Identifying Environmental Refuges (“Coldspots”) from Infection by Batrachochytrium Dendrobatidis of Amphibians in Eastern Europe †
Abstract
:1. Introduction
2. Materials and Methods
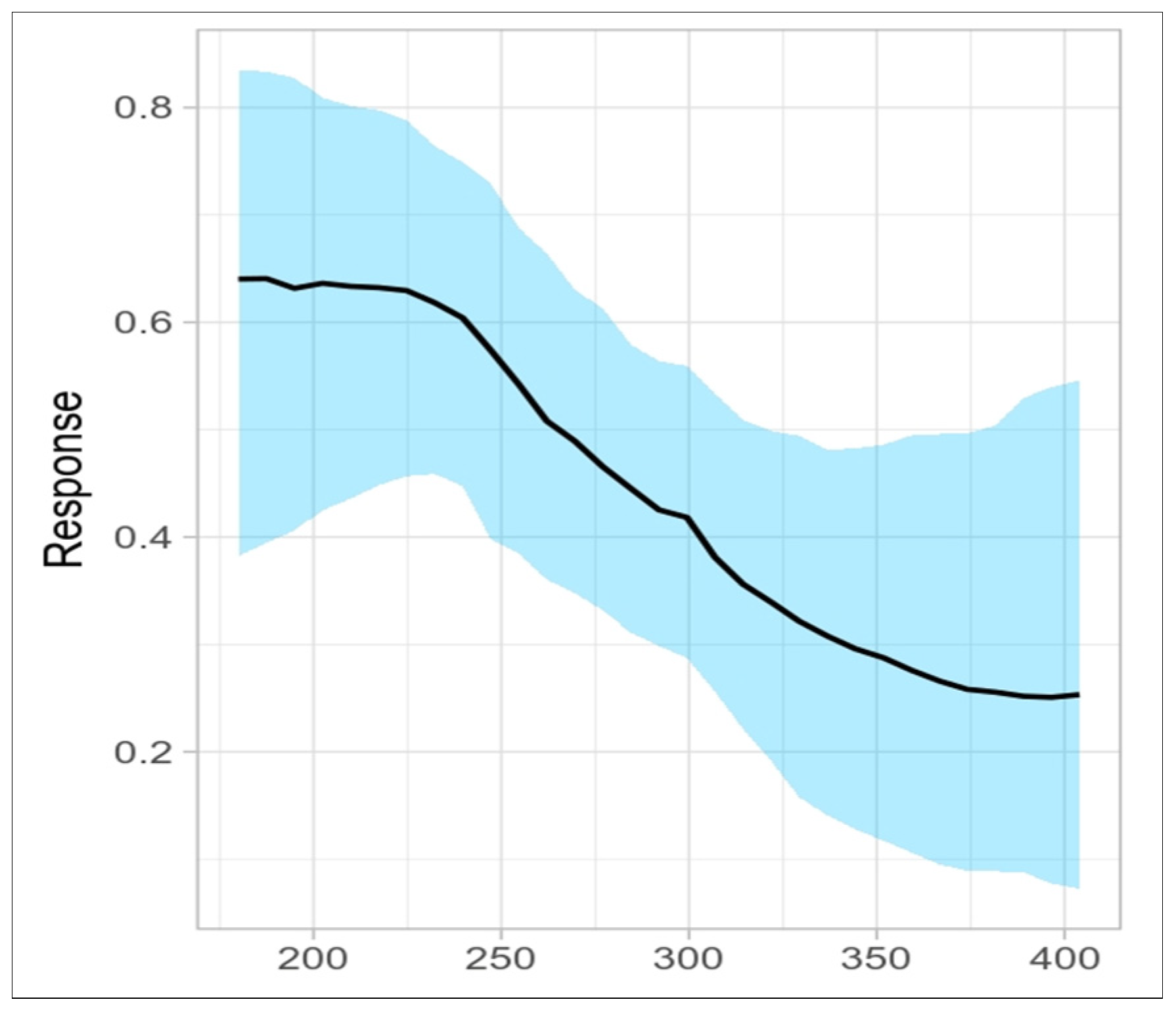
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SDM | species distribution model |
| BART | Bayesian additive regression trees |
| Bd | Batrachochytrium dendrobatidis |
| TSS | true skills statistic |
| AUC | area under the receiver operator curve |
References
- The International Union for Conservation of Nature (IUCN). Conservation International; NatureServe. An Analysis of Amphibians on the 2008 IUCN Red List. 2008. Available online: www.iucnredlist.org/amphibians (accessed on 21 July 2020).
