.; investigation, A.E.D.M.; resources, V.Y.I. and M.C.T.; writing—original draft preparation, A.E.D.M.; writing—review and editing, V.Y.I.; visualization, A.E.D.M. and V.Y.I.; supervision, V.Y.I. and M.C.T.; project administration, M.C.T. and V.Y.I.; funding acquisition, M.C.T. and V.Y.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a grant from la ValSe-Food (119RT0567), the Universidad Nacional de La Plata (UNLP) (11/X907), the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2020-01274) (Argentina), and the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP 2007) (Argentina).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors wish to thank Ingredion Inc. for the donation of high amylose corn starch.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Putseys, J.A.; Lamberts, L.; Delcour, A.J. Amylose-inclusion complexes: Formation, identity and physico-chemical properties. J. Cereal Sci. 2010, 51, 238–247. [Google Scholar] [CrossRef]
- Di Marco, A.E.; Ixtaina, V.Y.; Tomás, M.C. Inclusion complexes of high amylose corn starch with essential fatty acids from chia seed oil as potential delivery systems in food. Food Hydrocoll. 2020, 108, 106030. [Google Scholar] [CrossRef]
- Di Marco, A.E.; Ixtaina, V.Y.; Tomás, M.C. Effect of ligand concentration and ultrasonic treatment on inclusion complexes of high amylose corn starch with chia seed oil fatty acids. Food Hydrocoll. 2023, 136, 108222. [Google Scholar] [CrossRef]
- Guillen, M.D.; Cabo, N. Infrared spectroscopy in the study of edible oils and fats. J. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Cao, Z.; Woortman, A.J.; Rudolf, P.; Loos, K. Facile synthesis and structural characterization of amylose–fatty acid inclusion complexes. Macromol. Biosci. 2015, 15, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jia, X.; Miao, S.; Chen, B.; Lu, X.; Zheng, B. Structural and thermal properties of amylose–fatty acid complexes prepared via high hydrostatic pressure. Food Chem. 2018, 264, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Hou, H.; Liu, Y.; Chen, L.; Zheng, B. In vitro digestibility and structural control of rice starch-unsaturated fatty acid complexes by high-pressure homogenization. Carbohydr. Polym. 2021, 256, 117607. [Google Scholar] [CrossRef] [PubMed]
- Zabar, S.; Lesmes, U.; Katz, I.; Shimoni, E.; Bianco-Peled, H. Structural characterization of amylose-long chain fatty acid complexes produced via the acidification method. Food Hydrocoll. 2010, 24, 347–357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).