Abstract
The host selection behavior is essential to studies of plant–insect interaction, considered as a critical step to populations maintenance since it directly influences offspring development. This work describes the sexual and oviposition behavior of the invasive species Ceratitis capitata (Wied.) (Diptera: Tephritidae) in cashew apple (Anacardium occidentale L.). The results showed that from 5.645 behavioral patterns registered, for males and females, in the tests with papaya, mango, cashew apple, sprayed papaya extract, and sprayed mango extract, 3.719 were activities displayed by the males and 1.935 displayed by the females. As regards the female species, the walking activity on cashew apples differed between the morning and afternoon shifts (4.3 ± 2.58 and 1.5 ± 1.22). The oviposition behavior in mango fruits (11.16) differed from all the other treatments, except from papaya (6.38). However, the quantity of obtained adults was higher in papaya fruits (97) than in mango fruits (49), reducing with papaya (48) and mango (24) treatments exposed to the cashew apple extract. There are differences in the total number of obtained adults by treatment, showing that the cashew extract reduces the total number of adults obtained in papaya and mango treatments. The results obtained in this research are essential for advances in studies related to chemical ecology and behavior of Mediterranean fruit flies in semiarid fruits.
1. Introduction
Mating and host selection behavior of males and females are important for the investigations related to plant–insect interaction since they are directly influenced by the presence of plants in the area by chemical and physical signs [1,2,3].
The selection of host fruit for Ceratitis capitata (Wied.) (Diptera: Tephritidae) is displayed by the females, as it is a critical stage to the population survival by directly influencing the brood development [4]. In general, several hosts are evaluated in relation to the C. capitata females before the oviposition [5]. The oviposition behavior can be divided into four steps: (1) arrival, a stage when the insect seeks the most suitable fruit to lay the eggs, relying on visual stimuli, such as color, format, and size; (2) search, during which chemical and physical features of the fruit is analyzed; (3) puncture, a stage when the insect introduces the ovipositor in the fruit, not necessarily laying the eggs; (4) dragging, which occurs after the oviposition, when the female releases a signaling pheromone, in order for any other female to perceive that the fruit is already colonized [6]. The host selection occurs from the evaluation of different aspects, inasmuch as some fruit and plant characteristics can influence the host acceptance or rejection, which include the maturation stage of the fruit, phenological stage, and volatile production of the plant [7]. Size, color, form of the fruit, and the place where they are located in the plant also act as visual clues to localize and evaluate the fruit, supporting the choice of the more suitable ones for the oviposition [8].
The reports of C. capitata infesting cashew apple fruits in field conditions are rare, especially when focused on semiarid regions. The presence of allelopathic components, negatively acting in the adults and larvae, can influence the low collection of C. capitata colonized fruits in the field. Thus, it is necessary to invest in studies about the acceptance of such pseudo-fruit to the oviposition of C. capitata, based on a hypothesis that there is a negative influence from the fruit that leads the female to other hosts. In this way, we aimed to verify the sexual and oviposition behavior of C. capitata in cashew apple fruits, and the interference of such fruits in the behavior of males.
2. Experiments
Experiment characterization. Tests were carried out with fruit fly couples (C. capitata), Vienna 8 strain, between 10 and 12 days (the stage when the adults are sexually mature) in order to evaluate their behavior. To this end, visual notes were collected over 12 h in tests. Mature fruits were used from three species: cashew apple (Anacardium occidentale L.); mango (Mangifera indica L.); papaya (Carica papaya L.).
The fruit flies were obtained from the previous rearing ([9], Methodology) in the Applied Entomology Laboratory from the Federal University of Semiarid (UFERSA), Brazil, where all the experiments were also carried out.
Tests. Tests were prepared with cashew apple fruits, papaya, and mango without any type of treatment, and another set of tests with papaya and mango sprayed with cashew apple fruit extract. The essays were conducted in plastic cages (60 cm in all dimensions). For the treatment essays, before the introduction of the fruits in the cages, they were sprayed with cashew apple juice, previously squeezed and stored in 500 mL plastic containers. Then, the papaya and mango fruits received 2 mL of such extract, in a way where all the fruit surfaces were covered. From this point on, the fruits were only introduced in the cage after the extract was completely dried.
