Sub-Chronic Toxicological Evaluation of the Sesquiterpene Lactone-Enriched Fraction of Tithonia diversifolia (Hemsley) A. Gray in Experimental Rats †
Abstract
:1. Introduction
2. Experimental
2.1. Collection and Extraction of Plant Material
2.2. Fractionation and Chromatographic Separation
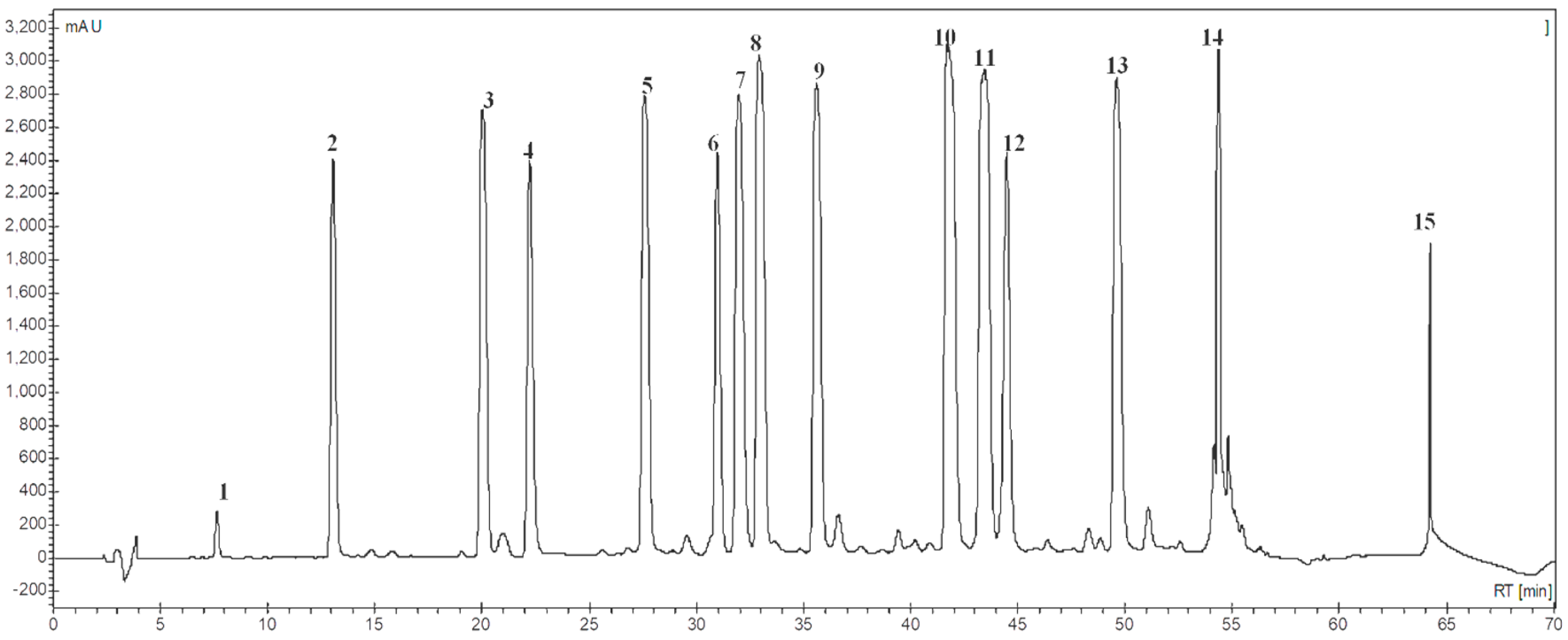
2.3. LC-MS Dereplication of the STL Fraction
2.4. Bioassay Dosing Schedule
2.5. Toxicological Evaluation
2.6. Data Analysis
3. Results and Discussion
3.1. Phytochemical Analysis
3.2. Toxicity of STL Fraction
3.3. Clinical Pathological Examination
3.4. Histopathological Examination of Liver
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ihedioha, T.E.; Asuzu, I.U.; Anaga, A.O.; Ihedioha, J.I.; Nnadi, C.O. Bioassay-guided fractionation, isolation and characterization of hepatotherapeutic 1, 3, diortho-galloyl quinic acid from the methanol leaf extract of Pterocarpus santalinoides. J. Ethnopharmacol. 2023, 301, 115864. [Google Scholar] [CrossRef] [PubMed]
- Diovu, E.O.; Ayoka, T.O.; Onah, C.M.; Nnadi, C.O. Biochemical and histological insights of 1,4-polyisoprene isolated from Sphenocentrum jollyanum Pierre (Menispermaceae) stem in wound healing activity in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2023, 307, 116248. [Google Scholar] [CrossRef]
- Onoja, S.O.; Nnadi, C.O.; Udem, S.C.; Anaga, A.O. Potential antidiabetic and antioxidant activities of a heliangolide sesquiterpene lactone isolated from Helianthus annuus L. leaves. Acta Pharm. 2020, 70, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.A.F.M.; Matos, A.F.; Volante, A.C.; Cunha, G.O.; Gualtieri, S.C. Insecticidal activity from leaves and sesquiterpene lactones of Tithonia diversifolia (Helms.) A. Gray (Asteraceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae). S. Afr. J. Bot. 2022, 144, 377–379. [Google Scholar] [CrossRef]
- Fangue-Yapseu, G.Y.; Mouafo-Tchinda, R.A.; Kenne, M.F.; Onomo, P.E.; Djocgoue, P.F. Allelopathic effect of three wild plants (Azadirachta indica, Tithonia diversifolia, and Thevetia peruviana) on tomato (Lycopersicum esculentum Mill.) growth and stimulation of metabolites involved in plant resistance. Am. J. Plant Sci. 2021, 12, 285–299. [Google Scholar] [CrossRef]
- Ayoka, T.O.; Nwachukwu, N.; Ene, A.C.; Igwe, C.U.; Nnadi, C.O. Hepatocurative and histopathological evaluations in albino rats exposed to Vitex doniana alkaloids. Lett. Appl. NanoBioSci. 2023, 12, 56. [Google Scholar]
- Osagie-Eweka, S.E.D.; Orhue, N.E.J.; Omogbai, E.K.I.; Amaechina, F.C. Oral acute and sub-chronic toxicity assessment of aqueous leaf extract of Simarouba glauca DC (Paradise tree). Toxicol. Rep. 2021, 8, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ayoka, T.O.; Nwachukwu, N.; Ene, A.C.; Igwe, C.U.; Nnadi, C.O. The hepatocurative effects of Zanthoxylum zanthoxyloides alkaloids on tetrachloromethane-induced hepatotoxicity on albino rats. Indian J. Clin. Biochem. 2022. [Google Scholar] [CrossRef]
