The Best of Both Worlds? Hybridization Potentiates Exotic Bohemian Knotweed’s (Reynoutria × bohemica) Impacts on Native Plant and Faunal Communities †
Abstract
:1. Introduction
2. Material & Methods
2.1. Study Sites and Sampling
2.2. Data Analysis
3. Results
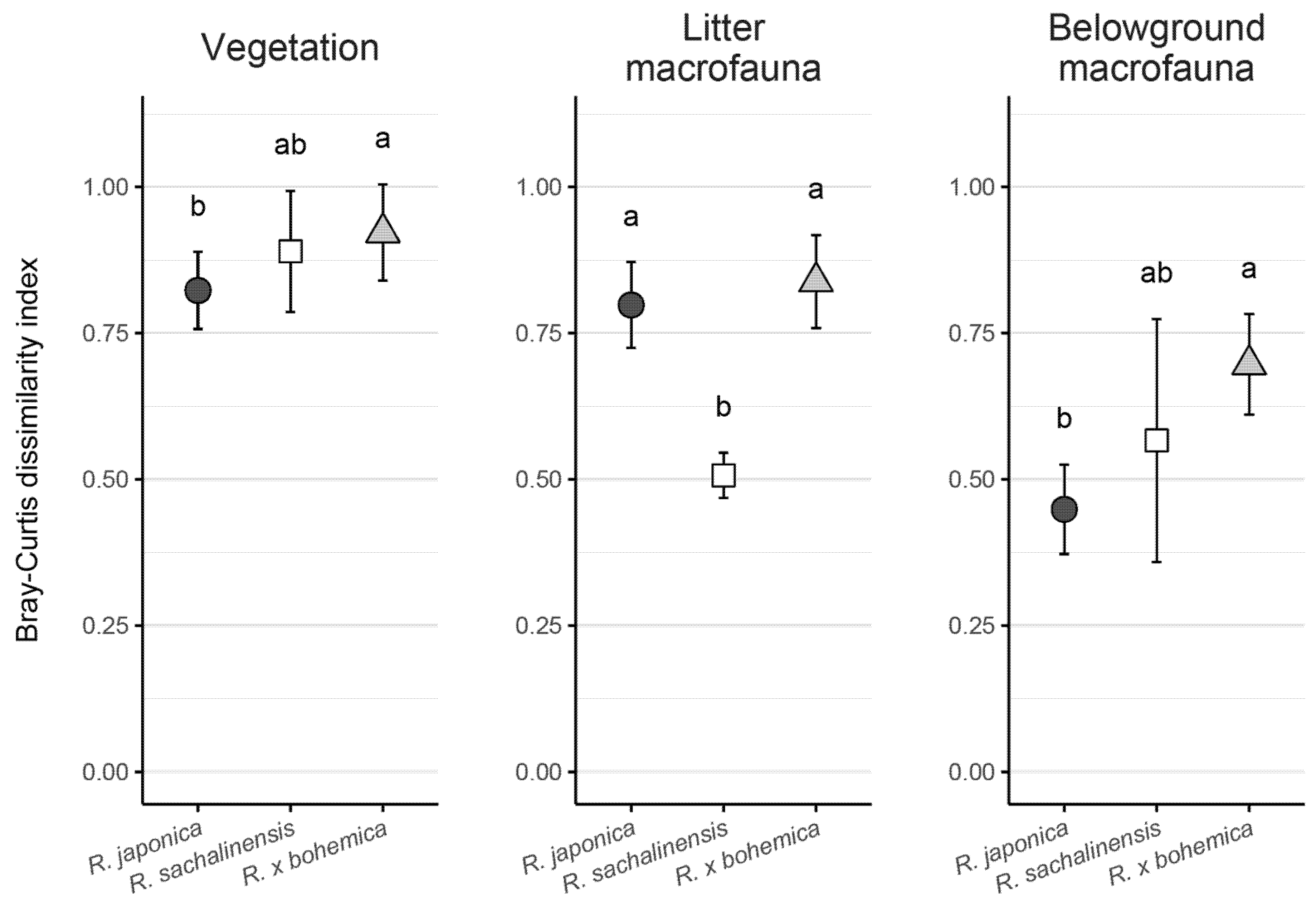
3.1. Impact of Knotweed Invasion on Taxonomic Richness of Native Communities
3.2. Impact of Knotweed Invasion on Composition of Native Communities
4. Discussion
4.1. Effects of Asian Knotweed Invasion on Native Plant Communities
4.2. Effects of Asian Knotweeds on Aboveground Macroinvertebrate Communities
4.3. Effects of Asian Knotweeds on Belowground Macroinvertebrates
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Luque, G.M.; Bellard, C.; Bertelsmeier, C.; Bonnaud, E.; Genovesi, P.; Simberloff, D.; Courchamp, F. The 100th of the world’s worst invasive alien species. Biol. Invasions 2014, 16, 981–985. [Google Scholar] [CrossRef]
- Invasive Species Specialist Group Polygonum Cuspidatum Sieb. & Zucc. (= Fallopia japonica (Houtt. Dcne.)). Global Invasive Species Database. International Union for Conservation of Nature. Available online: http://www.iucngisd.org/gisd/species.php?sc=91 (accessed on 25 April 2019).
- Bailey, J.P.; Conolly, A.P. Prize-winners to pariahs—A history of Japanese Knotweed s.l. (Polygonaceae) in the British Isles. Watsonia 2000, 23, 93–110. [Google Scholar]
- Barney, J.N. North American History of Two Invasive Plant Species: Phytogeographic Distribution, Dispersal Vectors, and Multiple Introductions. Biol. Invasions 2006, 8, 703–717. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Gruber, W.; Meerts, P. Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations. Écoscience 2007, 14, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Pyšek, P.; Moravcová, L.; Jarošik, V.; Zákravský, P. Potential phytotoxic and shading effects of invasive Fallopia (Polygonaceae) taxa on the germination of native dominant species. NeoBiota 2011, 9, 31–47. [Google Scholar] [CrossRef] [Green Version]
- Horáčková, J.; Juřičková, L.; Šizling, A.L.; Jarošík, V.; Pyšek, P. Invasiveness Does Not Predict Impact: Response of Native Land Snail Communities to Plant Invasions in Riparian Habitats. PLoS ONE 2014, 9, e108296. [Google Scholar] [CrossRef] [Green Version]
- Chmura, D.; Tokarska-Guzik, B.; Nowak, T.; Woźniak, G.; Bzdęga, K.; Koszela, K.; Gancarek, M. The influence of invasive Fallopia taxa on resident plant species in two river valleys (southern Poland). Acta Soc. Bot. Pol. 2015, 84, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Stefanowicz, A.M.; Stanek, M.; Nobis, M.; Zubek, S. Few effects of invasive plants Reynoutria japonica, Rudbeckia laciniata and Solidago gigantea on soil physical and chemical properties. Sci. Total Environ. 2017, 574, 938–946. [Google Scholar] [CrossRef]