Inside each cage, artificial food, water, and five fruits were offered. Three couples of C. capitata were then released per fruit, amounting to 15 couples in total in each cage. They were separated 24 h before the beginning of the experiment. After the acclimatization of the couples in the cages, behavior data were collected every 30 min, for 12 h, totaling 24 observations per repetition. Each observation lasted 3 min. The first observations occurred at 06:30 and finished at 18:00. The essays were conducted in controlled conditions (25° + 2°, 60% relative humidity, and 12 h of phtophase).
All the activities of males and females were counted and registered. The main activities were observed as follows: feeding, water consumption, attraction (toward the males), courtship, mating, walking on the fruit, and oviposition (toward the females). Activities in which the insects repeatedly presented the proboscides, touching the food and water container, were considered as feeding and water consumption activity. As regards the attraction behavior, it was checked if the males were releasing a drop of pheromone; as courtship behavior, the moment when males and females were interacting, when there is the partner choice to, eventually, mate, was observed. The flies that walked on the cage surface without any interaction with other individuals or were simply inactive were registered as rest.
After the last observation, and in order to allow the larval development, the fruits were removed from the cages, stored in plastic containers, filled with a thin layer of vermiculite, simulating the soil condition. After 12 days, the vermiculite was removed and was searched for late pupae and larvae in the fruits. Then, adults were counted after the emergence.
Data analysis. The individual behavioral patterns were evaluated for each treatment, dividing them into shifts. The morning shift included the activities observed in the first period (06:30–12:00), while the afternoon shift was considered the rest of the time (13:30–18:00). Initially, the confidence interval of the mean per shift was observed, followed by the P value. Then, a t-test was performed for mean comparison, discriminating which activities/treatments had differences between morning and afternoon shifts.
Regarding the evaluation of mean comparison for the behavioral patterns between the treatments, initially the Shapiro–Wilk test was used to check if the data is normally distributed. After confirming data normality, an F test (ANOVA) was applied, running the global mean comparison. Then, the Tukey test was applied for multiple comparisons. On the results that did not present data normality, Kruskal–Wallis tests were performed, thus comparing if the distribution functions were the same for all the treatments tested. In these cases, the Dunn test with Bonferroni correction was performed for multiple comparisons. In the comparison of adults obtained per each treatment, a chi-squared test was used, evaluating if data frequencies were equal. In the case of null hypothesis rejection, multiple comparisons were made in column pairs. For all analyses, it was adopted a significance level of 5%. The statistical methods were performed in R [10] and the graphs were constructed in Excel.
3. Results
In total, 5654 activities for males and females were recorded in papaya, mango, cashew apple fruits, as well as papaya and mango sprayed with cashew extract. From this number, 719 were displayed by the males and 1935 by females (Figure 1 and Figure 2). The treatment that presented a higher quantity of activities was mango (1388), with 868 recordings for males and 520 for females, followed by the cashew apple treatment (1297—males: 836; females: 461), papaya (1206—males: 741; females: 465), sprayed mango (899—males: 647; females: 252), and sprayed papaya (864—males: 627; females: 237). For all treatments, there was a reduction in activities during the first hours of the afternoon shift, presenting an increase after 15 p.m. (15:00). Such variation was higher for the males when compared with the females.
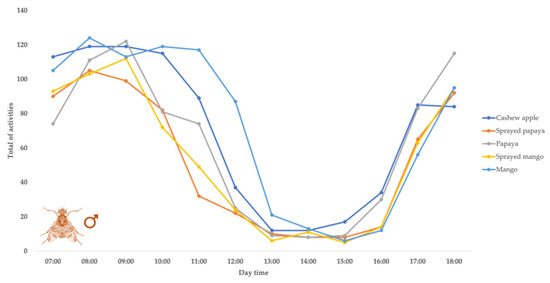
Figure 1.