- Scheele, B.C.; Pasmans, F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; De la Riva, I.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Garner, T.W.J.; Walker, S.F. Global Emergence of Batrachochytrium dendrobatidis and Amphibian Chytridiomycosis in Space, Time, and Host. Annu. Rev. Microbiol. 2009, 63, 291–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rödder, D.; Kielgast, J.; Bielby, J.; Schmidtlein, S.; Bosch, J.; Garner, T.W.; Veith, M.; Walker, S.; Fisher, M.C.; Lötters, S. Global amphibian extinction risk assessment for the panzootic chytrid fungus. Diversity 2009, 1, 52–66. [Google Scholar] [CrossRef]
- Liu, X.; Rohr, J.R.; Li, Y.M. Climate, vegetation, introduced hosts and trade shape a global wildlife pandemic. Proc. Biol. Sci. 2013, 280, 20122506. [Google Scholar] [CrossRef] [PubMed]
- Zumbado-Ulate, H.; García-Rodríguez, A.; Vredenburg, V.T.; Searle, C. Infection with Batrachochytrium dendrobatidis is common in tropical lowland habitats: Implications for amphibian conservation. Ecol. Evol. 2019, 9, 4917–4930. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.A.; Tasse Taboue, G.C.; Ekane MM, P.; Robak, M.; Sesink Clee, P.R.; Richards-Zawacki, C.; Fokam, E.B.; Fuashi, N.A.; Anthony, N.M. Distribution modeling and lineage diversity of the chytrid fungus Batrachochytrium dendrobatidis (Bd) in a central African amphibian hotspot. PLoS ONE 2018, 13, e0199288. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.A.; Palen, W.J.; Mooers, A.Ø. Amphibian species traits, evolutionary history and environment predict Batrachochytrium dendrobatidis infection patterns, but not extinction risk. Evol Appl. 2017, 10, 1130–1145. [Google Scholar] [CrossRef] [PubMed]
- Schatz, A.M.; Kramer, A.M.; Drake, J.M. Accuracy of climate-based forecasts of pathogen spread. R. Soc. Opensci. 2017, 4, 160975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martinez-Meyer, E.; Nakamura, M.; Araújo, M. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011; p. 314. [Google Scholar]
- Hulleman, W.G.; Vos, R.A. Modeling Abiotic Niches of Crops and Wild Ancestors Using Deep Learning: A Generalized Approach. bioRxiv 2019, 826347. [Google Scholar] [CrossRef]
- Carlson, C.J. ‘embarcadero’: Species distribution modelling with Bayesian additive regression trees in R. Methods Ecol. Evol. 2020, 11, 850–858. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Env. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. The climate of North America according to a new classification. Geogr. Rev. 1931, 21, 633–655. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An approach toward a rational classification of climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Imhoff, M.L.; Bounoua, L. Exploring Global Patterns of Net Primary Production Carbon Supply and Demand Using Satellite Observations and Statistical Data. J. Geophys. Res. 2006, 111, S12. [Google Scholar] [CrossRef]
- Krausmann, F.; Erb, K.H.; Gingrich, S.; Haberl, H.; Bondeau, A.; Gaube, V.; Lauk, C.; Plutzar, C.; Searchinger, T.D. Global human appropriation of net primary production doubled in the 20th century. Proc. Natl. Acad. Sci. USA 2013, 110, 10324–10329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, P.W.; Brawner, M.D.; Raffel, T.R.; Rohr, J.R.; Olson, D.H.; Blaustein, A.R. Shifts in temperature influence how Batrachochytrium dendrobatidis infects amphibian larvae. PLoS ONE 2019, 4, e0222237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowski, J.S.; Annis, S.L.; Longcore, J.E. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia 2004, 96, 9–15. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tytar, V.; Nekrasova, O.; Pupins, M.; Skute, A.; Marushchak, O.; Čeirāns, A.; Kozynenko, I. Identifying Environmental Refuges (“Coldspots”) from Infection by Batrachochytrium Dendrobatidis of Amphibians in Eastern Europe. Biol. Life Sci. Forum 2021, 2, 36. https://doi.org/10.3390/BDEE2021-09505
Tytar V, Nekrasova O, Pupins M, Skute A, Marushchak O, Čeirāns A, Kozynenko I. Identifying Environmental Refuges (“Coldspots”) from Infection by Batrachochytrium Dendrobatidis of Amphibians in Eastern Europe. Biology and Life Sciences Forum. 2021; 2(1):36. https://doi.org/10.3390/BDEE2021-09505
Chicago/Turabian StyleTytar, Volodymyr, Oksana Nekrasova, Mihails Pupins, Arturs Skute, Oleksii Marushchak, Andris Čeirāns, and Iryna Kozynenko. 2021. "Identifying Environmental Refuges (“Coldspots”) from Infection by Batrachochytrium Dendrobatidis of Amphibians in Eastern Europe" Biology and Life Sciences Forum 2, no. 1: 36. https://doi.org/10.3390/BDEE2021-09505
APA StyleTytar, V., Nekrasova, O., Pupins, M., Skute, A., Marushchak, O., Čeirāns, A., & Kozynenko, I. (2021). Identifying Environmental Refuges (“Coldspots”) from Infection by Batrachochytrium Dendrobatidis of Amphibians in Eastern Europe. Biology and Life Sciences Forum, 2(1), 36. https://doi.org/10.3390/BDEE2021-09505