- Maerz, J.C.; Blossey, B.; Nuzzo, V. Green Frogs Show Reduced Foraging Success in Habitats Invaded by Japanese knotweed. Biodivers. Conserv. 2005, 14, 2901–2911. [Google Scholar] [CrossRef]
- Gerber, E.; Krebs, C.; Murrell, C.; Moretti, M.; Rocklin, R.; Schaffner, U. Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats. Biol. Conserv. 2008, 141, 646–654. [Google Scholar] [CrossRef]
- Stoll, P.; Gatzsch, K.; Rusterholz, H.-P.; Baur, B. Response of plant and gastropod species to knotweed invasion. Basic Appl. Ecol. 2012, 13, 232–240. [Google Scholar] [CrossRef]
- Claeson, S.M.; LeRoy, C.J.; Barry, J.R.; Kuehn, K.A. Impacts of invasive riparian knotweed on litter decomposition, aquatic fungi, and macroinvertebrates. Biol. Invasions 2014, 16, 1531–1544. [Google Scholar] [CrossRef]
- Kappes, H.; Lay, R.; Topp, W. Changes in Different Trophic Levels of Litter-dwelling Macrofauna Associated with Giant Knotweed Invasion. Ecosystems 2007, 10, 734–744. [Google Scholar] [CrossRef]
- Maurel, N.; Salmon, S.; Ponge, J.-F.; Machon, N.; Moret, J.; Muratet, A. Does the invasive species Reynoutria japonica have an impact on soil and flora in urban wastelands? Biol. Invasions 2010, 12, 1709–1719. [Google Scholar] [CrossRef] [Green Version]
- Tamura, M.; Tharayil, N. Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol. 2014, 203, 110–124. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setälä, H.; Van Der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Wolfe, B.E.; Klironomos, J.N. Breaking New Ground: Soil Communities and Exotic Plant Invasion. Bioscience 2005, 55, 477–487. [Google Scholar] [CrossRef]
- Bardgett, R.D. The Biology of Soil: A Community and Ecosystem Approach; Bardgett, R.D., Ed.; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Knops, J.M.H.; Tilman, D. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 2009, 12, 1029–1039. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.M.; Crutsinger, G.M.; Gross, K.; Haarstad, J.; Tilman, D. Plant diversity and the stability of foodwebs. Ecol. Lett. 2011, 14, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Tanner, R.A.; Varia, S.; Eschen, R.; Wood, S.; Murphy, S.T.; Gange, A.C. Impacts of an Invasive Non-Native Annual Weed, Impatiens glandulifera, on Above- and Below-Ground Invertebrate Communities in the United Kingdom. PLoS ONE 2013, 8, e67271. [Google Scholar] [CrossRef] [Green Version]
- Maceda-Veiga, A.; Basas, H.; Lanzaco, G.; Sala, M.; de Sostoa, A.; Serra, A. Impacts of the invader giant reed (Arundo donax) on riparian habitats and ground arthropod communities. Biol. Invasions 2016, 18, 731–749. [Google Scholar] [CrossRef]