Behavioral pattern distribution of Ceratitis capitata males displayed in 12 h light with different oviposition means, by no-choice tests in 25 ± 2 °C temperature and 60% RU environment.
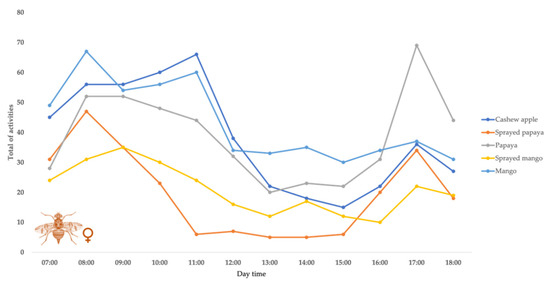
Figure 2.
Behavioral pattern distribution of Ceratitis capitata females displayed in 12 h light with different oviposition means, by no-choice tests in 25 ± 2 °C temperature and 60% RU environment.
As regards the males, the most frequent behavior was attraction, showing more than 50% of all daily activities displayed by this group. This behavior was generally observed in the upper part of the cage. On the other hand, for the females, the most frequent activity was courtship (30%). In this case, the only exception was for papaya, which had the highest number of recordings of walking on the fruit (30.11%).
As regards the females, the main activity was courtship. After such behavior, the walking behavior on the fruits occurred with high frequency in the cases of papaya and mango with natural treatments, as well as for the sprayed papaya. Following these activities was feeding for cashew apple and sprayed mango treatments.
Next, whether the behavioral patterns for each treatment would be different between the shifts was evaluated. It was observed that for cashew apple fruit treatment, in relation to females, there was a mean difference between the morning and afternoon shifts, respectively, regarding water consumption, courtship, and walking on the fruit. For the males tested in such treatment, there was a difference between the shifts in terms of attraction and courtship. In all cases, the higher values of recorded activities were for the morning shift (Table 1).

Table 1.
Comparative analysis of behavioral patterns (mean + SD) between shifts (morning and afternoon) for females and males of Ceratitis capitata displayed in 12 h light with different oviposition means, by no-choice tests in 25 ± 2 °C temperature and 60% RU environment.
In the papaya fruit treatment, there was a difference for females only in courtship behavioral patterns, with means for morning and afternoon shift, respectively, values of 11 ± 1.1 e 6.5 ± 2.35, while the difference between shifts for the male insects occurred in attraction and courtship behaviors. On the other hand, for the sprayed papaya fruit, the behavioral patterns that did not occur to an equivalent extent for the females were courtship and mating, with mean values for the morning and afternoon shifts, respectively. In male insects, besides mating, there were three patterns that showed differences between shifts: feeding, attraction, and courtship.
The behavioral patterns were also compared among the treatments. As regards the females, the feeding pattern was higher in the cashew apple treatment; there was a difference between this treatment and the patterns found in sprayed papaya and mango, with no difference among the other interactions. Regarding the water consumption pattern, a difference between the cashew apple treatment and other treatments was observed. A similar result was found for courtship pattern in the cashew apple treatment, differing from the other treatments, which did not present differences among them. The mating behavior differed between natural mango and sprayed mango treatments, as well as mango and cashew apple treatments. The papaya in natural treatment and sprayed papaya did not present a difference from the others. On the other hand, regarding the walking behavior on papaya, it presented differences from the cashew apple, sprayed papaya, and sprayed mango treatments but did not differ from the treatment with mango. The other treatments did not present statistical differences among them (Table 2).

Table 2.
Comparative analysis of behavioral patterns (mean + SD) of females and males of Ceratitis capitata displayed in 12 h light with different oviposition means, by no-choice tests in 25 ± 2 °C temperature and 60% RU environment.
As regards the oviposition behavioral pattern, the treatment with mango (11.16 ± 3.71) differed from all the other treatments, except papaya (6.83 ± 3.12). This only differed from the cashew apple treatment, in which oviposition was not observed. The sprayed papaya (1.33 ± 1.63) treatment differed from cashew apple and mango treatments, while the sprayed mango (1.50 ± 1.64) only differed from the mango with natural treatment, not presenting, therefore, significant differences from the other treatments.