- Alam, N.; Najnin, H.; Islam, M.; Shakya, S.; Khan, I.M.; Zaidi, R. Biochemical and histopathological analysis after sub-chronic administration of oxyresveratrol in Wistar rats. Drug Chem. Toxicol. 2021, 46, 166–175. [Google Scholar] [CrossRef]
- Baruah, N.C.; Sharma, R.P.; Madhusudanan, K.P.; Thyagarajan, G.; Herz, W.; Murari, R. Sesquiterpene lactones of Tithonia diversifolia. Stereochemistry of the tagitinins and related compounds. J. Org. Chem. 1979, 44, 1831–1835. [Google Scholar] [CrossRef]
- Onuoha, C.H.; Ala, A.A. Effects of aqueous leaf extracts of Tithonia diversifolia and Moringa oleifera on hematological, biochemical, and histopathological parameters in albino rats. J. Med. Plants Res. 2020, 14, 331–342. [Google Scholar]

| Parameter/Groups | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| AST (i/uL) | 11.85 ± 0.05 a | 11.47 ± 0.08 a | 10.90 ± 0.46 b | 11.93 ± 0.06 a |
| ALT (i/uL) | 11.77 ± 0.17 a | 11.49 ± 0.02 b | 10.92 ± 0.16 c | 11.97 ± 0.09 a |
| ALP (iu/L) | 51.79 ± 1.81 a | 47.22 ± 2.41 b | 42.76 ± 3.64 c | 53.33 ± 3.19 a |
| PCV (%) | 40.67 ± 1.15 a | 39.33 ± 1.15 a | 38.67 ± 1.53 a | 40.67 ± 1.15 a |
| RBC (×106/μL) | 7.33 ± 0.58 a | 7.43 ± 0.60 a | 6.67 ± 0.42 b | 7.50 ± 0.50 a |
| Hb (g/dL) | 10.24 ± 0.35 a | 9.91 ± 0.22 a | 9.05 ± 0.89 b | 10.47 ± 0.11 a |
| WBC (×106/μL) | 9.00 ± 0.87 a | 7.73 ± 0.50 b | 7.27 ± 0.64 b | 9.40 ± 0.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egbule, D.K.; Oji, A.P.; Nnadi, C.O. Sub-Chronic Toxicological Evaluation of the Sesquiterpene Lactone-Enriched Fraction of Tithonia diversifolia (Hemsley) A. Gray in Experimental Rats. Biol. Life Sci. Forum 2023, 24, 1. https://doi.org/10.3390/IECT2023-14801
Egbule DK, Oji AP, Nnadi CO. Sub-Chronic Toxicological Evaluation of the Sesquiterpene Lactone-Enriched Fraction of Tithonia diversifolia (Hemsley) A. Gray in Experimental Rats. Biology and Life Sciences Forum. 2023; 24(1):1. https://doi.org/10.3390/IECT2023-14801
Chicago/Turabian StyleEgbule, Daniel K., Akudo P. Oji, and Charles O. Nnadi. 2023. "Sub-Chronic Toxicological Evaluation of the Sesquiterpene Lactone-Enriched Fraction of Tithonia diversifolia (Hemsley) A. Gray in Experimental Rats" Biology and Life Sciences Forum 24, no. 1: 1. https://doi.org/10.3390/IECT2023-14801
APA StyleEgbule, D. K., Oji, A. P., & Nnadi, C. O. (2023). Sub-Chronic Toxicological Evaluation of the Sesquiterpene Lactone-Enriched Fraction of Tithonia diversifolia (Hemsley) A. Gray in Experimental Rats. Biology and Life Sciences Forum, 24(1), 1. https://doi.org/10.3390/IECT2023-14801