- Lecerf, A.; Patfield, D.; Boiché, A.; Riipinen, M.P.; Chauvet, E.; Dobson, M. Stream ecosystems respond to riparian invasion by Japanese knotweed (Fallopia japonica). Can. J. Fish. Aquat. Sci. 2007, 64, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.F.; Chapuis, E.; Loeuille, N. Impacts of Invasive Species on Food Webs: A Review of Empirical Data. In Advances in Ecological Research; Academic Press Inc.: Cambridge, MA, USA, 2017; Volume 56, pp. 1–60. [Google Scholar]
- McCary, M.A.; Mores, R.; Farfan, M.A.; Wise, D.H. Invasive plants have different effects on trophic structure of green and brown food webs in terrestrial ecosystems: A meta-analysis. Ecol. Lett. 2016, 19, 328–335. [Google Scholar] [CrossRef]
- Zhang, P.; Li, B.; Wu, J.; Hu, S. Invasive plants differentially affect soil biota through litter and rhizosphere pathways: A meta-analysis. Ecol. Lett. 2019, 22, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Abgrall, C.; Forey, E.; Chauvat, M. Soil fauna responses to invasive alien plants are determined by trophic groups and habitat structure: A global meta-analysis. Oikos 2019, 128, 1390–1401. [Google Scholar] [CrossRef]
- Barney, J.N.; Tharayil, N.; DiTommaso, A.; Bhowmik, P.C. The Biology of Invasive Alien Plants in Canada. 5. Polygonum cuspidatum Sieb. & Zucc. [= Fallopia japonica (Houtt.) Ronse Decr.]. Can. J. Plant Sci. 2006, 86, 887–906. [Google Scholar] [CrossRef] [Green Version]
- Locandro, R. Reproduction Ecology of Fallopia Japonica. Ph.D. Thesis, Rutgers University, New Brunswick, NJ, USA, 1973. [Google Scholar]
- Brock, J.; Wade, M. Regeneration of Japanese knotweed (Fallopia japonica) from rhizome and stems: Observation from greenhouse trials. In Proceedings of the 9th international Symposium on the Biology of Weed, Dijon, France, 16–18 September 1992; pp. 85–94. [Google Scholar]
- Palmer, J.P. Fallopia Japonica (Japanese Knotweed) in Wales. Ecol; Centre for Agriculture and Bioscience International: Wallingford, UK, 1994; pp. 159–172. [Google Scholar]
- Vastano, B.C.; Chen, Y.; Zhu, N.; Ho, C.-T.; Zhou, Z.; Rosen, R.T. Isolation and Identification of Stilbenes in Two Varieties of Polygonum cuspidatum. J. Agric. Food Chem. 2000, 48, 253–256. [Google Scholar] [CrossRef]
- Rouifed, S.; Piola, F.; Spiegelberger, T. Invasion by Fallopia spp. in a French upland region is related to anthropogenic disturbances. Basic Appl. Ecol. 2014, 15, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Bímová, K.; Mandák, B.; Pyšek, P. Experimental study of vegetative regeneration in four invasive Reynoutria taxa (Polygonaceae). Plant Ecol. 2003, 166, 1–11. [Google Scholar] [CrossRef]
- Parepa, M.; Fischer, M.; Krebs, C.; Bossdorf, O. Hybridization increases invasive knotweed success. Evol. Appl. 2014, 7, 413–420. [Google Scholar] [CrossRef]