Regarding the development of flies in the tested fruits, 218 adults were counted, with a higher quantity obtained from papaya fruits (97), followed by mango (49), sprayed papaya (48), and sprayed mango (24). No adults were obtained from the cashew apple fruits. Based on the comparison of total adults per treatment, papaya treatment differed from the others in terms of insect development. The mango fruit treatment did not differ from the sprayed papaya, presenting a significant difference (aside from papaya) from the sprayed mango. The sprayed papaya presented a difference (aside from papaya) from the sprayed mango treatment.
4. Discussion
The behavioral patterns of fruit flies have been studied, with the purpose of helping to control such pests, since they reveal the pests’ daily carried-out activities. On the other hand, these patterns show the times when and under which conditions the flies display certain activities, making pest control more efficient and rational. These behaviors depend on biotic and abiotic factors, such as the presence of natural enemies, population density, temperature, humidity, and host availability, also acting on population regulation [11,12,13].
The pattern of walking on the fruit is linked to the evaluation of its qualities by the females, which perform punctures to assess the condition of the fruit and whether or not it is suitable for laying eggs [1,6,14]. There was a reduction in activities of the females in treatments with sprayed fruits, suggesting a negative effect on the action of walking and oviposition, as papaya and mango fruits with natural treatments presented greater acceptability by females.
In general, insects that have a phytophagous feeding habit with a wider range of hosts have adaptive advantages, with some hosts considered as primary or preferential, which present better conditions for the development of offspring; however, in the absence of these hosts, others are explored, also called secondary [15]. The oviposition activity is directly linked to the availability of these hosts in the area and also to the type of host (primary or secondary).
A preference of females for the tested exotic fruits (papaya and mango) with natural treatment was also observed. The results of the present study reveal cashew apples as a non-preferred host for C. capitata; despite having records of development in this cashew apples in the field (usually with few specimens collected), females avoid laying eggs when exposed only to this fruit, and when exposed to conditions allowing for choice selection, they preferred exotic hosts [16,17].
For oviposition, there was a preference for fresh mango and papaya fruits, followed by treatments with the same fruits exposed to cashew extract, suggesting that this exposure reduced the attractiveness of the fruits. No oviposition was observed in cashew fruits. The hierarchy of preference of C. capitata for hosts was observed in two distinct populations, with papaya as the preferred host among the tested fruits, followed by mango, orange, and apple fruits [18].
Trawling activity was not observed in the present study. However, the occurrence of this phenomenon is directly linked to the oviposition [6]. In natural conditions, two peaks of oviposition were observed for C. capitata: The smallest peak recorded in the early hours of the morning (20%) and the second peak, in the late afternoon (71%) [19]. This activity is related to the release of pheromones that the females use to signal that the fruit is infested, causing other females to seek other hosts to lay their eggs [6]. This behavior is observed in several studies with several species of tephritid and is known to be closely related to the size of the fruit and also to the intra- or interspecific competition of larvae [14]. The relationship between the number of offspring and the size of the fruit or local-host density has been revealed in several studies, e.g., [20,21,22].
It was observed that males and females showed more frequent activities in the first hours and in the late afternoon, oscillating during the 12 h of light. Records of rest/inactivity were more frequent for females than for males. However, in all treatments, this phenomenon represented the largest portion observed for both sexes, with a high difference from the other observed behaviors, which was not used for the analyses. This pattern was observed by [19] in studies on apple and fig orchards, differing between sex and the place of occurrence (fruit for females and leaves for males). Any activity can be attributed as a response to some stimulus; thus, the females initially tend to remain inactive until the perception of the stimuli emitted by the males or by the fruits.