- Krebs, C.; Gerber, E.; Matthies, D.; Schaffner, U. Herbivore resistance of invasive Fallopia species and their hybrids. Oecologia 2011, 167, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Urgenson, L.S.; Reichard, S.H.; Halpern, C.B. Community and ecosystem consequences of giant knotweed (Polygonum sachalinense) invasion into riparian forests of western Washington, USA. Biol. Conserv. 2009, 142, 1536–1541. [Google Scholar] [CrossRef]
- Bailey, J.P.; Wisskirchen, R. The distribution and origins of Fallopia × bohemica (Polygonaceae) in Europe. Nord. J. Bot. 2004, 24, 173–199. [Google Scholar] [CrossRef]
- Tiébré, M.-S.; Bizoux, J.-P.; Hardy, O.J.; Bailey, J.P.; Mahy, G. Hybridization and morphogenetic variation in the invasive alien Fallopia (Polygonaceae) complex in Belgium. Am. J. Bot. 2007, 94, 1900–1910. [Google Scholar] [CrossRef] [Green Version]
- Mandák, B.; Pyšek, P.; Bímová, K. History of the invasion and distribution of Reynoutria taxa in the Czech Republic: A hybrid spreading faster than its parents. Preslia 2004, 76, 15–64. [Google Scholar]
- Tiébré, M.S.; Saad, L.; Mahy, G. Landscape dynamics and habitat selection by the alien invasive Fallopia (Polygonaceae) in Belgium. Biodivers. Conserv. 2008, 17, 2357–2370. [Google Scholar] [CrossRef] [Green Version]
- Vuković, N.; Šegota, V.; Alegro, A.; Koletić, N.; Rimac, A.; Dekanić, S. “Flying under the radar”—How misleading distributional data led to wrong appreciation of knotweeds invasion (Reynoutria spp.) in Croatia. BioInvasions Rec. 2019, 8, 175–189. [Google Scholar] [CrossRef]
- Walls, R.L. Hybridization and plasticity contribute to divergence among coastal and wetland populations of invasive Hybrid Japanese knotweed s.l. (Fallopia spp.). Estuaries Coasts 2010, 33, 902–918. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Schwarzländer, M.; Grevstad, F.S.; Haverhals, M.A.; Bourchier, R.S.; Miller, T.W. Extreme differences in population structure and genetic diversity for three invasive congeners: Knotweeds in western North America. Biol. Invasions 2014, 16, 2127–2136. [Google Scholar] [CrossRef]
- Duquette, M.-C.; Compérot, A.; Hayes, L.F.; Pagola, C.; Belzile, F.; Dubé, J.; Lavoie, C. From the Source to the Outlet: Understanding the Distribution of Invasive Knotweeds along a North American River. River Res. Appl. 2016, 32, 958–966. [Google Scholar] [CrossRef]
- Pyšek, P.; Brock, J.H.; Bímová, K.; Mandak, B.; Jarošík, V.; Koukolíková, I.; Pergl, J.; Stepanek, J. Vegetative regeneration in invasive Reynoutria (Polygonaceae) taxa: The determinant of invasibility at the genotype level. Am. J. Bot. 2003, 90, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Buhk, C.; Thielsch, A. Hybridisation boosts the invasion of an alien species complex: Insights into future invasiveness. Perspect. Plant Ecol. Evol. Syst. 2015, 17, 274–283. [Google Scholar] [CrossRef]
- Gillies, S.; Clements, D.R.; Grenz, J. Knotweed (Fallopia spp.) Invasion of North America Utilizes Hybridization, Epigenetics, Seed Dispersal (Unexpectedly), and an Arsenal of Physiological Tactics. Invasive Plant Sci. Manag. 2016, 9, 71–80. [Google Scholar] [CrossRef]
- Baker, H.G. The Evolution of Weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants? Euphytica 2006, 148, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, C. The impact of invasive knotweed species (Reynoutria spp.) on the environment: Review and research perspectives. Biol. Invasions 2017, 19, 2319–2337. [Google Scholar] [CrossRef]
- Skubała, P.; Mierny, A. Invasive Reynoutria taxa as a contaminant of soil. Does it reduce abundance and diversity of microarthropods and damage soil habitat? Pestycydy 2009, 1–4, 57–62. [Google Scholar]
- Mincheva, T.; Barni, E.; Varese, G.C.; Brusa, G.; Cerabolini, B.; Siniscalco, C. Litter quality, decomposition rates and saprotrophic mycoflora in Fallopia japonica (Houtt.) Ronse Decraene and in adjacent native grassland vegetation. Acta Oecologica 2014, 54, 29–35. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Milcu, A.; Sabais, A.C.W.; Bessler, H.; Brenner, J.; Engels, C.; Klarner, B.; Maraun, M.; Partsch, S.; Roscher, C.; et al. Plant Diversity Surpasses Plant Functional Groups and Plant Productivity as Driver of Soil Biota in the Long Term. PLoS ONE 2011, 6, e16055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callaway, R.; Ridenour, W. Novel weapons: A biochemically based hypothesis for invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B. Similarities in the impact of three large invasive plant species on soil seed bank communities. Biol. Invasions 2010, 12, 1671–1683. [Google Scholar] [CrossRef]
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 2018, 127, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.P.; Bímová, K.; Mandák, B. Asexual spread versus sexual reproduction and evolution in Japanese Knotweed s.l. sets the stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Prinzipien Einer Systematik der Pflanzengesellschaften auf Floristischer Grundlage; St. Gallische Naturwissenschaftliche Gesellschaft: St. Gallen, Switzerland, 1921. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing. Available online: https://cran.microsoft.com/snapshot/2014-09-08/web/packages/dplR/vignettes/xdate-dplR.pdf (accessed on 1 March 2021).
- RStudio Team. RStudio: Integrated Development for R. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.651.1157&rep=rep1&type=pdf#page=14 (accessed on 1 March 2021).
- Dray, S.; Dufour, A.B. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.3-1; John Wiley & Sons: SonsHoboken, NJ, USA, 2019. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-5. 2019. Available online: https://www.researchgate.net/publication/258996451_Vegan_Community_Ecology_Package_R_Package_Version_20-10 (accessed on 1 March 2021).