In order to carry out oviposition, some barriers must be overcome. The peel is a physical barrier, configuring itself as a limiting factor and a possible primary characteristic in the evaluation of the fruits by the females. This is mainly due to the size of the ovipositor, which may not be large enough to overcome this barrier, characterizing the fruit as not suitable for laying eggs [23]. The fruits studied in the present investigation (papaya, mango, and cashew apple) did not have thick skins, and therefore, this factor could not be considered as influencing the results. Among them, mango had the greatest thickness. Nonetheless, for this fruit (without the treatment with the cashew extract), the highest levels of oviposition (11.16 ± 3.71) were observed, showing that this factor did not determine the occurrence of such activity in the results. Although the preference of exotic fruits for C. capitata is reported, species of Anastrepha have a preference for native fruits, showing coevolutionary adaptations between these species and their hosts [24]. In tests with tropical fruits, using the same strain of C. capitata from the present study, a preference for oviposition was observed in guava (Psidium guajava L.), soursop (Annona muricata L.), and acerola (Malpighia glabra L.) fruits, followed by carambola (Averrhoa carambola L.), Malay apple (Syzygium malaccensis L.), cashew, and yellow cajá (Spondias mombin L.). The oviposition behavior was attributed to a preference associated with the nutritional value of the fruit, providing optimal conditions for the development of the offspring [25].
In studies that evaluated the compounds present in cashew, it was possible to observe a large quantity of ethanol, since this fruit undergoes fermentation very quickly. However, when fruit-based baits were tested in the field, no differences were found in the attractiveness between cashew, hydrolyzed protein, mango, and papaya baits for the first two days tested, showing differences between the protein and all others based on fruit on the seventh day of exposure [26,27].
It was observed that the activity of attracting males preferably occurs in the early morning, showing a reduction in the following hours. The activity increases again in the late afternoon, differing between shifts for all evaluated treatments. Similar results, with the same fruit flies species, were found in natural environment conditions, presenting two peaks throughout the day: a larger peak in the morning and another in the late afternoon [19,28]. The same phenomenon is registered for Anastrepha fraterculus (Wiedemann), which, in semi-field conditions, showed higher activity between 07:00 and 11:00, whereas males of Anastrepha obliqua (Macquart) preferentially displayed the activities between 13:00 and 19:00 [29]. In subsequent studies, a study [30] showed the highest frequency of sexual activity of A. obliqua in the afternoon, with the time between 15:30 and 16:30 as the preferred time for courtship and mating. The daytime behavioral pattern for Anastrepha zenildae Zucchi is also known [31].
From the cashew apple fruits, emergences were not recorded. These results suggest that cashew does not represent a host with suitable characteristics for females, and the extract sprayed on the exotic fruits, which are its preferred hosts, has a negative influence on the oviposition behavior, reducing its frequency when compared with fresh fruits. In tests involving the effect of eight tropical fruits on the biology and behavior of C. capitata, it was observed that guava, star fruit, and soursop fruits showed the best performance regarding the biological parameters evaluated, with the cycle varying from 14 to 18 days, while fruits of cashew and acerola showed regular development with a cycle of 19 to 20 days [25].
5. Conclusions
Cashew fruits are not attractive to C. capitata females, with no record of oviposition and development in the tested conditions. Males displayed the attraction behavior preferentially in the early hours of the day, which is not influenced by the available fruit but by courtship, treated here as the moment of interaction between males and females when there is the choice of mate for copulation, differing between the morning and afternoon shifts and also between treatments with different fruits. Papaya fruits had a higher number of offspring. Cashew extract, when sprayed on fruits that are the preferred hosts for females (papaya and mango), reduced the frequency of total activities, with less oviposition, and, consequently, gave rise to fewer adults than fresh fruits. The results obtained in this research are essential for advances in studies related to chemical ecology and behavior of Mediterranean fruit flies in semiarid fruits.