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Brabec, J.; Pyšek, P. Establishment and survival of three invasive taxa of the Genus Reynoutria (Polygonaceae) in mesic mown meadows: A field experimental study. Folia Geobot. 2000, 35, 27–42. [Google Scholar] [CrossRef]
- Vrchotová, N.; Šerá, B. Allelopathic properties of knotweed rhizome extracts. Plant Soil Environ. 2008, 54, 301–303. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, C.; Xiong, S.; Johansson, M.E.; Vought, L.B.-M. Effects of leaf-litter accumulation on riparian plant diversity across Europe. Ecology 1999, 80, 1770–1775. [Google Scholar] [CrossRef]
- Xiong, S.; Nilsson, C. Dynamics of leaf litter accumulation and its effects on riparian vegetation: A review. Bot. Rev. 1997, 63, 240–264. [Google Scholar] [CrossRef]
- Mincheva, T.; Barni, E.; Siniscalco, C. From plant traits to invasion success: Impacts of the alien Fallopia japonica (Houtt.) Ronse Decraene on two native grassland species. Plant Biosyst. 2016, 150, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Bardon, C.; Poly, F.; Haichar, F.E.Z.; Le Roux, X.; Simon, L.; Meiffren, G.; Comte, G.; Rouifed, S.; Piola, F. Biological denitrification inhibition (BDI) with procyanidins induces modification of root traits, growth and N status in Fallopia x bohemica. Soil Biol. Biochem. 2017, 107, 41–49. [Google Scholar] [CrossRef]
- Staentzel, C.; Rouifed, S.; Beisel, J.-N.; Hardion, L.; Poulin, N.; Combroux, I. Ecological implications of the replacement of native plant species in riparian systems: Unexpected effects of Reynoutria japonica Houtt. leaf litter. Biol. Invasions 2020, 22, 1917–1930. [Google Scholar] [CrossRef]
- Hejda, M.; Pyšek, P.; Jarošík, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Hurd, L.E.; Mellinger, M.V.; Wolf, L.L.; McNaughton, S.J. Stability and diversity at three trophic levels in terrestrial successional ecosystems. Science 1971, 173, 1134–1136. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Homage to Santa Rosalia or Why Are There So Many Kinds of Animals? Am. Nat. 1959, 93, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Strong, D.R.J.; Lawton, J.H.; Southwood, T.R.E. Insects on Plants: Community Patterns and Mechanisms; Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Tilman, D.; Reich, P.B.; Knops, J.; Wedin, D.; Mielke, T.; Lehman, C. Diversity and productivity in a long-term grassland experiment. Science 2001, 294, 843–845. [Google Scholar] [CrossRef] [Green Version]
- Polis, G.A.; Strong, D.R. Food web complexity and community dynamics. Am. Nat. 1996, 147, 813–846. [Google Scholar] [CrossRef]
- Topp, W.; Kappes, H.; Rogers, F. Response of ground-dwelling beetle (Coleoptera) assemblages to giant knotweed (Reynoutria spp.) invasion. Biol. Invasions 2008, 10, 381–390. [Google Scholar] [CrossRef]
- Song, Y.; Wang, P.; Li, G.; Zhou, D. Relationships between functional diversity and ecosystem functioning: A review. Acta Ecol. Sin. 2014, 34, 85–91. [Google Scholar] [CrossRef]
- Scherber, C.; Eisenhauer, N.; Weisser, W.W.; Schmid, B.; Voigt, W.; Fischer, M.; Schulze, E.D.; Roscher, C.; Weigelt, A.; Allan, E.; et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 2010, 468, 553–556. [Google Scholar] [CrossRef]
- Korthals, G.W.; Smilauer, P.; Van Dijk, C.; Van Der Putten, W.H. Linking above- and below-ground biodiversity: Abundance and trophic complexity in soil as a response to experimental plant communities on abandoned arable land. Funct. Ecol. 2001, 15, 506–514. [Google Scholar] [CrossRef]
- Henneron, L.; Aubert, M.; Archaux, F.; Bureau, F.; Dumas, Y.; Ningre, F.; Richter, C.; Balandier, P.; Chauvat, M. Forest plant community as a driver of soil biodiversity: Experimental evidence from collembolan assemblages through large-scale and long-term removal of oak canopy trees Quercus petraea. Oikos 2017, 126, 420–434. [Google Scholar] [CrossRef]
- Ehrenfeld, J.G. Effects of Exotic Plant Invasions on Soil Nutrient Cycling Processes. Ecosystems 2003, 6, 503–523. [Google Scholar] [CrossRef]
- van Hengstum, T.; Hooftman, D.A.P.; Oostermeijer, J.G.B.; van Tienderen, P.H. Impact of plant invasions on local arthropod communities: A meta-analysis. J. Ecol. 2014, 102, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Schaffers, A.P.; Raemakers, I.P.; Sýkora, K.V.; ter Braak, C.J.F. Arthropod assemblages are best predicted by plant species composition. Ecology 2008, 89, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Belnap, J.; Phillips, S.L. Soil biota in an ungrazed grassland: Response to annual grass (Bromus tectorum) invasion. Ecol. Appl. 2001, 11, 1261–1275. [Google Scholar] [CrossRef]
- Callaway, R.M.; Thelen, G.C.; Rodriguez, A.; Holben, W.E. Soil biota and exotic plant invasion. Nature 2004, 427, 731–733. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.M.; Dudley, T.L. Reduction of riparian arthropod abundance and diversity as a consequence of giant reed (Arundo donax) invasion. Biol. Invasions 2003, 5, 167–177. [Google Scholar] [CrossRef]

| Invasive_Species | Site | Spatial Coordinates | Soil Type | pH | Organic_C Content (%) | Total_N Content (%) | Native Litter Amount (cm) | Invaded Litter Amount (cm) |
|---|---|---|---|---|---|---|---|---|
| Reynoutria × bohemica | Belbeuf | 49.3908692N, 1.12413817E | Loam | 7.90 ± 0.13 | 4.26 ± 1.95 | 0.278 ± 0.125 | 0.4 ± 0.4 | 9.8 ± 2.1 |
| R. × bohemica | Gouville | 48.8417835N, 1.00837984E | Silt loam | 6.03 ± 0.33 | 2.64 ± 0.63 | 0.254 ± 0.058 | 2.9 ± 1.2 | 5.6 ± 1.5 |
| R. × bohemica | Oissel | 49.3564571N, 1.12406613E | Sandy loam | 6.27 ± 0.59 | 1.42 ± 0.37 | 0.124 ± 0.030 | 0 | 14.9 ± 3.5 |
| R. japonica | Sylvains-les-Moulins | 48.8839602N, 1.08344571E | Silt loam | 6.76 ± 0.17 | 5.46 ± 0.70 | 0.551 ± 0.070 | 5.6 ± 2.2 | 3.4 ± 0.8 |
| R. japonica | Vimoutiers | 48.9396947N, 0.18810666E | Sandy loam | 7.46 ± 0.57 | 2.71 ± 0.48 | 0.260 ± 0.039 | 1.4 ± 0.9 | 3.1 ± 1.0 |