Author Contributions
R.d.S.B. undertook the statistical analysis. All the other authors conceived and designed the experiments, performed the experiments, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordination of Improvement of Higher Education Personel (CAPES-Brazil).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bernays, E.A.; Chapman, R.F. Host-Plant Selection by Phytophagous Insects; Chapman and Hall: New York, NY, USA, 1994; 312p. [Google Scholar]
- Joachim-Bravo, I.S.; Guimarães, A.N.; Magalhães, T.C. Influência de substâncias atrativas no comportamento alimentar e na preferência de oviposição de Ceratitis capitata (Diptera: Tephritidae). Sitientibus Ser. Ci. Biol. 2001, 1, 60–65. [Google Scholar]
- Loaiza, J.C.M.; Céspedes, C.L. Compuestos volatiles de plantas. Origen, emission efectos, análisis y aplicaciones. Rev. Fitotec. Mex. 2007, 30, 327–351. [Google Scholar]
- Sugayama, R.L.; Kovaleski, A.; Liedo, P.; Malavasi, A. Colonization of a new fruit crop by Anastrepha fraterculus (Diptera: Tephritidae) in Brasil: A demografhic analysis. Environ. Entomol. 1988, 27, 642–648. [Google Scholar] [CrossRef]
- Zucchi, R.A. Taxonomia, Moscas-das-Frutas de Importância Econômica no Brasil: Conhecimento Básico e Aplicado; Malavasi, A., Zucchi, R.A., Eds.; Holos: Ribeirão Preto, Brazil, 2000; pp. 13–24, 327. [Google Scholar]
- Sugayama, R.L.; Malavasi, A. Ecologia Comportamental. In Moscas-Das-Frutas de Importância Econômica no Brasil: Conhecimento Básico e Aplicado; Malavasi, A., Zucchi, R.A., Eds.; Holos: Ribeirão Preto, Brazil, 2000; pp. 103–108, 327. [Google Scholar]
- Szentesi, A.; Greany, P.D.; Chambers, D.L. Oviposition behavior of laboratory-reared and wild Caribbean fruit flies (Anastrepha suspensa; Diptera: Tephritidae): I. Selected chemical influences. Entomol. Exp. Appl. 1979, 26, 227–238. [Google Scholar] [CrossRef]
- De Azevedo, F.R.; Santos, C.A.M.; Nere, D.R.; Moura, E.S.; Azevedo, R. Influência da cor e forma dos frutos artificiais e quadrantes da copa da goiabeira sobre a atração de Anastrepha spp. para oviposição. Rev. Cient. Elet. Agro. 2013, 23, 34–45. [Google Scholar]
- Zucoloto, F.S. Feeding habits of Ceratitis capitata: Can larvae recognize a nutritional effective diet? J. Insect Physiol. Oxf. 1987, 33, 349–353. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2013; Available online: http://www.R-project.org (accessed on 15 September 2016).
- Silveira-Neto, S.; Nakano, O.; Barbin, D.; Villa Nova, N.A. Manual de Ecologia dos Insetos; Ceres: Piracicaba, Brazil, 1976; 419p. [Google Scholar]
- Celedonio, H.; Aluja, M.; Liedo, P. Adult population of Anastrepha species (Diptera: Tephritidae) in tropical orchard habitats of Chiapas, Mexico. Environ. Entomol. 1995, 24, 861–869. [Google Scholar] [CrossRef]
- Aluja, M.; Ordano, M.; Guillén, L.L.; Rull, J. Understanding long-term fruit fly (Diptera: Tephritidae) population dynamics: Implications for areawide management. J. Econ. Entomol. 2012, 105, 823–836. [Google Scholar] [CrossRef]
- Papaj, D.R.; Averill, A.L.; Prokopy, R.J.; Wong, T.T.Y. Host-marking pheromone and use of previously established oviposition sites by the Mediterranean fruit fly (Diptera: Tephritidae). J. Insect. Behav. 1992, 5, 583–598. [Google Scholar] [CrossRef]
- Fitt, G.P. The influence of a shortage of host on the specificity of oviposition behavior in species of Dacus (Diptera: Tephritidae). Physiol. Entomol. 1986, 11, 133–143. [Google Scholar] [CrossRef]
- Canal, N.A.D.; Alvarenga, C.D.; Zucchi, R.A. Análise faunística de espécies de moscas-das-frutas (Diptera: Tephritidae) em Minas Gerais. Sci. Agric. 1998, 55, 15–24. [Google Scholar] [CrossRef]
- Pirovani, V.D.; Martins, D.S.; Souza, S.A.S.; Uramoto, K.; Ferreira, P.S.F. Moscas-das-frutas (Diptera: Tephritidae), seus parasitoides e hospedeiros em viçosa, zona da mata mineira. Arq. Inst. Biol. 2010, 77, 727–733. [Google Scholar] [CrossRef]
- Joachim-Bravo, I.S.; Silva-Neto, A.M. Aceitação e preferência de frutos para oviposição em duas populações de Ceratitis capitata (Diptera: Tephritidae). Iheringia Sér. Zool. 2004, 94, 171–176. [Google Scholar] [CrossRef][Green Version]
- Hendrichs, J.; Hendrichs, M.A. Mediterran fruit fly (Diptera: Tephritidae) in nature: Location and diel pattern of feeding and other activitis on fruiting nonfruittting host and nonhost. Ann. Entomol. Soc. Am. 1990, 83, 632–641. [Google Scholar] [CrossRef]
- Pilson, D.; Rausher, M.D. Clutch size adjustment by a swalllowtail butterfly. Nature 1988, 333, 361–363. [Google Scholar] [CrossRef]
- Lemasurier, A.D. Costs and benefits off egg clustering in Pieris brassicae. J. Anim. Ecol. 1994, 63, 677–685. [Google Scholar] [CrossRef]
- Fox, C.W.; Martin, J.D.; Thakar, M.S.; Mousseau, T.A. Clutch size manipulations in two seed beetles: Consequences for progeny fitness. Oecologia 1996, 108, 88–94. [Google Scholar] [CrossRef]
- Hickel, E.R. Espessura da polpa como condicionante do parasitismo de moscas-das-frutas (Diptera: Tephritidae) por Hymenoptera: Braconidae. Ciênc. Rural 2002, 32, 1005–1009. [Google Scholar] [CrossRef]
- Malavasi, A.; Morgante, A.J. Biologia de “moscas-das-frutas” (Diptera, Tephritidae). I. Índices de infestação em diferentes hospedeiros e localidades. Rev. Bras. Biol. 1980, 40, 17–24. [Google Scholar]
- Costa, A.M.; Amorim, F.O.; Anjos-Duarte, C.S.; Joachim-Bravo, I.S. Influence of different tropical fruits on biological and behavioral aspects of the Mediterranean fruit fly Ceratitis capitata (Wiedemann) (Diptera, Tephritidae). Rev. Bras. Entomol. 2011, 55, 355–360. [Google Scholar] [CrossRef]
- Garruti, D.S.; Franco, M.R.B.; Silva, M.A.A.P. Evaluation of volatile compounds from cashew apple (Anacardium occidentale L.) juice by the osme gas chromatography/olfactometry techinique. J. Sci. Food Agric. 2003, 83, 1455–1462. [Google Scholar] [CrossRef]
- Medeiros, M.A.A. Atratividade de Iscas Alimentares na Captura de Insetos em Armadilhas McPhail; Tese de doutorado, UFERSA: Mossoró, Brazil, 2009; 101p. [Google Scholar]
- Whittier, T.S.; Kaneshiro, K.; Prescott, L.D. Mating behavior of mediterranean fruit flies (Diptera: Tephritidae) in natural enviroment. Ann. Entomol. Soc. Am. 1992, 85, 214–218. [Google Scholar] [CrossRef]
- Malavasi, A. Estudo de Duas Espécies Crípticas do Gênero Anastrepha (Diptera: Tephritidae); Tese de Livre-Docência, IB/USP: São Paulo, Brazil, 1984; 189p. [Google Scholar]
- Henning, F.; Matioli, S.R. Mating time of the West fruit fly Anastrepha obliqua (Macquart) (Diptera: Tephritidae) under laboratory conditions. Neotrop. Entomol. 2006, 35, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.M.; Rocha, L.L.; Souza, M.L.; Mendes, N.H.D.; Souza, J.M.G.A. Escolha de parceiros sexuais em Anastrepha zenildae (Zucchi, 1979, Diptera: Tephritidae): Papel dos caracteres morfológicos. Biotemas 2013, 26, 113–120. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).