| R. sachalinensis | Harfleur | 49.514873N, 0.191627E | Loam | 7.82 ± 0.14 | 3.52 ± 1.52 | 0.264 ± 0.066 | 4.6 ± 2.2 | 7.7 ± 2.7 |
| R. sachalinensis | Quillebeuf | 49.473142N, 0.524756E | Silt loam | 8.00 ± 0.06 | 3.56 ± 1.00 | 0.324 ± 0.082 | 0.8 ± 0.8 | 0.7 ± 0.5 |
| Site | Vegetation | Aboveground Macroinvertebrates | Belowground Macroinvertebrates | |||
|---|---|---|---|---|---|---|
| Richness | Richness | Abundance | Richness | Abundance | ||
| Belbeuf | Uninvaded | 23 | 6 | 2.00 ± 1.00 | 6 | 2.33 ± 0.58 |
| Invaded (Rb) | 8 | 7 | 26.00 ± 14.10 | 2 | 2.00 ± 1.73 | |
| Gouville | Uninvaded | 12 | 3 | 0.67 ± 1.15 | 11 | 8.00 ± 2.00 |
| Invaded (Rb) | 7 | 12 | 15.70 ± 14.60 | 11 | 12.00 ± 9.54 | |
| Oissel | Uninvaded | 31 | 7 | 2.67 ± 1.15 | 9 | 14.00 ± 1.00 |
| Invaded (Rb) | 1 | 9 | 13.30 ± 6.81 | 4 | 4.33 ± 2.89 | |
| Sylvains-lès-Moulins | Uninvaded | 11 | 4 | 0.67 ± 1.15 | 7 | 3.33 ± 1.53 |
| Invaded (Rj) | 7 | 9 | 2.67 ± 2.89 | 4 | 6.00 ± 6.08 | |
| Vimoutiers | Uninvaded | 24 | 3 | 0.33 ± 0.58 | 6 | 1.67 ± 1.53 |
| Invaded (Rj) | 19 | 6 | 0.33 ± 0.58 | 4 | 2.00 ± 0.00 | |
| Harfleur | Uninvaded | 24 | 10 | 37.3 ± 49.9 | 11 | 24.30 ± 12.70 |
| Invaded (Rs) | 5 | 6 | 8.67 ± 3.06 | 6 | 6.33 ± 2.31 | |
| Quillebeuf | Uninvaded | 15 | 8 | 2.00 ± 1.73 | 9 | 5.67 ± 3.79 |
| Invaded (Rs) | 11 | 5 | 0.00 ± 0.00 | 7 | 4.67 ± 1.53 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupert, M.; Margerie, P.; Forey, E.; Chauvat, M.; Bureau, F.; Aubert, M.; Prével, S.; Langlois, E.; Vincenot, L. The Best of Both Worlds? Hybridization Potentiates Exotic Bohemian Knotweed’s (Reynoutria × bohemica) Impacts on Native Plant and Faunal Communities. Biol. Life Sci. Forum 2021, 2, 20. https://doi.org/10.3390/BDEE2021-09471
Neupert M, Margerie P, Forey E, Chauvat M, Bureau F, Aubert M, Prével S, Langlois E, Vincenot L. The Best of Both Worlds? Hybridization Potentiates Exotic Bohemian Knotweed’s (Reynoutria × bohemica) Impacts on Native Plant and Faunal Communities. Biology and Life Sciences Forum. 2021; 2(1):20. https://doi.org/10.3390/BDEE2021-09471
Chicago/Turabian StyleNeupert, Markus, Pierre Margerie, Estelle Forey, Matthieu Chauvat, Fabrice Bureau, Michaël Aubert, Stève Prével, Estelle Langlois, and Lucie Vincenot. 2021. "The Best of Both Worlds? Hybridization Potentiates Exotic Bohemian Knotweed’s (Reynoutria × bohemica) Impacts on Native Plant and Faunal Communities" Biology and Life Sciences Forum 2, no. 1: 20. https://doi.org/10.3390/BDEE2021-09471
APA StyleNeupert, M., Margerie, P., Forey, E., Chauvat, M., Bureau, F., Aubert, M., Prével, S., Langlois, E., & Vincenot, L. (2021). The Best of Both Worlds? Hybridization Potentiates Exotic Bohemian Knotweed’s (Reynoutria × bohemica) Impacts on Native Plant and Faunal Communities. Biology and Life Sciences Forum, 2(1), 20. https://doi.org/10.3390/BDEE2021-09471








